Abstract
Various lines of published evidence have already demonstrated the impact of TRPV1 agonists on energetic metabolism through the stimulation of the sympathetic nervous system (SNS). This study presents a trial investigating if stimulation of the two related sensory receptors TRPA1 and TRPM8 could also stimulate the SNS and impact the energetic metabolism of healthy subjects. The trial was designed to be double-blinded, randomized, cross-over, placebo-controlled with healthy subjects and the impact on the energetic metabolism and the autonomic nervous system (ANS) of cinnamaldehyde, capsaicin and a cooling flavor was measured during the 90 min after ingestion. Energy expenditure and substrate oxidation were measured by indirect calorimetry. An exploratory method to measure ANS activity was by facial thermography and power spectral analysis of heart rate variability using ECG was also used. Following cinnamaldehyde ingestion, energy expenditure was increased as compared to placebo. Furthermore, postprandial fat oxidation was maintained higher compared to placebo after cinnamaldehyde and capsaicin ingestion. Similar peripheral thermoregulation was observed after capsaicin and cinnamaldehyde ingestion. Unlike capsaicin, the dose of cinnamaldehyde was not judged to be sensorially ‘too intense’ by participants suggesting that Cinnamaldehyde would be a more tolerable solution to improve thermogenesis via spicy ingredients as compared to capsaicin.
Similar content being viewed by others
Introduction
It is generally accepted that the increasing prevalence of obesity and overweight worldwide can be attributed to an imbalance between energy intake and energy expenditure1. To prevent from a positive energy balance leading to body weight gain, one fundamental approach would be to enhance energy expenditure. Physical activity, basal metabolic rate (MR) and diet induced thermogenesis are the three components promoting energy expenditure2. It has been reported that natural ingredients such as caffeine, different varieties of tea or extracts from chili peppers such as capsiate or capsaicin are able to increase thermogenesis3 and that the increased energy expenditure observed results from sustained sympathetic nervous system activity originating from several different mechanisms3.
Capsaicin (the pungent ingredient of red chilli pepper) has a non-pungent analog molecule found in the sweet pepper capsicum variety CH-19, which is called capsiate. Several published studies summarized in a review by Ludy et al.4 have shown the impact of both capsaicin and capsiate on energy expenditure and fat oxidation5,6,7,8,9,10. Capsaicin stimulates the sympathetic nervous system (SNS) and increases the energetic metabolism in humans through sensory nerve stimulation. Since capsaicin increases catecholamine secretion via the activation of the central nervous system11, it is proposed that its thermogenic effect is due to β-adrenergic stimulation of brown adipose tissue (BAT) metabolic function. Both capsaicin and capsiate are agonists of the Transient Receptor Potential Vanilloid 1 (TRPV1), a broadly tuned cation channel12,13. Thermogenic and metabolic effects of capsaicin and capsiate are hypothesised to be due to the activation of TRPV1. Indeed, capsaicin prevents obesity of mice fed with high fat diet, and evidence is provided with knock out (KO) animals that this effect is mediated by TRPV114. A recent study shows that TRPV1 KO mice are more sensitive to body weight gain and become more insulin resistant under high fat diet than wild type animals (WT)15. Taken together these studies seem to position TRPV1 as a dominant player in the regulation of energy balance.
TRPV1 is expressed in a subset of nociceptor sensory neurons innervating the mouth and the gut, and is involved in temperature and chemical sensing. Besides capsaicin and capsiate, it also responds to numerous natural molecules (e.g. vanilloids, gingerol, zingerone, eugenol or piperine) as well as to both temperatures above 42 °C and low pH16. Within the TRP channel family, two other channels are activated by various compounds found in plants that are used as spices: TRPA1 and TRPM8. TRPA1 responds to many irritant and pungent molecules found in plants (e.g. mustard oil, garlic, cinnamon, cloves or ginger) as well as temperatures below 17 °C17. TRPM8 is activated by compounds such as eucalyptol, menthol or linalool as well as by temperatures below 25 °C18.
Converging evidences are showing that TRPA1 and TRPM8 may, like TRPV1, impact energy balance and metabolism. In rodents it has been observed that agonists of TRPV1, TRPA1 and TRPM8 increase thermogenesis19. Indeed intragastric administration of capsaicin, menthol or cinnamaldehyde in mice, increases colonic temperature19. It has also been shown that, in rats, intramuscular injections of either capsaicin or isothiocyanate, a TRPA1 agonist, stimulate BAT function, which is the key effector of diet induced thermogenesis20. Moreover, TRPA1 agonists have been shown to induce adrenaline secretion21. As some obese animal models are presenting impaired thermogensis22 BAT transplantation can reverse obesity23. TRPA1, as a BAT function stimulator, would therefore appear to be a good pathway to target obesity. Recently, chronic administraiton of a TRPA1 agonist, cinnamaldehyde, has been shown to reduce body weight gain and improve insulin sensitivity of diet-induced obese mice. However, no evidence was obtained related to either long term regulation of BAT activity or adrenaline secretion24.
TRPM8 was shown to be expressed in BAT and its stimulation enhances thermogenesis via an up-regulation of the uncoupling protein 1 (UCP1) and consequently reduces body weight gain of diet induced obese animals25. Additionally, it was shown that adipocytes are able to directly sense cold temperature and up-regulate expression of genes involved in thermogenesis such as UCP1, in a β-adrenergic independent pathway26.
Based on this pre-clinical evidence we hypothesized that stimulation of TRPA1 and TRPM8 pathways could, as with the stimulation of TRPV1, increase thermogenesis promoting energy expenditure and thereby exert an antiobesity effect. The primary objective of this exploratory clinical trial was to compare the impact on energy expenditure of a relevant agonist of each of the three TRP channels of interest, respectively: capsaicin, cinnamaldeyhde and a cooling flavor versus placebo. Secondary objectives were to compare their impact on substrate oxidation and on the activation of the SNS measured by heart rate variability (HRV) analysis and facial thermography as respectively gold standard and exploratory methods.
Results
Ingredient selection
The objective of this trial was to assess whether the stimulation of TRPA1 and TRPM8 pathways could be as efficient as the stimulation of TRPV1 by capsaicin or capsiate4 in impacting energy balance. Based on the literature, cinnamaldehyde was selected as the most representative TRPA1 agonist: since it activates specifically TRPA1 with an EC50 of 60 μM27. Its specificity on the human form of the receptor was confirmed in vitro (data not shown). To identify an efficient and specific agonist of hTRPM8, different ingredients with cooling properties were evaluated in vitro. Following these initial tests, a cooling flavor (CF) that specifically activates hTRPM8 with an EC50 of 0.07% (Fig. 1) was selected. As a positive control, capsaicin was evaluated in parallel to cinnamaldehyde and CF in this trial12.
By calcium imaging the effect and specificity of the cooling flavor has been evaluated on cells expressing hTRPA1, hTRPV1 or hTRPM8. The cooling flavor activates specifically hTRPM8 in a dose-dependent way. Data are represented by the mean of relative fluorescence ± s.e.m, n = 6. EC50 =~0.07% (=half maximal effective concentration).
The requirement to administrate a safe and tolerable dose determined the concentrations tested for each ingredient. Based on a safety assessment and on informal sensory evaluation these were 1 mg of capsaicin, 70 mg of cinnamaldehyde and 0.2 ml of CF in 200 ml of tomato juice. The doses represent concentrations of 16 μM, 2.6 mM and 0.1% respectively.
Trial population
The trial utilized a cross-over design, in which all subjects who completed the study received, in different visits, all the treatments and the placebo but in a double-blinded and randomized order (see Table 1 for the randomized sequences of treatment). In total, 19 healthy subjects, all of whom were ‘moderate spicy food eaters’, were recruited and randomized; but only 16 completed the study (Table 1). Nevertheless, all of the completed sessions were included in the statistical analysis regardless of whether the subject completed the full protocol or not. In consequence, the n per treatment varies from 16 to 18 depending on the session at which the particular subject dropped-out.
Three subjects dropped-out: one because of discomfort, one withdrew consent and ECG signal was not measurable in one of the sessions of a third one. The population’s mean age was 32 years with a BMI of 22.43 kg/m2 (Table 1). Each treatment was evaluated over the 90 min following sample ingestion in two different sessions A and B, each with the same design but different recordings (Fig. 2A).
Energy expenditure
In Fig. 3, energy expenditure is represented as the distribution of the data averaged over 10 min intervals (Fig. 3A). The difference of each treatment relative to the placebo was evaluated as the time standardized area under the curve (AUC) after ingestion until the end of the session (Fig. 3D). When compared to the placebo, cinnamaldehyde increased energy expenditure (unadjusted p < 0.05), whereas no significant differences were observed for capsaicin or CF. Extrapolating over the 90 min of recording, the difference of energy expenditure between cinnamaldehyde treatment and placebo would represent 3.6 kcal (i.e. 2.4 k Cal/hr).
Effect of treatment on Energy expenditure (A,D), fat oxidation (B,E) and CHO oxidation (C,F). (A–C) Data distribution averaged over 10 min +- SD. (D–F) Comparison of treatment AUC (time standardized) +– SE. Cinnamaldehyde significantly increases AUC of energy expenditure compared to placebo (D). Both cinnamaldehyde and capsaicin significantly increase fat oxidation AUC compared to placebo (E). (*p < 0.05; Unadjusted p-values computed using mixed model, n = 16–18).
Fat and carbohydrate (CHO) oxidation
Figure 3B,C shows, fat and CHO oxidation data. These are plotted as the distribution of data over 10 min intervals and the time standardized difference of each treatment compared to placebo as AUC after ingestion until the end of the session (Fig. 3E,F). A higher level of fat oxidation is observed after the ingestion of capsaicin and cinnamaldehyde compared to placebo (unadjusted p < 0.05). However, no statistically significant difference of fat oxidation was observed after the ingestion of the CF compared to the placebo. None of the treatments induced any significant changes of CHO oxidation compared to the placebo (Fig. 3F).
Heart rate variability
For each treatment, participants went through two sessions A and B, where the administration and measurement protocol was the same but the recorded parameters were different (Fig. 2).
ECG was logged during both sessions in order to evaluate ANS activity. Three parameters were calculated using power spectral analysis of the heart rate variability collected from ECG recordings in session A and B. These were: SNS index, PNS index and the VLF/TP ratio averaged over 10 minute intervals (see supplementary Tables S1 to S6 for data distribution). Sessions A and B were analyzed both separately and pooled together. Table 2 shows the statistical analysis of the comparison of AUCs of ANS parameters, each treatment being referenced to the placebo. AUCs of PNS index, SNS index and VLF/TP were compared between groups for each session A or B and the union of both sessions.
None of these parameters showed any consistent differences over both sessions A and B, except a reduced SNS index and VLF/TP induced by the CF compared to placebo statistically significant for session B only. The respective values were: −142.944 ± 67.928 (SE), p = 0.0378 (unadjusted p-value) and −3.072 ± 1.235 (SE), p = 0.0435 (adjusted p-value) (see complete analysis on Table 2).
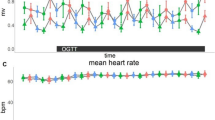
Blood pressure and heart rate
Mean arterial pressure, diastolic and systolic blood pressure (BP) were measured and calculated from the measures taken each 15 minutes during session B (time points: T0, T15, T30, T42, T57, T72, T87, T102, T117, T132) (see supplementary Tables S7–S9 for data distribution). Table 3 details the statistical analysis of the comparison of AUCs after ingestion until the end of the session of the different BP variables; each treatment being compared to the placebo. No significant difference was observed. Heart rate was measured using the ECG for each subject at each visit. To determine the effect of the different treatment, data were analyzed over 15 minutes after ingestion to capture fast changes of ANS activity. Figure 4 represents effect size of each treatment compared to placebo; no significant difference was observed.
Effect of treatment on heart rate. Comparisons of different treatments with placebo at 0, 5, 10 and 15 minutes after ingestion. Data over 1 minute were aggregated and expressed as effect size +– SE. No significant differences are observed between placebo and any treatment, all p-value being >0.221 (p-values were computed using a mixed linear model, n = 16–18).
Facial thermography
Vasoconstriction and reduced blood flow into peripheral capillary vessels of the face, and sweating on the forehead, are phenomena that could reflect activation of the SNS under stress conditions or spicy gustatory stimulation28,29. For all products, subjects’ facial temperature was measured every second and averaged over different zones (Fig. 2B).
AUCs were compared between groups for each zone for the entire post ingestion period (Table 4). Cinnamaldehyde significantly increased chin temperature (Zone-10) compared to placebo for a prolonged time after ingestion with an adjusted p-value of 0.0486 compared to placebo.
Additionally, for each zone, data were analyzed over the 15 minute post-ingestion period in order to capture fast changes of temperature induced by product ingestion The temperature significantly increased in Zone-2 (nose) after cinnamaldehyde or capsaicin intake (Fig. 5A) and in Zone-12 (left cheek) immediately after intake of cinnamaldehyde (Fig. 5B).
Nose (A) and left cheek (B) temperature. Comparisons of different treatments with placebo at 0, 5, 10 and 15 minutes after ingestion. Data over 1 minute were aggregated and expressed as effect size +− SE. (*Adjusted p-value < 0.05; **Adjusted p-value < 0.001; p-values were computed using a mixed linear model while adjusted for multiplicity using Holm’s method, n = 16–18).
Post-ingestion evaluation of the samples
Intensity and chemesthetic properties of products were evaluated after ingestion using a sensory questionnaire in each session. The answers were consistent over sessions and cinnamaldehyde, capsaicin and CF could be distinguished by their sensory properties (data not shown). At the concentrations tested, cinnamaldehyde was judged less intense than capsaicin (Fig. 6) and CF was judged ‘not intense’ to ‘mildly intense’ relative to the placebo (data not shown).
Discussion
In the present exploratory clinical trial it has been observed that a single ingestion of cinnamaldehyde (70 mg/200 ml; 350 ppm) significantly increases energy expenditure by a magnitude of about 3.6 kcal over the period of the experiment (90 min) when compared to placebo.
Moreover, both capsaicin (1 mg/200 ml; 4.88 ppm) and cinnamaldehyde induced an enhanced postprandial fat oxidation (a magnitude of about 556.2 mg and 512.7 mg respectively), over the period of the experiment (90 min) when compared to the placebo. Cinnamaldehyde increased chin temperature in the overall treatment, and both capsaicin and cinnamaldehyde increased nose temperature until 15 minute after the treatment. However, only cinnamaldehyde unilaterally increased cheek temperature in the first minutes after ingestion.
Capsaicin and capsiate, two TRPV1 agonists, have been extensively studied for their impact on weight management4. However, in terms of weight loss the expected magnitude of the effect produced by a hedonically acceptable dose of capsaicin is quite small30. Compared to the study of Ludy and Mattes30, in the present study, half of the dose of capsaicin, which was still perceived as intense, was used (Fig. 6). Indeed, in the study presented here, 1 mg of capsaicin had no effect on energy expenditure. Nevertheless, informal sensory testing prior to the clinical trial indicated that this dose proposed in 200 ml (4.88 ppm) was the highest acceptable. Therefore, capsaicin doses required to reach a significant effect cmetabolism would be out of the hedonic range for moderate spicy food eaters. On the other hand, the dose of cinnamaldehyde used in this study has not been judged as intense as the capsaicin one by the majority of the participants, and it gave significant results on energy expenditure compared to placebo, as well as similar results as capsaicin on fat oxidation.
The present study did not show any modification of ANS activity by heart rate variability analysis (ANS variables and heart rate). However, the increase of nose skin temperature induced by capsaicin can be interpreted as a sympathetic vasodilatation effect responding to thermogenesis to promote heat diffusion, as observed in rats by an increase of tail temperature19. The same effect was observed after cinnamaldehyde ingestion on nose and left cheek temperature, but the opposite effect on rat tail temperature has been reported with cinnamaldehyde and allyl isothiocyanate (AITC), another TRPA1 agonist19.
In the overall treatment an increase of chin temperature after cinnamaldehyde ingestion was observed compared to placebo. This temperature increase might reflect an increased blood flow in the big vessel crossing the chin. The increased blood flow could be explained by either 1) an increased cardiac output under the control of SNS, stimulated by cinnamaldehyde inducing adrenaline secretion, or, 2) by vasodilatation of this big vessel induced by the inhibition of L-type calcium channels. Indeed it was shown recently that cinnamaldehyde can inhibit L-type calcium channels independently of TRPA1, inducing vasorelaxation and decreasing blood pressure31.
The anti-obesity effect of long term treatment with cinnamaldehyde, as a TRPA1 agonist, in mice was not associated with any upregulation of BAT function, UCP gene expression or catecholamine secretion24. However, in the present study data suggest that shortly after the ingestion of cinnamaldehyde thermogenesis and SNS are stimulated. Clearly, there is a discrepancy between short term and long term treatment effects. Interestingly, the opposite effects are observed with TRPM8 agonists. In rodents it has been shown that long term TRPM8 activation with menthol increases resting metabolic rate, inducing thermogenesis via up-regulation of UCP-1 in brown adipose tissue25; whereas the CF, activating TRPM8 efficiently in vitro, does not impact energy expenditure in the present study. It may be speculated that the stimulation of the two cold sensing TRPA1 and TRPM8 pathways may lead to different and complementary responses, a rapidly induced thermogenesis and a long term adaptation of resting metabolism.
Recently, Gregersen et al.32, published a study where they measured, with comparable design, the effect on diet induced thermogenesis, appetite, energy intake and energy balance of raw spices (mustard, horseradish, black pepper and ginger) ingested as part of a meal. They observed a tendency of mustard to increase diet induced thermogenesis measured by indirect calorimetry. As mustard contains AITC, it can be suggested that the effect they observed is due, as proposed for cinnamaldehyde in the present study, to the stimulation of TRPA1 in peripheral sensory fibers and the subsequent sympathetic nerve activity, catecholamine secretion and thermogenesis stimulation. However, in the Gregersen et al.32 study, they did not observe any effect on catecholamine. Possibly the small effect they observed is due to the fact that they use raw spices instead of isolated active ingredients.
Taken together, the present results indicate that cinnamaldehyde would be a more tolerable solution to improve thermogenesis via spicy ingredients than capsaicin. The present concentration of cinnamaldehyde (350 ppm) is in the range of its occurrence as a flavoring in food. Cinnamaldehyde potentially is more powerful than capsaicin at improving energy expenditure and fat oxidation through thermogenic effects. Even though the effect appears subtle, a cumulative approach (combining dietary, exercise and behavioral aspects) is believed to be the most efficient for sustainable weight loss or maintenance.
Further confirmatory as well as long term studies are needed to assess the impact of a chronic ingestion of cinnamaldehyde or TRPA1 agonists on metabolism, insulin sensitivity and body weight.
Materials and Methods
Participants
Healthy men between 20 and 50 years old with 19 < BMI < 25 and weight greater than 60 kg were enrolled in the study. Sample size was determined to be sufficient with 15 subjects, in a cross-over exploratory study to calculate effect size. Screening of candidates was done through a questionnaire on spicy food habits to select moderate spicy food eaters. The clinical trial was approved by a competent and independent ethics committee (Commission Cantonale (VD) d’Ethique pour la Recherche sur l’Etre Humain) and participants gave their written informed consent. This trial has been conducted according to the principles and rules laid down in the Declaration of Helsinki and its subsequent amendments.
Trial design
The study design was a double-blinded, randomized, cross-over, placebo-controlled, exploratory clinical trial. Randomization lists were produced using William’s design for a cross-over study using R version 2.8.1. The order of sessions was randomized and balanced for each subject and was determined at the beginning of the study by the statistician. The order of sessions was given for each subject to the person responsible of sample preparation who was the only person to know the order of the sessions and treatments and a code for each sample was applied. The person performing the recording was different from the person preparing the product.
For each measure, subjects were tested after an overnight fast. They were requested to use their car or public transportation to come to the investigation site in order to keep morning physical activity levels as low as possible. Moreover, consumption of caffeine has not been permitted after the lunch meal (12 a.m.). Furthermore, subjects were asked to refrain from spice consumption 2 days prior to each session. Each sample was evaluated in two different sessions A and B, with the same design but different data recording (Fig. 2A).
Each session started at 8:00 a.m. with a 30 minutes resting period and measurement preparation. During all the testing sessions subjects were seated comfortably. Room temperature was in the 20 °C to 22 °C range. Subjects were instructed to remain quiet and still, but they were allowed to watch TV. Parameters were recorded 30 minutes before and 90 minutes after ingestion of each sample (Fig. 2A). The clinical trial was registered in Clinicaltrials.gov ID: NCT02193438.
Treatment
All active TRP agonists were dissolved in 200 ml liquid tomato juice (Granini, Eckes-Granini, Henniez, Switzerland) at room temperature and served with a slice of bread. Placebo was 200 ml commercial tomato juice. Active ingredients per 200 ml were 1 mg of capsaicin (Spectrum chemicals, Gardena, CA, USA), 0.2 ml of cooling flavor (QB-113-979-5; Givaudan, Dübendorf, Switzerland) or 70 mg of cinnamaldehyde (Sigma-Aldrich, St-Louis, MO, USA). All ingredients were in vitro validated in cell lines expressing hTRPV1, hTRPA1 or hTRPM8 according to the calcium imaging method described in Riera et al.33.
Indirect calorimetry
Energy expenditure was measured by indirect calorimetry using a ventilated canopy connected to a MAX-II gas analyzer (Max II metabolic system, AEI technologies, Naperville, IL, USA). The equation used by this device to evaluate energy expenditure was the simplified Weir formula34.
REE = (3.941 x VO2) + (1.106 x VCO2), where:
EE = energy expenditure (kcal/min)
VO2 = rate of oxygen consumption (l/min) and
VCO2 = rate of carbon dioxide production (l/min)
After having answered questions from the compliance questionnaire participants were comfortably placed in semi-recumbent position in a reclining bed, at an angle allowing the participant to ingest the test solution without having to adjust position between metabolic rate measurement periods and ingestion. A canopy was installed over participant’s head. Tests started ~08:30 a.m. following a stabilization period allowing FeCO2 (expired fraction of CO2 in %) values to reach physiologic range.
Substrate oxidation (carbohydrates and fat utilization) and the respiratory quotient (RQ) were calculated from the VO2 and VCO2 values obtained with the MAX-II gas analyzer during the same timeline than energy expenditure. VO2 and VCO2 values were corrected for protein oxidation. The following equations were used to calculate the amount of fat and carbohydrates oxidized35:
PO = (0.8xbody weightx1000)/1440
NPVO2 = VO2-POx1.01031
NPVCO2 = VCO2-POx0.84361
FAT = (NVPO2-NVPCO2)/(2.01494x(1-0.707))
CHO = (NVPCO2-0.707x NVPO2)/(0.82821x(1-0.707))
RQ = VCO2/VO2
PO = protein oxidation (mg/min),
NPVO2 = non protein O2 consumption (ml/min),
NPVCO2 = non protein CO2 consumption (ml/min),
FAT = fat oxidation (mg/min) and
CHO =carbohydrate oxidation (mg/min)
RQ = Respiratory quotient
Power spectral analysis of heart rate variability
To evaluate the activity of the autonomic nervous system (ANS) power spectral analysis of the inter-beat interval (R-R) on electrocardiogram recordings (ECG) was used. The ECG signal was obtained from electrodes (Biocom 5000 Bluetooth ECG Recorder, Poulsbo, WA, USA) placed in the CM5 position on subjects. The monitored signal was computed using the HRVLive! Software (Biocom Technologies, Poulsbo, WA, USA). Periodically fluctuating components included in the HRV and their amplitudes were calculated over time into a power spectral curve. To evaluate the activity of ANS, the analysis was performed at a very low frequency (VLF) range (0.007–0.04 Hz), low frequency (LF) range (0.04–0.15 Hz), high frequency (HF) range (0.15–0.4 Hz) and the total power (TP) (0.007–0.4 Hz). HF reflects parasympathetic (PNS) influence on heart beat; LF displays mainly sympathetic (SNS) influence and partly vagal activity36. VLF reflects SNS activity related to energy metabolic regulation. VLFs are increased through thermogenic stimuli such as cold exposure and food or spicy food ingestion5,37.
Indices of SNS and PNS activities were calculated as:
SNS index = (VLF + LF)/HF
PNS index = HF/TP
VLF and VLF/TP are defined as the absolute and relative thermogenic SNS activities. Heart rate data (bpm) were also extracted from this recording and analyzed for the 15 minutes after ingestion.
The ECG signal was recorded continuously. ANS activity was analyzed during the 30 minutes before sample ingestion and the 90 minutes post-sample ingestion.
Blood pressure
Systolic (SP) and diastolic (DP) blood pressure were measured every 15 minutes before the ingestion (T0, T15, T30) and after (T42, T57, T72, T87, T102, T117, T132) by a nurse, using a standard blood pressure monitor (inflatable arm cuff) during same session as the indirect calorimetry measure. Mean arterial pressure was calculated as: MAP = SP/3 + 2DP/3.
Facial thermography
Infrared thermal imaging of the face was used as an exploratory, non-invasive recording method of sympathetic nervous system activity. Using a FLIR A325 infrared camera (FLIR Systems, Inc, Emitec AG, Rotkreuz, Switzerland) subjects were continuously recorded at a distance of ~1 meter. Thermographic imaging software, FLIR ResearchIR (FLIR Systems, Inc) was used to acquire images. The recording was performed by acquiring 1 image/second.
Infrared Image analysis
Facial thermography data were extracted using in-house algorithms developed within the Matlab framework (The Mathworks Inc., Natick, MA, USA). First, a graphical user interface (GUI) was created to help defining manually a temperature threshold highlighting primarily subject’s faces. An alignment of the face was then computed and twelve regions of interest (ROIs) drawn manually using the GUI (Fig. 2B). Face alignment/orientation correction was computed image by image using a binary mask based on manually set temperature thresholds. Holes in the binary mask were filled and the region with the greatest contiguous area was selected. The selected region centroid was determined using the built-in Matlab region properties tool (regionprops). With the same tool, an ellipse was fitted within the region to obtain the angular orientation of the ellipse’s major axis compared to the x-axis. The original image was then shifted to center the region centroid at the origin (0,0) and rotated at an angle aligning the major axis of the fitted ellipse to the y-axis. Face alignments were computed for all images of the recorded session and average temperatures within ROIs extracted. Alignment performance was checked visually over the whole recorded session and discrepancies corrected by re-setting new regions and re-extracting the data in extreme cases (e.g. if a subject showed strong forward or background head movements not captured by the alignment algorithm).
Data management
A paper case report form (CRF) was used. All data required in the protocol and captured by the investigator were transcribed on the CRFs. All questionnaires were joined to the CRF.
Indirect calorimetric data was automatically generated by the MAX-II program on an excel sheet. Heart rate power spectra analysis data (VLF, LF, HF, TP, HR for each second of recording) was exported into an excel file automatically by the HRV Live! program. Facial area temperatures were analyzed as described above and exported into an excel file. All generated data excel files were downloaded into the clinical data management system (CDMS, ClintrialTM).
Statistical analysis
For energy expenditure, fat and CHO oxidation, the raw data was first smoothed (using loess) then the time-standardized AUC were calculated as the integral over the time after ingestion until the end of the experimental session divided by the length of this time interval. All analyses were carried out using linear mixed-effects models with a random subject-wise intercept to take into account the correlation between repeated measures.
For ANS variables, blood pressure and thermography the area under the curve (AUC) was computed after ingestion for each subject. AUCs were then compared between groups considering a linear mixed-effects model with the baseline measurement and the treatment group as fixed effects and the subject as random effect. For ANS parameters, the session was added as a fixed effect and an overall F-test was computed in order to compare the groups over the sessions. P-values were adjusted for multiple comparisons according to Bonferroni.
Additional analyses were performed to evaluate short term (within 15 minutes) effects of treatments on facial temperature and heart rate. To analyze fast changes, data were aggregated over 1 minute for before, right after ingestion (0–1 minute), after 5 minutes, after 10 minutes and after 15 minutes. Outliers were removed before aggregation. All analyses were carried out using linear mixed-effects models with a random subject-wise intercept to take into account the correlation between repeated measures. For facial temperature analysis, P-values were computed using a linear mixed-effects model while adjusted for multiplicity using Holm’s method.
Sample sensory evaluation and gut comfort evaluation
Pleasantness and intensity of the chemesthetic stimuli was evaluated during the ingestion period using a questionnaire. Similarly, a questionnaire was used to evaluate gut comfort after the recording session.
Trial registration
Clinicaltrials.gov ID: NCT02193438 “Physiologic Effect of Spices Ingestion” registered on July 2014.
Additional Information
How to cite this article: Michlig, S. et al. Effects of TRP channel agonist ingestion on metabolism and autonomic nervous system in a randomized clinical trial of healthy subjects. Sci. Rep. 6, 20795; doi: 10.1038/srep20795 (2016).
References
Mitchell, N. S., Catenacci, V. A., Wyatt, H. R. & Hill, J. O. Obesity: overview of an epidemic. Psychiatr. Clin North Am 34, 717–732 (2011).
Donahoo, W. T., Levine, J. A. & Melanson, E. L. Variability in energy expenditure and its components. Curr Opin. Clin Nutr Metab Care 7, 599–605 (2004).
Hursel, R. & Westerterp-Plantenga, M. S. Thermogenic ingredients and body weight regulation. Int. J. Obes. (Lond) 34, 659–669 (2010).
Ludy, M. J., Moore, G. E. & Mattes, R. D. The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem. Senses 37, 103–121 (2012).
Matsumoto, T. et al. Effects of capsaicin-containing yellow curry sauce on sympathetic nervous system activity and diet-induced thermogenesis in lean and obese young women. J Nutr Sci. Vitaminol. (Tokyo) 46, 309–315 (2000).
Yoshioka, M. et al. Effects of red-pepper diet on the energy metabolism in men. J Nutr Sci . Vitaminol. (Tokyo) 41, 647–656 (1995).
Yoshioka, M., St-Pierre, S., Suzuki, M. & Tremblay, A. Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br. J Nutr 80, 503–510 (1998).
Yoshioka, M. et al. Effects of red pepper on appetite and energy intake. Br. J. Nutr. 82, 115–123 (1999).
Yoshioka, M., Doucet, E., Drapeau, V., Dionne, I. & Tremblay, A. Combined effects of red pepper and caffeine consumption on 24 h energy balance in subjects given free access to foods. Br. J Nutr 85, 203–211 (2001).
Lim, K. et al. Dietary red pepper ingestion increases carbohydrate oxidation at rest and during exercise in runners. Med. Sci. Sports Exerc. 29, 355–361 (1997).
Watanabe, T., Kawada, T. & Iwai, K. Effect of capsaicin pretreatment on capsaicin-induced catecholamine secretion from the adrenal medulla in rats. Proc. Soc. Exp. Biol. Med. 187, 370–374 (1988).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Iida, T. et al. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 44, 958–967 (2003).
Zhang, L. L. et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res 100, 1063–1070 (2007).
Lee, E. et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J 29, 3182–3192 (2015).
Vetter, I. & Lewis, R. J. Natural product ligands of TRP channels. Adv. Exp. Med. Biol. 704, 41–85 (2011).
Story, G. M. et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003).
Bautista, D. M. et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 (2007).
Masamoto, Y., Kawabata, F. & Fushiki, T. Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice. Biosci. Biotechnol. Biochem. 73, 1021–1027 (2009).
Yoshida, T., Yoshioka, K., Wakabayashi, Y., Nishioka, H. & Kondo, M. Effects of capsaicin and isothiocyanate on thermogenesis of interscapular brown adipose tissue in rats. J Nutr Sci Vitaminol. (Tokyo.) 34, 587–594 (1988).
Iwasaki, Y., Tanabe, M., Kobata, K. & Watanabe, T. TRPA1 agonists–allyl isothiocyanate and cinnamaldehyde–induce adrenaline secretion. Biosci. Biotechnol. Biochem. 72, 2608–2614 (2008).
Batt, R. A. & Hambi, M. Development of the hypothermia in obese mice (genotype ob/ob). Int J Obes 6, 391–397 (1982).
Liu, X. et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology. 156, 2461–2469 (2015).
Camacho, S. et al. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci Rep 5, 7919 (2015).
Ma, S. et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J. Mol. Cell Biol. 4, 88–96 (2012).
Ye, L. et al. Fat cells directly sense temperature to activate thermogenesis. Proc. Natl. Acad. Sci USA 110, 12480–12485 (2013).
Bandell, M. et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004).
Nakayama, K., Goto, S., Kuraoka, K. & Nakamura, K. Decrease in nasal temperature of rhesus monkeys (Macaca mulatta) in negative emotional state. Physiol Behav. 84, 783–790 (2005).
Drummond, P. D. Mechanisms of physiological gustatory sweating and flushing in the face. J Auton. Nerv. Syst. 52, 117–124 (1995).
Ludy, M. J. & Mattes, R. D. The effects of hedonically acceptable red pepper doses on thermogenesis and appetite. Physiol Behav. 102, 251–258 (2011).
Alvarez-Collazo, J. et al. Cinnamaldehyde inhibits L-type calcium channels in mouse ventricular cardiomyocytes and vascular smooth muscle cells. Pflugers Arch. 466, 2089–99 (2014).
Gregersen, N. T. et al. Acute effects of mustard, horseradish, black pepper and ginger on energy expenditure, appetite, ad libitum energy intake and energy balance in human subjects. Br. J Nutr 109, 556–563 (2013).
Riera, C. E., Vogel, H., Simon, S. A., Damak, S. & le Coutre, J. Sensory attributes of complex tasting divalent salts are mediated by TRPM5 and TRPV1 channels. J. Neurosci. 29, 2654–2662 (2009).
Weir, J. B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol 109, 1–9 (1949).
Livesey, G. & Elia, M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr 47, 608–628 (1988).
Pagani, M. et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 59, 178–193 (1986).
Matsumoto, I., Emori, Y., Ninomiya, Y. & Abe, K. A comparative study of three cranial sensory ganglia projecting into the oral cavity: in situ hybridization analyses of neurotrophin receptors and thermosensitive cation channels. Brain Res. Mol. Brain Res. 93, 105–112 (2001).
Acknowledgements
This work would not have been possible without the involvement of the Metabolic Unit team at Nestlé Research Center, Lausanne. We also thank all the subjects for their commitment. We like to thank Dr. Adam Burbidge for the helpful review of the manuscript as well as Dr. Maria Laura Gosoniu for the revision of the statistics.
Author information
Authors and Affiliations
Contributions
S.M. and J.l.C. conceived the scientific approach and the project. S.M. and M.B. designed the study. M.B. was the principal investigator. S.M., M.B. and J.M.M. performed the experiments. M.L., A.T. and R.M. contributed to the analysis of the results. S.C. and M.B. contributed to the interpretation of the results. J.l.C. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Michlig, S., Merlini, J., Beaumont, M. et al. Effects of TRP channel agonist ingestion on metabolism and autonomic nervous system in a randomized clinical trial of healthy subjects. Sci Rep 6, 20795 (2016). https://doi.org/10.1038/srep20795
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20795
This article is cited by
-
Sex differences in estimates of cardiac autonomic function using heart rate variability: effects of dietary capsaicin
European Journal of Applied Physiology (2023)
-
Cinnamaldehyde supplementation acts as an insulin mimetic compound improving glucose metabolism during adolescence, but not during adulthood, in healthy male rats
Hormones (2023)
-
New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel
Scientific Reports (2020)
-
Mammalian cold TRP channels: impact on thermoregulation and energy homeostasis
Pflügers Archiv - European Journal of Physiology (2018)
-
Cinnamaldehyde exerts vasculoprotective effects in hypercholestrolemic rabbits
Naunyn-Schmiedeberg's Archives of Pharmacology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.