Abstract
A number of studies have shown that systemic 5-HT6 receptor antagonists can improve learning and memory, but the mechanism for these observations is not known. As striatum normally expresses 5-HT6 receptors abundantly and is important in consolidating stimulus-response learning, we used targeted gene delivery to further increase the expression of 5-HT6 receptors in rat striatum and then examined learning. Increased 5-HT6 expression had no effect on performance in the Morris water maze, a hippocampal-dependent learning paradigm, and did not alter the latency to approach or consume sucrose tablets. However, rats with increased 5-HT6 expression failed to acquire a reward-based instrumental learning task, a striatum-dependent learning model, during 3 days of successive sessions as compared to sham surgery or GFP-expressing control rats. This behavioral deficit was observed in rats overexpressing 5-HT6 receptors in the dorsomedial striatum, but not in rats with increased dorsocentral striatal expression. The 5-HT6 receptor-associated deficit was reversed by administration of a 5-HT6 antagonist, SB-258585, before each training session. When animals learned the instrumental learning task before gene transfer, increased 5-HT6 receptor expression had no effect on long-term recall or performance of the task or on extinction of operant responding. Thus, 5-HT6 receptor activity in rat striatum disrupts acquisition of new instrumental learning but does not impair memory or performance of reward-motivated behavior once established.
Similar content being viewed by others
INTRODUCTION
Serotonin has received increasing attention as a modulator of learning and memory mechanisms, but the multiplicity of serotonin receptors and the difficulty in selectively modulating some of these receptors has impeded the understanding of how this neurotransmitter modulates critical learning behaviors. 5-HT6 receptors are one of the most recently identified serotonin receptor subtypes (Monsma et al, 1993; Plassat et al, 1993; Ruat et al, 1993), and are coupled to a G stimulatory protein, inducing cAMP through adenylate cyclase stimulation (Monsma et al, 1993). Several in situ hybridization studies have shown robust mRNA expression in rat and human striatum, nucleus accumbens, cortex and olfactory tubercle, with moderate expression in the hippocampus and thalamus (Gerard et al, 1996; Ward and Dorsa, 1996), whereas expression in mouse brain is very low compared to rat or human brain (Hirst et al, 2003). Immunohistochemical localization of 5-HT6 receptors has been detected in the above regions as well as the cerebellum and substantia nigra (Gerard et al, 1997; Hamon et al, 1999).
Over the last 10 years, increasing evidence indicates that 5-HT6 receptors play a role in memory consolidation (Meneses, 2001; Rogers and Hagan, 2001; Woolley et al, 2001; Foley et al, 2004; King et al, 2004; Mitchell and Neumaier, 2005). SB-271046, a 5-HT6 antagonist, has been shown to improve memory consolidation of passive avoidance, novel object recognition and autoshaping (Meneses, 2001; Woolley et al, 2001; Foley et al, 2004). In general, it appears that 5-HT6 blockade is more consistently effective in alleviating memory deficits rather than increasing memory in normally functioning animals.
Another 5-HT6 selective antagonist, BGC20-761, enhances memory consolidation and reverses scopolamine-induced memory deficit in social and visuospatial memory tasks (Mitchell et al, 2006). The mechanism of enhanced consolidation is not completely understood; however, microdialysis studies have shown that 5-HT6 inhibition increases acetylcholine and glutamate release in the cortex and hippocampus (Dawson et al, 2001; Riemer et al, 2003). Although a number of different antagonists have been tested, 5-HT6 receptor activation in learning has not been investigated, and the anatomical basis for 5-HT6 receptor effects on memory and learning have not been examined.
The striatum has the highest density of 5-HT6 expression in rat brain (Gerard et al, 1997; East et al, 2002), where receptors have been identified on medium spiny neurons (Ward and Dorsa, 1996), which make 95% of the neurons in this region. The dorsomedial striatum has been shown to be important for instrumental learning such as habituation to lever pressing (Hartley and Burgess, 2005), which may be influenced by serotoninergic activity (Harrison et al, 1999). Therefore, this region seemed like a logical place to investigate the effects of altered 5-HT6 expression on learning behavior. To examine the contribution of 5-HT6 receptors in the striatum to instrumental learning, we used viral-mediated gene transfer to increase 5-HT6 receptor expression followed by behavioral testing. This approach has the advantage of regional, temporal, and pharmacological specificity that could not have been easily achieved by other means, and does not disrupt the spatial and temporal patterning of information involved in endogenous serotonin neurotransmission. The present results suggest that serotonin neurotransmission via striatal 5-HT6 receptors has a potent effect on instrumental learning.
MATERIALS AND METHODS
Construction of the 5-HT6 Viral Vector
We used a modified version of the herpes simplex virus amplicon that expresses a desired transgene and green fluorescent protein (GFP) under separate transcriptional control that has been previously described (Clark et al, 2002). A hemagglutinin (HA) epitope tag coding sequencing was added to the N-terminus of the rat 5-HT6 receptor sequence by PCR cloning. This PCR product was subcloned using the Zero Blunt TOPO PCR Cloning Kit (Invitrogen, Carlsbad, CA). From the TOPO plasmid, the inserted PCR product was cut out using BglII and AvrII sites engineered into the PCR primers. This fragment was inserted into the viral amplicon behind the HSV promoter; GFP expression was under the control of the CMV I/E promoter/enhancer. The HA-5-HT6 sequence of the pHSV-HA6/GFP amplicon was confirmed in its entirety. The pHSV-HA6/GFP amplicon (Figure 2a) was packaged into HSV viral particles as previously described (Clark et al, 2002) yielding approximately 1 × 108 infective units/ml.
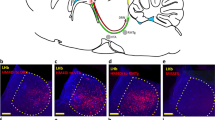
5-HT6 immunohistochemistry transfected striatal sections. (a) Amplicon map of pHSV-HA6/GFP; GFP expression is driven by a CMV promoter and HA-5-HT6 expression by an HSV promoter. (b) Photomicrograph of striatal infusion site: GFP (green) and 5-HT6 immunohistochemical staining (red); generally infected neurons lie within 200–300 μm of the center of the injection site. scale bar indicates 50 μm; (c) no antibody control tissue, scale bar indicates 10 μm; (d) 5-HT6 antibody in control striatum that received GFP-only vector; (e) 5-HT6-like immunostaining after pHSV-HA6/GFP gene transfer into striatum. Note that punctate 5-HT6 immunolabeling of endogenous receptors is apparent in control or uninfected neurons, while immunostaining is significantly increased in neurons after infection with pHSV-HA6/GFP.
Confirmation of Transgene Function
Functional characteristics of the HA-modified 5-HT6 receptor was evaluated using a luciferase-based cAMP assay (Kohen et al, 2001). PC12 cells were seeded onto 24-well plates, and grown to 50% confluence in Dulbecco's modified Eagle's media supplemented with 12% fetal calf serum and ampicillin. Using FuGENE (Roche, Palo Alto, CA), cells were transfected with 0.5 ng/well of rat wild-type 5-HT6 or pHSV-HA6/GFP plasmid DNA. All wells were also transfected with 0.5 ng of plasmid containing the RSV-cAMP responsive element (CRE)-luciferase and 0.1 ng of plasmid containing the β-galactose gene (gifts from Mark Hamblin). At 24 h before the assay, media was aspirated and replaced with serum-free media. Triplicate wells were incubated with serial dilutions of 5-HT (Perkin-Elmer, Boston, MA) added to Optimem media (Invitrogen) for 4 h. Some wells were also treated with the 5-HT6 antagonist, BGC20-761 (Tsai et al, 2000; Mitchell et al, 2006). Cells were rinsed with phosphate-buffered saline (PBS) and lysed with a proprietory lysing buffer (Promega, Madison, WI). Luciferase activity was measured with a commercial assay system (steadylite, Promega), using a Perkin-Elmer Fusion microplate reader to detect luminescence. Luciferase luminescence was quantified as a percent increase over control (no 5-HT added). Transfection efficiency was measured by an X-gal assay. Briefly, the cells were rinsed several times with phosphate-buffered saline, fixed with 0.5% glutaraldehyde for 30 min, and incubated for 24 h in an X-gal Reaction buffer (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl, and 2 mM X-Gal in PBS).
Animals
In all, 160 male Long-Evans rats, 300 g in weight, were ordered from Charles River Laboratories and housed according to the guidelines of the IACUC of University of Washington. Rats were pair-housed, kept in a 12:12 light/dark schedule (lights on at 0600 hours) and fed ad lib food and water unless otherwise specified.
Stereotaxic Surgery
Surgery was performed under aseptic conditions and general anesthesia was maintained using 2–3% isoflurane. The scalp of the rat was shaved and wiped with Betadine and secured into a stereotaxic device (Stoelting, Woodale, IL) with blunt ear-bars. After a vertical scalp incision, the skin covering the skull was retracted and bregma was located. Jeweler's screws were drilled into two caudal regions of the skull to provide anchorage. Two single barrel 26-gauge guide cannulas with dummy cannulas in place (Plastics One, Roanoke, VA) were inserted at the dorsomedial striatum A/P −0.8, M/D ±2.8 from the bregma (Paxinos and Wats, 1982), advanced to 5.0 mm below dura, and secured with dental cement (Supplementary Figure 1). Rats from an additional set were implanted with cannulas in the dorsal central striatum A/P −0.2, M/D ±3.3 as an anatomical control (Supplementary Figure 2). The skin around the dental cement was sutured and antibiotic ointment was applied to the scalp. The cannulas were covered by screw-on plastic caps. Each rat received buprenorphine (s.c. 0.5 mg/kg) to reduce post-surgical pain. The rats were allowed to recover over the course of 3 days.
Viral Vector Microinjections and Feeding Schedule
We used a viral vector injection method modified for the striatum that has been previously described by this and other labs (Carlezon et al, 2000a, 2000b; Clark et al, 2002; Neumaier et al, 2002). At 4 days after surgery, the rats were started on a food-restricted diet of 20 g of rat chow pellets and ad lib water for 10 days before operant conditioning was started. During the reduced feeding period, the rats were housed singly so that their food intake could be accurately monitored. The rats were weighed daily to ascertain a consistent weight loss. The rats typically lost 10–15% of their original weight. While the rats were being tested, they were kept on the same diet, being fed at 1700 hours each day.
At 3 days before operant conditioning, rats were briefly anesthetized with 2% isoflurane and a 33-gauge infusion cannula was inserted through each implanted guide cannula and extended 1 mm below the tip of the guide cannula. Rats were infused bilaterally with 2 μl of either GFP-only (control; 200 000 infectious units in 10% sucrose) or HA6/GFP viral vector (200 000 infectious units in 10% sucrose) at a rate of 0.2 μl/min under the control of microprocessor-controlled pumps (World Precision Instruments, Sarasota, FL). The infusion cannula was left in place for at least 5 min after active infusion to ensure accurate dosing. Rats from one set, the ‘sham surgery’ group, were implanted with guide cannulas but did not receive viral infusions. These injection volumes and parameters produce a transgene expression zone of about 500 μm in length and 200 μm in width.
Operant Conditioning Procedure
The afternoon before testing, rats were presented with five sucrose pellets (45 mg, Bio-Serv, Frenchtown, NJ) and the intervals to approach and consumption of the first pellet were recorded. Rats from one set were briefly anesthetized 1 day before behavioral testing but did not receive surgery and acted as the ‘no surgery’ control group.
The animals were brought to the testing room at 0930 hours every day and left for an hour before training or testing. The rats were weighed before each conditioning session. Operant boxes (Med Associates, St Albans, VT) were equipped with a house light, a stimulus light above a retracting lever, and a food receptacle. All boxes were kept in sound-attenuating chambers (Med Associates) equipped with fans providing background noise and temperature control. Experimental events were controlled and data were recorded by MED-PC 4.0 (Med Associates) software on an IBM-compatible computer. Programs written in MedState Notation were used to train rats for reward-based operant conditioning and extinction.
The rat was placed in the operant chamber 5 min before the start of a session. Each session was initiated by the activation of the house light, which stayed on for the duration of the procedure. During the first training session (day 1), one sucrose pellet was presented in the food receptacle every 40 s. The rat received a total of 10 pellets over the course of 7 min before the first trial (lever presentation) began. The stimulus light over the retractable lever was activated at the same time the lever was presented. The stimulus light remained on and the lever was presented for 10 s. If the animal failed to press the lever during this time, the light turned off and the lever retracted. If the rat pressed the lever during the 10 s, a pellet was presented in the food receptacle and the lever was retracted. The lever was presented every 50 s for 50 trials (lever presentations). The rate of success was calculated as the number of successful lever presses as a ratio of the number of trials (lever presentations).
During the next training session of operant conditioning (day 2), three pellets were inserted into the receptacle during the first 2 min before the lever was presented for 40 trials. The last day of conditioning (day 3) follows the same procedure as day 2. Each rat was trained in a different box for each conditioning session. The boxes were cleaned with a detergent at the end of each session.
A separate over-training plus extinction schedule was also given to some rats. In this experiment, untreated rats were given one session per day of the instrumental training procedure as described above for 7 days in total. At this point, all rats were achieving at least 90% successful lever presses. After the seventh day, the rats were infused intrastriatally with either pHSV-GFP-only or pHSV-GFP/HA6 viral vector and allowed to recover for 2 days. Rats were then tested on the operant conditioning schedule once daily for 2 days to assess the effect of increased 5-HT6 expression on previously consolidated memory and performance of the lever-pressing task. Rats were then tested for extinction, where the rats were presented with the lever and cue light 50 times per session but no rewarding food pellet was delivered upon pressing of the lever. Rats were given one extinction session per day for 3 days.
Immediately after the end of the last session, rats were either narcotized with CO2, decapitated, and the brains removed for histological confirmation of cannula placement or anesthetized with a pentobarbital/xylazine solution and perfused transcardially with 1% heparin phosphate-buffered saline, then 4% paraformaldehyde (for immunohistochemical analysis). Each rat's brain was removed, post-fixed overnight in 4% paraformaldehyde, and then sliced into 40 μm sections with a vibratome. Sections were checked for cannula placement into the medial striatum and GFP fluorescence, indicating adequate viral infection and GFP expression in neurons.
Drug Treatment
In order to reverse the physiological effects of increased 5-HT6 expression, 5-HT6 antagonist were given to rats by various administration paradigms. Some pHSV-GFP-only and pHSV-HA6/GFP infused groups received the 5-HT6 antagonist, SB-258585 (i.p. 5 mg/kg, Sigma), or saline 10 min before the start of training on both day 1 and day 2 sessions, while some groups received the 5-HT6 antagonist, BGC20-761 (10 mg/kg i.p.), or SB-258585 (5 mg/kg i.p.) after the training session on day 2 only. A separate group of infused rats received SB-258585 intrastriatally via the guide cannula immediately after training on day 2. Rats were habituated to the infusion procedure for 2 days beforehand, by connecting plastic tubing to the awake rat's cannula guides. The rat was then allowed to move freely in its homecage. On the day of infusion, 1 μg per side of SB-258585 (in 2 μl of aCSF) was infused over the course of 10 min via a microprocessor-controlled pump.
Morris Water Maze
The apparatus used for behavior consisted of a galvanized steel tank, painted white, with dimensions 202 cm in diameter × 60 cm in height. A platform, also painted white and 34.5 cm in height × 11.5 cm in diameter was placed within the tank in one of the quadrants. The tanks was filled with warm water (24°C), made opaque by nontoxic, white tempura paint, to a depth of 36 cm. A solid-state monochromatic video camera recorded animal movement within the tank, data were then analyzed offline by the SMART Tracking program (San Diego Instruments). Visual cues (large posterboard geometric shapes) were placed in the south and east corners of the room.
The rats were habituated to the water maze the day before viral infusions by placing each animal into the tank (platform removed) and allowing it to swim for 90 s.
The learning trials began 3 days after viral infusion. Each rat was given four trials per day, with starting placements next to the north, south, east and west wall, always facing the inside of the tank. The rats were given 90 s to find and mount the platform. The rat was allowed to stand on the platform for 30 s before removal to a holding cage with a warming lamp. The next trial began 60 s later. If the rat failed to find the platform in 90 s, then it was guided to the platform. Trials were given for four cumulative days where starting placements for each trial were randomized. On the last day, an additional probe trial was performed (where the platform was removed) to assess spatial bias. In addition, a trial where the platform was 1 cm above the water was performed to test visual acuity. The SMART tracking program analyzed time to find the platform (latency), swim speed, swim path, and cumulative distance.
Immunohistochemistry
Floating sections (40 μm) were immersed in a 0.5% Triton-X PBS solution for 10 min, then blocked in 10% normal goat serum (NGS) Triton/PBS for 4 h. Sections were transferred in 5% NGS Triton/PBS containing 5-HT6 receptor antibody (1:500, rabbit, Novus, Littleton, CO) and gently agitated at 4°C for 72 h. After 4–5 rinses in PBS, sections were incubated with Alexa 568-conjugated goat anti-rabbit secondary antibody (1:200, Invitrogen). The sections were rinsed in several changes of PBS before being mounted on slides and cover-slipped with Vectashield mounting medium (Vectorlabs, Burlingame, CA). Slides were visualized with a Bio-Rad Radiance 2000 confocal system (Bio-Rad, Hercules, CA) and an associated Nikon fluorescence microscope using an argon/krypton laser and red laser diode.
For chromogenic staining, sections were blocked in NGS Triton/PBS, then placed in a 5-HT6 receptor antibody solution and incubated for 72 h at 4°C. The sections were rinsed, then immersed in biotinylated secondary antibody solution (1:200, goat anti-rabbit, Vector), followed by immersion in an avidin–biotin peroxidase solution (Vector Elite ABC kit) for 2 h and then reacted with diaminobenzidine (0.05%) with nickel (0.25%) and H2O2 (0.0015%). The sections were then mounted on glass slides, and dehydrated in increasing concentrations of ethanol. The slides were then immersed in xylene and cover-slipped with Permount.
Statistics
Data from the instrumental training sessions was analyzed for significance with a 2 × 2 and repeated measures ANOVA, followed by a Newman–Keuls post hoc test, using the Statistica suite, v.6 (Statsoft, Tulsa, OK), where significance was set at p<0.05. Morris water maze (MWM) data were analyzed for statistical significance using two-way (group × day) ANOVA followed by Newman–Keuls post hoc tests.
RESULTS
Activation of HA-Tagged 5-HT6 Receptors Produce Levels of cAMP Induction Similar to Wild Type
5-HT6 receptors are normally located in dendritic and ciliary processes of neurons, with little expression adjacent to the cell body (Gerard et al, 1997). In order to visualize neurons expressing transgenes, the GFP protein was coexpressed along with HA-5-HT6 receptor from the viral amplicon. GFP expression has been shown to be present throughout the cytosol in infected cells (Clark et al, 2002). To allow direct observation of transgenic 5-HT6 expression, we ‘tagged’ the 5-HT6 receptor with HA to identify 5-HT6-overexpressing cells. In order to confirm that the HA tag did not interfere with the pharmacological functioning of the receptor, a cAMP accumulation assay was performed comparing wild-type to tagged receptor.
We performed a cAMP accumulation assay visualized by luciferase to compare the functionality of the HA-tagged receptor. PC12 cells transfected with pHSV-HA6 or wild-type 5-HT6 receptor plasmid had no observable changes in health or growth. Transfection efficiency was about 30% per well based on GFP expression and HA immunostaining (data not shown). Cells transfected with luciferase and β-galactosidase plasmids only exhibited negligible increases in cAMP-induced luciferase luminescence when incubated with increasing concentrations 5-HT (data not shown). While there have been contradictory reports of endogenous 5-HT receptor expression in PC12 cells (Furukawa et al, 1992; Quinn et al, 2002), the absence of 5-HT-induced luminescence in luciferase-only treated cells indicates that PC12 cells do not exhibit endogenous serotonin receptors that stimulate adenylate cyclase in numbers significant enough to confound measurement of cAMP induction from transfected 5-HT6 receptors.
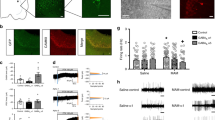
The expression of luciferase, coupled to the CRE element, did not differ appreciably in cells transfected with the wild-type or tagged 5-HT6 receptor, as shown by Figure 1. The EC50 of 5-HT for the wild-type receptor was 7.1 nM, while that for the tagged receptor was 6.9 nM, and are comparable to previous reports of wild-type rat 5-HT6 receptor EC50 as measured by cAMP accumulation assays (6.91 nM) (Boess et al, 1997). This indicates that HA-tagged 5-HT6 receptor shows similar affinity for 5-HT as wild type. Furthermore, a specific 5-HT6 antagonist, BGC20-761 (Mitchell et al, 2006), blocked 5-HT-induced luciferase expression equivalently in cells transfected with either wild-type or tagged 5-HT6 receptors (data not shown). Therefore, the presence of an amino terminal epitope tag did not appear to alter the pharmacology of the 5-HT6 receptor.
Luciferase assay measuring adenylate cyclase activity in PC12 cultures transfected with HA-tagged and or wild-type 5-HT6 receptor. Activity is expressed as a percentage of baseline luciferase luminescence, n=5 assays/dose. EC50 determinations were calculated with Sigma plot. Note that there was a modest amount of spare receptor activity in this overexpression system, but that the HA tag did not alter the functional characteristics of the 5-HT6 receptor.
Striatal Neurons Infected by HSV Amplicons Express HA-5-HT6 and GFP Proteins
Construction of the pHSV-HA6/GFP transgene amplicon is shown in Figure 2a. Expression of gene products from HSV viral vectors has been shown to peak about 3 days after injection and slowly decrease (Carlezon et al, 2000a). Furthermore, we have observed that expression levels of HA-5-HT1B receptor using the same vector in nucleus accumbens was comparable from days 2 to 10 after viral infection (data not shown), so transgene expression is quite stable over the duration of the behavioral experiments used in this report. At 6 days after intrastriatal infusion, brains injected with pHSV-GFP-only vector exhibited GFP-positive fluorescent cell bodies and processes in the medial striatum, as depicted in Figure 2b; no expression was apparent in cells with glial morphology. The area with greatest GFP expression was found directly below the guide cannula indentation, with moderate GFP expression observed adjacent to the guide cannula. Animals injected with the pHSV-HA6/GFP vector exhibited GFP expression in similar numbers of cells as the pHSV-GFP-only injected brains. Figure 2b shows increased 5-HT6 immunostaining within the medial striatum after injection of pHSV-HA6/GFP, both in the cell body and in the surrounding dendrites; most infected neurons show strong colocalized GFP fluorescence. Although immunostaining of 5-HT6 receptors can be seen throughout the striatum and cortex, immunostaining is primarily located within the dendrites of neurons. Figure 2d demonstrates the neuroanatomical localization of 5-HT6 immunohistochemical staining in control tissue, whereas Figure 2e shows increased staining in transfected tissue. Figure 2c shows the absence of specific staining in tissue processed without the 5-HT6 antibody. Figure 3 demonstrates striatal localization of the increased 5-HT6 expression directly beneath the infusion sites (Figures 3a and b).
Distribution of 5-HT6 expression following intrastriatal injection of HSV-HA6/GFP vector (a) Low magnification view of 5-HT6 immunohistochemical staining localization in the striatum. COR, cortex; STR, striatum. Scale bar indicates 500 μm; (b) high magnification view of 5-HT6 immunohistochemical staining localization below injection site. Scale bar indicates 500 μm. Note that increased 5-HT staining is apparent over a significant area within striatum; no GFP neurons were visible in similar section in cortex, and the tearing from the cannula occurs primarily at the time of dissection.
5-HT6 Receptor Overexpression in the Dorsomedial Striatum Decreased Learning of Operant Conditioning but had No Effect on Spatial Learning
Four groups of rats were food-restricted and trained to perform an instrumental learning procedure, pHSV-GFP-only or pHSV-HA6/GFP infused rats, as well as sham surgery rats and untreated rats, to serve as controls for viral infusion and surgery. After the viral vector infusions, rats were carefully monitored for changes in food intake and weight. No significant differences in feeding or weight were observed between groups. Additionally, there was no apparent neophobia or decreased appetite in any rat group, as tested by approach and consumption of sugar pellets 2 days after viral vector infusion (Figure 4a).
Increased expression 5-HT6 on behavior. Data are presented as means±SEM. (a) Effect of increased striatal 5-HT6 expression on mean time to approach and consumption of one 45 mg sugar pellet. n=6 for each group. (b) Effect of striatal 5-HT6 overexpression in conditioned learning: no surgery rats (n=8), sham: sham surgery (n=8), GFP, pHSV-GFP-only vector (n=12); 5-HT6, pHSV-HA6/GFP vector (n=12). *Significantly differently from GFP (p<0.05).
All treatment groups showed comparable, low rates of successful operant responses during the first session (Figure 4b). All treatment groups showed some increase in successful responses. On day 3, pHSV-GFP-only rats demonstrated significant increases in success rates (50±4%), but pHSV-HA6/GFP rats' performance did not improve (20±5%). Sham and no surgery rats exhibited sequential daily improvement in acquiring the instrumental task equivalent to that of the pHSV-GFP-only rats. There was an overall effect of days of training on the rate of successful operant responses for all groups (p<0.05, F(2,70)=25.2) except those with increased 5-HT6 expression; these animals were significantly different from GFP controls (p<0.05, F(3,39)=4.48), as well as the sham and untreated control groups.
In order to assess whether increased expression of striatal 5-HT6 affected the performance of lever pressing for food rewards, two groups of cannulated rats were trained to perform the instrumental learning task to a success rate of 90% or above and then pHSV-GFP-only or pHSV-HA6/GFP viral vectors were infused after training. In 2 days of subsequent probe tests, there was no difference in the number of responses between the two groups (Figure 5). This indicates that increased 5-HT6 expression in striatum did not block the performance of the food reward task once already learned. These rats were then given three additional daily sessions of extinction training during which lever presses were no longer rewarded. Both GFP-only control and increased 5-HT6 expressing animals had a similar rate of extinction learning as indicated by reduced rates of lever pressing.
Effect of 5-HT6 receptors on performance of previously acquired instrumental conditioning. Data are presented as means±SEM. Groups are as follows: pHSV-GFP-only (n=7): GFP or pHSV-HA6/GFP (n=7): 5-HT6. Data points PROBE 1 and PROBE 2 demonstrate lever-pressing rates of reward-based lever pressing on two separate days, whereas EXT 1, 2, and 3 demonstrate nonreward-based lever pressing over 3 days.
We also tested the effects of increased striatal expression of 5-HT6 receptors in a spatial learning task, the MWM (Morris, 1984). Rats with increased striatal 5-HT6 receptor expression learned to navigate the MWM at the same rate as pHSV-GFP-only control rats; more specifically, they showed no difference in memory of platform location over 4 days as compared to controls (Figure 6). Additionally, there were no differences in speed or path length between groups (data not shown).
Morris water maze training. (a) Probe test: percent time spent in platform quadrant and time spent in pool periphery (thigmotaxis). (b) Latency to reach the submerged platform. Data are presented as means±SEM. Groups are as follows: GFP, pHSV-GFP-only vector (n=7); 5-HT6, pHSV-HA6/GFP vector (n=7). Shown are means±SEM.
Learning Impairment from Increased 5-HT6 Receptor Expression in the Dorsomedial Striatum is Reversed by Administration of 5-HT6 Antagonists
Increased 5-HT6 expression prevented rats from acquiring the operant conditioning task during 3 days of training. To determine whether this could be attributed to overactivity of 5-HT6 receptors in the striatum following the training session, we administered the 5-HT6-selective antagonists (BGC20-761 or SB-258585) either intrastriatally or intraperitoneally immediately after the training session on day 2, but these two paradigms did not improve lever-pressing success rates in the pHSV-HA6/GFP-expressing rats (day 3 success rate—pHSV-HA6+SB258585 after day 2 session: 17±12%, pHSV-HA6+intrastriatal BGC20-761 on day 2: 22±11%). We next tested the effects of SB-258585 (5 mg/kg i.p.) vs saline given immediately before each conditioning session on days 1 and 2. pHSV-HA6/GFP and pHSV-GFP-only rats given pre-session saline performed similarly to each other on days 1 and 2. While saline-injected pHSV-HA6/GFP rats did not improve significantly on day 3 (14±3%), the saline-injected pHSV-GFP-only control rats' success rates increased (52±15%, p<0.05 (F(3,39)=3.66)) (Figure 7). These data replicate the results shown in Figure 3b. In contrast, the pHSV-HA6/GFP rats given pre-session SB-258585 displayed success rates comparable to both the saline-treated pHSV-GFP-only rats and the SB-258585-treated pHSV-GFP-only rats (p>0.05 (F(3,39)=1.56)) (Figure 7). As the animals were tested on day 3 in a drug-free state, the antagonist was probably influencing learning or memory consolidation during days 1 and 2 rather than performance on day 3.
Effect of striatal 5-HT6 overexpression and administration of a 5-HT6 antagonist, SB-258585, on instrumental learning. Data are presented as means±SEM. Groups: GFP SAL, pHSV-GFP-only vector+i.p. saline (n=12); 5-HT6 SAL, pHSV-HA6/GFP vector+i.p. saline (n=12); GFP SB, pHSV-GFP-only vector+SB-258585 (5 mg/kg, i.p.) (n=9); 5-HT6+SAL: pHSV-HA6/GFP vector+SB-258585 (5 mg/kg i.p.) (n=9). *p<0.05 vs GFP+SAL (F(3,39)=3.66).
In order to test whether SB-258585 modulates general motor activity, we measured noncontingent lever pressing in control rats expressing GFP that were administered SB-258585 (5 mg/kg i.p.) immediately before a session where the lever was presented but no reward was given after it was pressed. These rats did not show improvement in lever pressing over the course of 3 days (data not shown). This experiment demonstrates that SB-258585 did not affect motor activity levels as represented by spontaneous lever pressing.
Increased 5-HT6 Expression in the Dorsal Central Striatum has No Effect on Instrumental Conditioning
Two separate groups of rats were implanted with cannulas in either the dorsomedial striatum, the same region targeted in the above experiments, or the dorsal central region of the striatum. These rats were infused with either pHSV-GFP or pHSV-HA6 vectors. While increased expression of 5-HT6 receptors in the dorsomedial region was associated with impairment in the learning of the operant task as compared to control rats (post hoc Tukey test, p<0.043) (Figure 8), there was no impairment associated with increased 5-HT6 receptors in the dorsal central region; suggesting that 5-HT6 receptors in the dorsomedial region have a more profound effect on instrumental learning, at least under these conditions.
Increased expression of 5-HT6 on behavior: comparison of distinct striatal subregions. Data are presented as means±SEM. (a) Effect of increased 5-HT6 expression in the dorsomedial striatum. n=8 for each group. (b) Effect of increased 5-HT6 expression in the dorsal central striatum. n=11 for each group. *Significantly differently from GFP (p<0.05).
DISCUSSION
This is the first report of the behavioral effects of increased activation of 5-HT6 receptors in vivo through increased expression. Previous work from this lab has demonstrated the potential uses of viral-mediated gene transfer to investigate the functions of other serotonin receptors in discrete brain regions (Clark et al, 2002, 2004; Neumaier et al, 2002; Hoplight et al, 2006). We confirmed that the HA-tagged 5-HT6 receptor functioned identically to wild-type receptor in vitro, and the anatomical pattern of distribution of the transgenic 5-HT6 receptors appears to closely match the normal neuronal distribution of these receptors in striatum, albeit at higher levels. As altered receptor expression level may be important in pathophysiological mechanism, viral gene transfer offers a novel method for manipulating receptors in a brain region of interest, and may eventually be relevant for therapeutic interventions in the future. The synthesis of 5-HT6 selective agonists have been described (Beyer et al, 2005), but increased expression using viral vectors allows the investigation of the effects of endogenous 5-HT released during behavior; this preserves the information contained in the temporal and spatial patterning of 5-HT release and action at the site of increased receptor expression.
The 5-HT6 receptor has been implicated in memory function because antagonists can enhance memory consolidation (Rogers and Hagan, 2001; Woolley et al, 2001; Mitchell and Neumaier, 2005), however the effects of increased stimulation of 5-HT6 receptors on learning has not been previously reported. As 5-HT6 receptors are prominently expressed in rat and human striatum normally, we focused on this region using a stimulus-reward learning paradigm that depends on normal striatal function (Hartley and Burgess, 2005). Increased striatal expression of the 5-HT6 receptor had no effect on the number of successful lever presses on day 1 and day 2, suggesting that 5-HT6 activity did not impair initial exploratory behavior. However, on day 3, animals in all three control groups markedly increased their successful lever-pressing rate, whereas rats with increased 5-HT6 expression did not. This suggests that increased 5-HT6 expression reduced acquisition or consolidation of instrumental learning. However, the fact that the antagonist had significant effects only when it was given before a session points to an effect in acquisition.
It is unlikely that the impairment in learning in HA6/GFP-treated animals was due to nonspecific effects of increased 5-HT6 expression because SB-258585, a selective antagonist, reversed the impairment in these animals. We gave the antagonist before the conditioning session on days 1 and 2, but observed robust increase in successful responses on day 3; the improved learning was not simply an acute performance enhancement by the drug. We expected that giving a 5-HT6 antagonist immediately after the trial might enhance memory consolidation based on previous studies of these drugs on memory consolidation, but it was necessary to give the drug before the conditioning session to reverse the effects of increased 5-HT6 receptor expression. We did not see an increase in the performance in GFP rats given 5-HT6 antagonist as compared to those given saline, a result different from that of Meneses (2001), possibly due to the specific antagonist and dose used in the present study.
We also cannot rule out an effect of SB-258585 in other brain regions in reversing the effects of 5-HT6 overexpression in striatum, although it seems most likely to have been an effect in the striatum. While we did not detect a significant enhancement in operant learning in pHSV-GFP-only animals treated with SB-258585, a majority of previous studies have only detected a significant effect of 5-HT6 antagonists on memory consolidation using impairment experimental models such as scopolamine administration or aging (Foley et al, 2004; Mitchell and Neumaier, 2005; Mitchell et al, 2006). Thus, it is not surprising that the effects of SB-258585 were selective for the animals with increased 5-HT6 receptor expression and learning deficits.
In order to ascertain that this memory deficit affected instrumental learning involving striatal circuits, we trained rats infused with pHSV-HA6/GFP or pHSV-GFP-only viral vectors in a MWM. The MWM is a hippocampal-dependent learning task where visuospatial cues allow rats to navigate towards a hidden platform with a pool of water (Morris, 1984; Brandeis et al, 1989). Increased 5-HT6 expression did not impair memory retention of the platform location in a probe test, thus indicating that 5-HT6 expression is affecting primarily striatal-based learning and not attention or other global factors that might also impair spatial learning or general motor function.
While enhanced memory consolidation via systemic administration of 5-HT6 antagonists has received much attention (Mitchell and Neumaier, 2005), the present data focus on 5-HT6 receptors in striatum only, and the contribution of these localized receptors to acquisition vs consolidation needs to be addressed with additional experimentation. Other labs have shown that long-term retention of MWM training was improved with systemic administration of a 5-HT6 receptor antagonist (i.p.) or 5-HT6 antisense oligonucleotides (i.c.v.), but had no effect on acquisition (Woolley et al, 2001), as rats with decreased 5-HT6 receptor activity showed no difference in performance after several trials given on the same day, but did show improvement in trials given the day afterwards. However, a more recent study has demonstrated improvement in tasks measuring executive function, such as set-shifting, with administration of 5-HT6 antagonists (Hatcher et al, 2005). In contrast, no studies, to our knowledge, have demonstrated a role of 5-HT6 receptors in memory retrieval or extinction. Indeed, when rats over-trained on the instrumental learning task were infused with either pHSV-GFP-only or pHSV-HA6/GFP vector, we found no difference in performance of the previously learned task, demonstrating that increased levels of 5-HT6 receptors in the striatum had no effect on the recall or performance of acquired, well-learned tasks. Furthermore, when rats subsequently underwent extinction of the instrumental conditioning, the rate of extinction was the same in both groups, suggesting that this type of learning was not sensitive to increased 5-HT6 activity in striatum.
The striatum has been shown to be an important region for specific types of acquisition and memory consolidation, most notably learning involving habituated activity such as lever pressing (Yin et al, 2005). As stated previously, 5-HT6 receptors are localized in medium spiny GABAergic neurons expressing enkephalin, substance P, and dynorphin receptor subtypes (Ward and Dorsa, 1996). Such cell types comprise 95% of neurons within the striatum (Wilson and Groves, 1980). Based on the homogenous distribution of medium spiny neurons and the pattern of transgene expression, HSV-mediated expression of 5-HT6 receptors occurred predominantly in these striatal neurons. The HSV virus is neurotropic, gaining entry into neurons by binding to Nectin-1/HveC (Richart et al, 2003) and does not infect glial cells at an appreciable rate (Carlezon et al, 2000b); we have never observed any GFP in typical astrocytic cells. In a previous study involving medium spiny neurons in nucleus accumbens shell (Neumaier et al, 2002), we observed rare retrograde infection of neurons that project to the viral infusion site. Thus, we believe that increased 5-HT6 receptor expression was extremely localized to medium spiny neurons at the injection site in this study as well. Immunohistochemical distribution of 5-HT6 immunoreactivity within virally infected neurons in transfected brains was localized in the dendrites (as in endogenously expressing neurons) but was also distributed on the cell body more intensely than endogenous 5-HT6 receptors. Therefore, it is possible that this factor may also contribute to the changes in learning behavior. However, transgene receptors have been routinely detected in cell bodies after viral overexpression and may simply reflect the high receptor production during peak expression. Additionally, it is possible that a small number of other neuronal subtypes, such as cholinergic interneurons, may also be expressing transgene 5-HT6 receptors and that such expression is contributing to the deficit in instrumental learning. The development of viral vectors, which express genes only in certain neuronal phenotypes, would allow further addressing of specific subpopulations' roles in striatal functioning.
Recent studies have attempted to dissect the roles of specific subregions of the striatum, such as the dorsomedial and dorsolateral striatum, in instrumental learning. Featherstone and McDonald (2005) have shown that lesioning of the dorsolateral regions causes deficits in acquisition of a learning task while dorsomedial lesions produce errors of commission and loss of habitation to previously learned tasks. Based on evidence that the dorsomedial striatum is strongly linked to stimulus-response goal-directed learning (Rogers et al, 2001), this region may also influence habituation. As 5-HT6-infused rats did not show a deficit in lever pressing until day 3, when lever-pressing rates reach levels that indicate a habituated behavior, it appears that 5-HT6 overexpression in the dorsomedial striatum may be interfering with habituation of the goal-directed behavior. In contrast, when 5-HT6 receptors were overexpressed in the dorsal central region of the striatum, the rats demonstrated intact learning of the instrumental conditioning. Pharmacological blockade of the medial prefrontal cortex, a region which projects to the dorsomedial striatum (Sesack et al, 1989), produces also deficits in instrumental conditioning (Baldwin et al, 2000). The dorsal central region receives inputs from the agranular cortex and lateral orbital cortex, regions important for reversal learning but not set-shifting or habituation (Reep et al, 2003; Dalley et al, 2004), and thus it is likely that these subregional differences in corticostriatal projections may underlie the differential behavior observed (Rogers et al, 2001).
Increased stimulation of 5-HT6 receptors has been shown to enhance extracellular GABA levels in the striatum (Beyer et al, 2005). The decrease in habituated learning observed in the present study may be due to changes in local signaling networks, as increased GABAergic firing inhibits cholinergic interneuron activity (DeBoer and Westerink, 1994; Kikuchi et al, 1998), which has been shown to be integral to instrumental learning (Ragozzino, 2003). Alternatively, 5-HT6 overexpression may be inhibiting learning primarily through direct and indirect basal ganglia pathways (Graybiel, 1986). Both pathways are GABAergic circuits, which ultimately influence the cortex, which provides glutamatergic input to the striatum. Increased 5-HT6 receptor expression may in fact be strengthening GABAergic inhibition of the basal ganglia, thus suppressing the learning feedback loop. Studies have shown that 5-HT6 blockade increases glutamate and acetylcholine release in cortex and hippocampus (Riemer et al, 2003). Woolley et al (2004) has proposed that 5-HT6 inhibition causes a disinhibition of striatal direct and indirect circuits, and increased neuronal firing in the cortex. Thus in the case of increased 5-HT6 activity via viral vector gene transfer, there may be decreased cortical stimulation and feedback, which would attenuate learning through dampening of excitatory signaling. Further studies will need to be conducted in order to verify such a proposed mechanism, which presumes that virally mediated expression is primarily occurring in medium spiny neurons that naturally express 5-HT6 receptors. In any event, the experiments detailed in this report suggest that the 5-HT6 receptors in striatum may play a more important role in cognitive function than has previously been appreciated.
References
Baldwin AE, Holahan MR, Sadeghian K, Kelley AE (2000). N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav Neurosci 114: 84–98.
Beyer CE, Smith DL, Zhang G, Li P, Lin Q, Stock JR et al (2005). WAY-181187: neurochemical profile of a novel and selective 5-HT6 receptor agonist. Eur Neuropsychopharmacol 15 (Suppl 3): S382.
Boess FG, Monsma FJ, Carolo C, Meyer V, Rudler A, Zwingelstein C et al (1997). Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells. Neuropharmacology 36: 713–720.
Brandeis R, Brandys Y, Yehuda S (1989). The use of the Morris water maze in the study of memory and learning. Int J Neurosci 48: 29–69.
Carlezon Jr WA, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL et al (2000a). Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci 20: RC62.
Carlezon Jr WA, Nestler EJ, Neve RL (2000b). Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol 14: 47–67.
Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF (2002). Overexpression of 5-HT1B receptor in dorsal raphe nucleus using herpes simplex virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci 22: 4550–4562.
Clark MS, Vincow ES, Sexton TJ, Neumaier JF (2004). Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res 1007: 86–97.
Dalley JW, Cardinal RN, Robbins TW (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28: 771–784.
Dawson LA, Nguyen HQ, Li P (2001). The 5-HT(6) receptor antagonist SB-271046 selectively enhances excitatory neurotransmission in the rat frontal cortex and hippocampus. Neuropsychopharmacology 25: 662–668.
DeBoer P, Westerink BH (1994). GABAergic modulation of striatal cholinergic interneurons: an in vivo microdialysis study. J Neurochem 62: 70–75.
East SZ, Burnet PW, Leslie RA, Roberts JC, Harrison PJ (2002). 5-HT6 receptor binding sites in schizophrenia and following antipsychotic drug administration: autoradiographic studies with [125I]SB-258585. Synapse 45: 191–199.
Featherstone RE, McDonald RJ (2005). Lesions of the dorsolateral or dorsomedial striatum impair performance of a previously acquired simple discrimination task. Neurobiol Learning Memory 84: 159–167.
Foley AG, Murphy KJ, Hirst WD, Gallagher HC, Hagan JJ, Upton N et al (2004). The 5-HT(6) receptor antagonist SB-271046 reverses scopolamine-disrupted consolidation of a passive avoidance task and ameliorates spatial task deficits in aged rats. Neuropsychopharmacology 29: 93–100.
Furukawa K, Akaike N, Onodera H, Kogure K (1992). Expression of 5-Ht3 receptors in Pc12 cells treated with Ngf and 8-Br-Camp. J Neurophysiol 67: 812–819.
Gerard C, el Mestikawy S, Lebrand C, Adrien J, Ruat M, Traiffort E et al (1996). Quantitative RT-PCR distribution of serotonin 5-HT6 receptor mRNA in the central nervous system of control or 5,7-dihydroxytryptamine-treated rats. Synapse 23: 164–173.
Gerard C, Martres MP, Lefevre K, Miquel MC, Verge D, Lanfumey L et al (1997). Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res 746: 207–219.
Graybiel AM (1986). Neuropeptides in the basal ganglia. Res Publ Assoc Res Nerv Ment Dis 64: 135–161.
Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R et al (1999). Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology 21: 68S–76S.
Harrison AA, Everitt BJ, Robbins TW (1999). Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go/no-go conditional visual discrimination. Behav Brain Res 100: 99–112.
Hartley T, Burgess N (2005). Complementary memory systems: competition, cooperation and compensation. Trends Neurosci 28: 169–170.
Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ et al (2005). 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology 181: 253–259.
Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW et al (2003). Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol 64: 1295–1308.
Hoplight B, Sandygren N, Neumaier JF (2006). Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol 38: 73–79.
Kikuchi T, Wang Y, Sato K, Okumura F (1998). In vivo effects of propofol on acetylcholine release from the frontal cortex, hippocampus and striatum studied by intracerebral microdialysis in freely moving rats. Br J Anaesth 80: 644–648.
King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KC (2004). 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation—an effect sensitive to NMDA receptor antagonism. Neuropharmacology 47: 195–204.
Kohen R, Fashingbauer LA, Heidmann DEA, Guthrie CR, Hamblin MW (2001). Cloning of the mouse 5-HT6 serotonin receptor and mutagenesis studies of the third cytoplasmic loop. Mol Brain Res 90: 110–117.
Meneses A (2001). Role of 5-HT6 receptors in memory formation. Drug News Perspect 14: 396–400.
Mitchell ES, Hoplight BJ, Lear S, Neumaier JF (2006). BGC20-761, a novel tryptamine analog, enhances memory consolidation and reverses scopolamine-induced memory deficit in social and visuospatial memory tasks through a 5-HT6 receptor-mediated mechanism. Neuropharmacology 50: 412–420.
Mitchell ES, Neumaier JF (2005). 5-HT(6) receptors: a novel target for cognitive enhancement. Pharmacol Ther 108: 320–333.
Monsma Jr FJ, Shen Y, Ward RP, Hamblin MW, Sibley DR (1993). Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol 43: 320–327.
Morris R (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47–60.
Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon Jr WA (2002). Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci 22: 10856–10863.
Paxinos G, Watson C (1982). The Rat Brain in Stereotaxic Coordinates. Academic Press: Sydney.
Plassat JL, Amlaiky N, Hen R (1993). Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol Pharmacol 44: 229–236.
Quinn JC, Johnson-Farley NN, Yoon JY, Cowen DS (2002). Activation of extracellular-regulated kinase by 5-hydroxytryptamine(2A) receptors in PC12 cells is protein kinase C-independent and requires calmodulin and tyrosine kinases. J Pharmacol Exp Therap 303: 746–752.
Ragozzino ME (2003). Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol Learn Mem 80: 257–267.
Reep RL, Cheatwood JL, Corwin JV (2003). The associative striatum: organization of cortical projections to the dorsocentral striatum in rats. J Comp Neurol 467: 271–292.
Richart SM, Simpson SA, Krummenacher C, Whitbeck JC, Pizer LI, Cohen GH et al (2003). Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by Nectin-1/HveC. J Virol 77: 3307–3311.
Riemer C, Borroni E, Levet-Trafit B, Martin JR, Poli S, Porter RH et al (2003). Influence of the 5-HT6 receptor on acetylcholine release in the cortex: pharmacological characterization of 4-(2-bromo-6-pyrrolidin-1-ylpyridine-4-sulfonyl)phenylamine, a potent and selective 5-HT6 receptor antagonist. J Med Chem 46: 1273–1276.
Rogers DC, Hagan JJ (2001). 5-HT6 receptor antagonists enhance retention of a water maze task in the rat. Psychopharmacology (Berl) 158: 114–119.
Rogers RD, Baunez C, Everitt BJ, Robbins TW (2001). Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav Neurosci 115: 799–811.
Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R et al (1993). A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun 193: 268–276.
Sesack SR, Deutch AY, Roth RH, Bunney BS (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat—an anterograde tract-tracing study with phaseolus-vulgaris leucoagglutinin. J Comp Neurol 290: 213–242.
Tsai Y, Dukat M, Slassi A, MacLean N, Demchyshyn L, Savage JE et al (2000). N1-(Benzenesulfonyl)tryptamines as novel 5-HT6 antagonists. Bioorg Med Chem Lett 10: 2295–2299.
Ward RP, Dorsa DM (1996). Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol 370: 405–414.
Wilson CJ, Groves PM (1980). Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol 194: 599–615.
Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KC (2001). A role for 5-ht6 receptors in retention of spatial learning in the Morris water maze. Neuropharmacology 41: 210–219.
Woolley ML, Marsden CA, Fone KC (2004). 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord 3: 59–79.
Yin HH, Ostlund SB, Knowlton BJ, Balleine BW (2005). The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22: 513–523.
Acknowledgements
This study was supported by R01-16432 and a NARSAD independent investigator award (to JN) and a 5T32AG000057 (to EM). We thank Sheri Mizumori for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary information
Rights and permissions
About this article
Cite this article
Mitchell, E., Sexton, T. & Neumaier, J. Increased Expression of 5-HT6 Receptors in the Rat Dorsomedial Striatum Impairs Instrumental Learning. Neuropsychopharmacol 32, 1520–1530 (2007). https://doi.org/10.1038/sj.npp.1301284
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301284
Keywords
This article is cited by
-
Over-expression of 5-HT6 Receptor and Activated Jab-1/p-c-Jun Play Important Roles in Pilocarpine-Induced Seizures and Learning-Memory Impairment
Journal of Molecular Neuroscience (2019)
-
5-Hydroxytryptamine receptor 6 antagonist, SB258585 exerts neuroprotection in a rat model of Streptozotocin-induced Alzheimer’s disease
Metabolic Brain Disease (2018)
-
Serotonin 5-HT6 Receptor Antagonists in Alzheimer’s Disease: Therapeutic Rationale and Current Development Status
CNS Drugs (2017)
-
Striatal 5-HT6 Receptors Regulate Cocaine Reinforcement in a Pathway-Selective Manner
Neuropsychopharmacology (2016)
-
Acute effects of cocaine and cannabis on reversal learning as a function of COMT and DRD2 genotype
Psychopharmacology (2016)