Abstract
Anticipatory and consummatory dissociation of hedonic experience may manifest as anhedonia in schizophrenia. However, it is unclear if this temporal dissociation of pleasure experience is also relevant in other symptoms like social anhedonia in the schizophrenia disorder spectrum. The present study applied two incentive delay tasks involving different incentive types (money vs. social affective images) to a sample of 28 participants with elevated social anhedonia (SocAnh) and 38 healthy controls from a population of 476 college students. The results showed that the SocAnh group had comparable anticipatory sensitivity and consummatory pleasure towards monetary incentives as the controls; but they exhibited significant decrease in both anticipatory sensitivity and consummatory experience to positive social affective images. These findings demonstrate the presence of a domain-specific deficit in people with social anhedonia towards social affective information and suggest that incentive types could confound the findings on the dissociation of anticipatory vs. consummatory hedonic capacities.
Similar content being viewed by others
Introduction
Humans are innate with the intrinsic desire for social affiliation and interpersonal interaction1. However, there are great individual variations in this desire. People who suffer from social anhedonia are known to have a diminished motivation for social affiliation and a lack of reward from social incentives2. This hedonic deficit is a core feature of schizotypy3 and has been associated with risks for the development of schizophrenia4,5.
Individuals with higher levels of social anhedonia tend to have lower levels of social support and social functioning3. They have difficulties in processing emotional facial expressions6, responding to and expressing feelings7 and performing tasks requiring theory of mind8 or working memory9. The impairments in these critical affective and cognitive functions overlap to a great extent with those found in patients with schizophrenia. Therefore, it may be promising to further explore this important trait in order to know how the emotional alterations in schizophrenia extend across spectrum conditions and how hedonic deficits manifest in the early stage of schizophrenia spectrum disorders7.
Recent findings have revealed an “anhedonia paradox” in schizophrenia in that patients usually have diminished positive affect in self-report ratings and clinical interviews, but exhibit an intact ability to experience in-the-moment emotional stimuli in laboratory settings10. Several models have been proposed to account for this paradox, including theories of 1) anticipatory hedonic deficit, 2) emotion regulation deficit, 3) memory deficit, 4) representational deficit and 5) social-specific deficit11. There is evidence supporting the dissociation of anticipatory (“wanting”, the ability to predict future pleasure experience) vs. consummatory (“liking”, “on-line” pleasure experience) hedonic systems. It has been postulated that the negative symptoms of schizophrenia could be largely related to deficits in anticipatory anhedonia12, in that reduced brain activation to anticipatory reward is associated with higher severity of negative symptoms in schizophrenia13. However, this “anhedonia paradox” have been investigated mostly in non-social domains, while social anhedonia might be a more relevant hedonic deficit to explain the trait negative affect in schizophrenia14.
Interestingly, despite the strong link between social anhedonia and schizophrenia spectrum disorders, very little research has examined the role of anticipatory hedonic deficit in social anhedonia; and it remains unclear whether the same dissociation between anticipatory and consummatory hedonic experiences is also found in people with social anhedonia. Such a clarification may yield two benefits. First, it would provide us with insights about how early a hedonic deficit would occur in the schizophrenia spectrum, which could provide opportunities for early identification and intervention. Secondly, it would also deepen our understanding of the nature of anhedonia and whether there is any domain-specific characteristic in social anhedonia.
Here, based on previous findings, we recommend a behavioral experimental paradigm — the incentive delay task that could capture the dissociation of anticipatory and consummatory hedonic processes towards various types of stimuli. The incentive delay task has long been used to assess subjects' responsivity to the pursuit of gain and avoidance of loss15. Its special architecture with an anticipatory delay for the incentive and a feedback period after behavioral responses makes it particular suitable for the study of dissociation between anticipatory and consummatory hedonic processes. In a typical incentive task, participants are instructed to make a quick response after a delay period preceding a cue, which indicates what type (reward, punishment, or neutral) of feedback they might receive if they win. Neural activities in the delay period have been shown to be linked with anticipation and motivation brain regions, such as the nucleus accumbens and the anterior cingulate cortex15,16 and the response times preceding the delay reveal participants' readiness for response, thus serving as a behavioral index of anticipatory sensitivity17. On the other hand, the feedback period after the response is associated with participants' subjective experience of the incentives. Therefore, with multiple parameters, the incentive delay task could not only facilitate our understanding of the nature of anhedonia at the behavioral level, but also allow examination of the “active” pattern of motivational and hedonic systems.
However, previous studies using incentive delay tasks relied heavily on the monetary incentive type, which might not be analogous to our daily emotive environment18. In the present study, we developed a modified affective incentive delay (AID) task to investigate the nature of social anhedonia. The AID task19 utilizes direct emotional stimuli from the International Affective Picture Set (IAPS)20 rather than monetary incentives. The diverse nature of the IAPS affords us an opportunity to look into specific domains of emotional responsivity, such as responses to social affective images. A specific emotional image set with social significance (e.g. reflecting social interaction, affiliation and activities, etc.) was selected from the IAPS in this study. We employed both the monetary incentive delay (MID) task and the AID task to examine differences in the motivational patterns to various incentive types in individuals with higher and lower levels of social anhedonia. We aimed to test whether the dissociation of anticipatory and consummatory anhedonia pattern is relevant in social anhedonia and how this “anhedonia paradox” would be reflected at the behavioral level.
If the “anhedonia paradox” were relevant in social anhedonia, we would expect to observe decreased sensitivity to pursue positive feedback in the incentive delay tasks in terms of RTs and subjective forecasting, but intact ability to experience the affect of feedbacks in the tasks in terms of subjective ratings, in individuals with elevated social anhedonia. Moreover, we also postulated that the AID task would be more sensitive than the MID task in detecting such a pattern.
Results
Self-report measures
As revealed in Table 1, significant differences between the control and SocAnh groups were observed in the scores of the Chapman Social Anhedonia Scale (CSAS; t(64) = −18.12, p < .001) and the Chapman Physical Anhedonia Scale (CPAS; t(64) = −3.69, p < .001); the cognitive perceptual (t(64) = −3.98, p < .001), interpersonal (t(64) = −7.08, p < .001), disorganization (t(64) = −5.33, p < .001) and total scores of the Schizotypal Personality Questionnaire (SPQ; t(64) = −6.85, p < .001); as well as the Temporal Experience of Pleasure Scale (TEPS) anticipatory pleasure scores (abstract: t(64) = 2.30, p < .05; total: t(64) = 2.13, p < .05). However, there was no difference between these groups in the TEPS consummatory pleasure scores and in anticipatory and consummatory ratings in the MID task. For the AID task, although the SocAnh and the control groups did not differ in anticipatory pleasure ratings, SocAnh individuals had reduced positive affect after receiving a reward (t(64) = 2.19, p < .05).
MID and AID task accuracy
The overall proportions of successful presses (accuracy, ACC) during target presentation did not differ between the AID (66.0% ± 7.6%) and the MID (67.1% ± 4.9%) tasks. Performance in both tasks did not differ between the SocAnh group (ACCAID = 65.9% ± 4.3%, ACCMID = 66.1% ± 5.3%) and the control group (ACCAID = 66.1% ± 9.2%, ACCMID = 68.1% ± 5.2%). Furthermore, ACC did not differ between the two groups under reward, punishment, or neutral conditions in both tasks (all ps > .05).
Reaction times in MID and AID tasks
A primary 2 (incentive type: monetary vs. affective) × 2 (group: SocAnh vs. control) × 3 (condition: reward vs. punishment vs. neutral) repeated-measure ANOVA showed that incentive type had a significant main effect on participants' RTs (F(1,64) = 12.00, p < .01, η2p = .158), which were generally shorter for the MID task (M = 212.56 ms) than the AID task (M = 218.85 ms). Experimental conditions also had a robust significant main effect on RTs (F(2,64) = 40.22, p < .001, η2p = .386), which were shorter under reward condition (M = 210.35 ms) than under neutral condition (M = 224.75 ms). Although group had no apparent main effect on RTs (F(1,64) = 1.86, p = .18, η2p = .028), marginal significant interactions were observed between incentive type, experimental condition and group (F(2,57) = 3.04, p = .051, η2p = .045).
To delineate specific effects, additional 2 (group: SocAnh vs. control) × 3 (condition: reward vs. punishment vs. neutral) repeated-measured ANOVAs were applied to the MID and the AID tasks separately. In the MID task, only condition had a significant effect (F(2,64) = 71.55, p < .001, η2p = .528), while no other main effect or interaction effect was observed. Therefore, in the MID task, participants generally reacted more quickly in the reward and punishment conditions compared with the neutral condition, as shown in Table 2. However, in the AID task, condition and group did not have a significant main effect, while a salient interaction effect was observed (F(2,64) = 7.21, p < .01, η2p = .186). Post hoc analysis with Bonferroni adjustment showed that the RTs were significantly different between the reward and the neutral conditions in the control group (Reward vs. Neutral: −8.80 ms, p = .001), indicating higher anticipatory sensitivity to pursue positive social affective outcomes in normal healthy controls. However, the SocAnh group did not exhibit such a pattern (p = .197). In addition, the SocAnh group reacted significantly more slowly than the control group in the reward condition (SocAnh vs. Control: 17.79 ms, p = .019), indicating a diminished sensitivity to pursue positive feedback in socially anhedonic individuals, compared to the controls.
Again for differences in RTs, the SocAnh group showed a larger difference between the reward and neutral conditions than the control group in the AID task (p < .001). However, no such pattern was observed in the MID task (see Table 2).
Correlation analysis
Correlations between variables of interest were summarized in Table 3. Differences in RTs in the AID task were more correlated with self-reported measurements than those in the MID task. In particular, differences in RTs between the reward and neutral conditions in the AID task were positively correlated with CSAS (r = .404, p = .001) and SPQ (r = .253, p = .035) scores. The anticipatory and consummatory pleasure scores in the TEPS did not correlate with behavioral performance in both the MID and AID tasks. However, the TEPS anticipatory pleasure score was negatively correlated with CSAS (r = .281, p = .019) and CPAS (r = −.356, p = .002) scores, while the TEPS consummatory pleasure score was correlated only with the CPAS score (r = −.499, p < .001).
Discussion
This study provides the first behavioral evidence showing domain-specific anticipatory and consummatory hedonic deficits in individuals with social anhedonia. Although there was no difference in anticipatory ratings for incentives between the SocAnh and the control groups, in agreement with the anticipatory deficit theory, SocAnh individuals exhibited diminished anticipatory responsivity towards social affective rewards in terms of having slower RTs in the reward (vs. neutral) condition. In addition, after controlling for participants' IQ scores, WMC and simple RT, we found that the SocAnh group performed as well as the control group in the MID task, suggesting that SocAnh individuals had intact motivational functions. However, they performed worse in the AID task, which included social affective images as incentives. These results suggest that the anticipatory deficits in individuals with social anhedonia may be domain-specific (limited to social affective stimuli).
Contrary to the “anhedonia paradox,” we found that SocAnh individuals had significantly reduced affect ratings towards positive social stimuli, but not towards monetary incentives. In fact, this finding is consistent with previous studies showing that social anhedonia is associated with reduced self-report positive affect and less emotional responsivity and expressivity7,21. However, this is not generalizable to every incentive type. This is probably because the utility-based monetary incentives are more individual-relevant than social affective stimuli. Studies have found that money could prime an individualistic mindset and decrease distress with social exclusion22. Therefore, it is possible that monetary incentives empowered participants' sense of self-focus, which motivated them to seek for more monetary rewards and avoid monetary losses. Alternatively, SocAnh individuals might also have a higher “hedonic threshold” for social affective information than for monetary incentives, resulting in lower consummatory ratings for social affective rewards.
The results so far confirmed the sensitivity of AID measurements for the detection of individual differences in anticipatory sensitivity and consummatory hedonic experience in those with elevated social anhedonia. However, Smoski and colleagues19 found that monetary incentives, compared with affective images, resulted in greater activation of motivation-related brain regions in major depression. Here we postulate that this may be a result of different paradigm architecture and stimuli nature. In their study, the AID task included only two conditions (reward and neutral) with the positive images set that did not guarantee social significance. In addition, patients with major depression are not expected to produce the same results as individuals with social anhedonia. For instance, Pechtel and colleagues23 showed that individuals with remitted depression had blunted reward responsiveness towards both monetary and social feedback. However, different from this overall decrease in reward responsiveness in depression, socially anhedonia individuals might have a more domain-specific deficit. Delineating such a different pattern could facilitate specific and effective interventions for social anhedonia.
The observed effects of anticipatory and consummatory hedonic deficits in individuals with social anhedonia have clear relevance. First, it provides the first experimental evidence for anticipatory hedonic deficit in people with social anhedonia and also supports the observation that the “in-the-moment” hedonic experience toward social affective rewards is also compromised in individuals with schizophrenia-spectrum pathology. It therefore further supports the social-specific hedonic deficit theory which states that emotion deficits are largely restricted to specific types of stimuli or specific domains11. Thus, the “anhedonia paradox” observed in the literature might be biased by extensive focus on physical anhedonia. Further, as mentioned above, our results may provide insight for possible interventions for social anhedonia. A guide to increase motivation for social interaction as well as affective experience is recommended to be included in the therapeutic efforts to bring changes to both trait and state social anhedonia24.
This study had several limitations. First, our relatively small sample size (N = 476) might have introduced bias in the subsequent laboratory study. A larger representative sample from various sources is needed for future study. Secondly, the correlative nature of this study precluded the identification of causal relationships between anticipatory and consummatory hedonic deficits, social anhedonia and schizotypal personality symptoms. A longitudinal study design would better address these issues. Furthermore, future exploration should take into account the difference between physical and social anhedonia. Another independent sample with higher physical anhedonia would be helpful in delineating whether the deficits in AID task are only limited to SocAnh individuals. Finally, the present study was limited to behavioral measures. It would be interesting to examine the potential distinct neural mechanisms of anticipation and consummation of social vs. monetary rewards in individuals with social anhedonia.
Methods
Participants
Subjects were selected from 206 males and 270 females from different departments at a university in Beijing who completed a set of questionnaires, consisting of the Chinese version of the revised Chapman Physical Anhedonia Scale (CPAS) and the revised Chapman Social Anhedonia Scale (CSAS)25. This survey was administered in a large group format in classes. The ethics committee of the Institute of Psychology, Chinese Academy of Sciences approved this study and students who agreed to take part in the study completed the survey after giving written informed consent.
Based on CSAS scores, two groups of individuals were selected, namely, a socially anhedonic (SocAnh) group and a control group. The SocAnh group consisted of participants who scored beyond 1.28 SD (upper 10%) from the same-sex sample mean on the CSAS, while the control group was selected randomly from those scored no higher than 0.5 SD off the same-sex sample mean. In our sample (n = 476), the mean CSAS scores for males and females were 11.45 (SD = 6.81) and 10.26 (SD = 6.18) respectively, which were consistent with a previous study that sampled 870 Chinese college students25.
Afterwards, potential participants were invited to take part in a laboratory study with monetary compensation. Those who eventually participated in the study gave their informed consent and were screened for personal or family history of physical diseases and mental disorders. The final sample consisted of 28 SocAnh participants and 38 controls. There was no significant difference in gender, age, estimated IQ, working memory capacity and simple reaction time between the SocAnh and the control groups (see Table 4). In addition, there was no difference in terms of CSAS and CPAS scores and age (ps > .05) between participants who agreed to take part in the subsequent study and those who did not.
Procedure and measures
Prior to the tasks, the abridged Chinese version of the Wechsler Adult Intelligence Scale (WAIS-R)26 was administered to estimate participants' IQ scores. Afterwards, participants completed the standardized change localization task27 to measure their working memory capacity (WMC). In addition, a simple reaction time (RT) task was also applied to the subjects. They were asked to press a button as soon as they saw a target on the screen (30 trials). Estimated IQ, WMC and simple RT were measured to ensure that any possible group differences in terms of RTs could not be attributed to differences in intellectual abilities, cognitive maintenance capacity and motor preparation. Subsequently, participants completed the MID and the AID tasks and filled out self-reported questionnaires and were then debriefed. The sequence of these two tasks was counterbalanced across participants.
Monetary incentive delay (MID) task
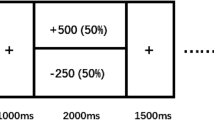
In the MID task, participants received points of reward or loss based on RTs to the target15. The cumulative number of points was transformed into money at the end of the study28. Under the reward condition, participants received five points for a win and 0 point for a loss. Under the punishment condition, participants received 0 point for a win but could avoid the loss of five points. Under the neutral condition, participants received 0 point for their performance, regardless of RTs. Participants were required to respond as quickly as possible in each trial. Task conditions and trial timing are summarized in Figure 1.
Sample trials of monetary incentive delay (MID) and affective incentive delay (AID) tasks under reward, punishment and neutral conditions.
Each trial consists of a cue (250 ms), an anticipatory rating task for possible feedback (until response), an anticipatory delay period (2,000–2,500 ms), a target display period (150–500 ms, adjusted according to individual mean response times) and a feedback display (MID, 1,650 ms; AID, 3,000 ms) and a consummatory rating task for the actual feedback (until response). A triangular cue indicates a positive trial, a square indicates a negative trial and a circle indicates a neutral trial. For the rating scale, 1 = “not happy at all,” 9 = “very happy.” Images applied in the AID task were drawn from the IAPS. However, the images in this figure are not from the IAPS, but are comparable to the ones used in the task. We appreciate the contribution of this figure by Mr. Weizhen Xie. Images in this figure were taken by Mr. Wiezhen Xie and coauthors.
Each trial consisted of: (1) a 250 ms cue indicating the conditions of reward, punishment, or neutral; (2) anticipatory rating to possible result of the trial in which participants reported how they anticipate feeling about the feedback from “not happy at all ( = 1)” to “very happy ( = 9)” on a 9-point scale; (3) delay period during which a white cross was presented for 2000–2500 ms; (4) the appearance of a blue target requiring rapid press of a button on the keyboard, the duration of which was adjusted on the basis of the individual's mean RT and accumulative ACC in the previous trials to guarantee a total accuracy rate of approximately 66.7%; (4) a 1650-ms feedback that indicated whether the performance was a “win” and displayed the number of points gained or lost along with accumulative points; and (5) a consummatory rating in which participants were asked on a 9-point scale to report how they actually felt when they saw the feedback from “not happy at all ( = 1)” to “very happy ( = 9).” Each participant completed 30 trials under each condition, presented in a random sequence with breaks in every 30 trials.
Revised affective incentive delay (AID) task
Similar to the MID task, the revised AID task also had three conditions. Under the reward condition, participants were presented with a positive image when their RTs were sufficiently short or otherwise a neutral image. Under the punishment condition, participants saw a negative image when their RTs were not sufficiently short or otherwise received a neutral feedback. Under the neutral condition, participants saw a neutral image regardless of RTs. The basic architecture of trial timings was similar with the MID task, except that the feedback period was longer (3000 ms) to ensure the emotion-eliciting effect of the images (see Figure 1).
A pilot study with 54 participants (27 males) tested and validated the images in terms of valence and arousal on a 9-point scale20. For the positive and negative images, they were also rated for whether the image included social factors (social interaction, e.g. parents play with babies; affiliation, e.g. intimate couples; and activities, e.g. family union), ranging from “don't include social factors ( = 1)”, “not sure ( = 2)” and “include social factors ( = 3).” Satisfying images were those with social factors, which included 30 positive images and 30 negative images. We also selected 30 neutral images. The pleasant images had a mean (SD) normative valence and arousal ratings of 7.41 (0.33) and 6.08 (0.54), respectively, including social scenes and photographs of children or adults. The unpleasant images had a normative mixed-gender mean (SD) valence and arousal ratings of 2.50 (0.34) and 6.31 (0.36), respectively, including social scenes and images of crying babies and sad faces. The neutral subset included images of everyday objects, such as a cup, bottle and lamp, with a normative mixed-gender mean (SD) valence and arousal ratings of 5.07 (0.29) and 3.41 (0.22), respectively.
Revised Chapman Social Anhedonia Scale (CSAS)
This scale assesses deficits in the ability to experience pleasure from non-physical stimuli such as other people, talking or exchanging expressions of feelings. It contains 40 True-or-False items and a higher score on the CSAS indicates more severe social anhedonia.
Revised Chapman Physical Anhedonia Scale (CPAS)
This scale was designed to measure a deficit in the ability to experience pleasure from typical physical stimuli such as food, sex and settings. It contains 61 True-or-False items and similarly, high scores indicate severe physical anhedonia. Both of the Chinese versions of the CSAS and the CPAS were tested and validated by Chan and colleagues25.The Cronbach's alpha coefficient of CSAS and CPAS were .84 and .80 respectively.
Schizotypal Personality Questionnaire (SPQ)
Modeled on the DSM-III-R criteria for schizotypal personality disorder29, the SPQ was widely used to screen for schizotypal personality disorder in the general population30. Higher scores mean greater severity in schizotypal personality symptoms. The Cronbach's alpha coefficient was .89 in the study.
Temporal Experience of Pleasure Scale (TEPS)
This scale measures individual trait dispositions in both anticipatory and consummatory experiences of pleasure. We used the Chinese version of the TEPS30, which has 20 items capturing four factors: consummatory contextual, consummatory abstract, anticipatory contextual and anticipatory abstract. Higher scores suggest better hedonic capability. The Cronbach's alpha coefficients were .74 for the whole scale and .60–.76 for the four factors.
Data analysis
Statistical analyses were performed using SPSS (17.0 for Windows; SPSS Inc., Chicago, IL, USA). Raw RTs and differences in RTs were analyzed using repeated-measures ANOVA. For each individual, trial RT > 3 SDs above the individual mean RT or below 100 ms were excluded. Smaller differences in RTs indicate stronger anticipatory responsivity to pursue positive feedback (Reward vs. Neutral) or avoid negative feedback (Punishment vs. Neutral).
References
Baumeister, R. F. & Leary, M. R. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 117, 497–529 (1995).
Kwapil, T. R., Silvia, P. J. & Myin-Germeys, I. The social world of the socially anhedonic: Exploring the daily ecology of asociality. J. Res. Pers. 43, 103–106 (2009).
Blanchard, J. J., Collins, L. M., Aghevli, M., Leung, W. W. & Cohen, A. S. Social anhedonia and schizotypy in a community sample: The Maryland Longitudinal Study of Schizotypy. Schiz. Bull. 37, 587–602 (2011).
Kwapil, T. R., Miller, M. B., Zinser, M. C., Chapman, J. & Chapman, L. J. Magical ideation and social anhedonia as predictors of psychosis proneness: A partial replication. J. Abnorm. Psychol. 106, 491–495 (1997).
Kwapil, T. R. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. J. Abnorm. Psychol. 107, 558–565 (1998).
Germine, L. T., Garrido, L., Bruce, L. & Hooker, C. Social anhedonia is associated with neural abnormalities during face emotion processing. NeuroImage 58, 935–945 (2011).
Leung, W. W., Couture, S. M., Blanchard, J. J., Lin, S. & Llerena, K. Is social anhedonia related to emotional responsivity and expressivity? A laboratory study in women. Schiz. Res. 124, 66–73 (2010).
Villatte, M., Monestes, J. L. & McHugh, L. Assessing deictic relational responding in social anhedonia: A functional approach to the development of Theory of Mind impairments. Int. J. Behav. Cons. Ther. 4, 360–373 (2008).
Gooding, D. C. & Tallent, K. A. Spatial, object and affective working memory in social anhedonia: an exploratory study. Schiz. Res. 63, 247–260 (2003).
Pizzagalli, D. A. The ‘anhedonia paradox’ in schizophrenia: Insights from affective neuroscience. Biol. Psychiat. 67, 899–901 (2010).
Cohen, A. S., Najolia, G. M., Brown, L. A. & Minor, K. S. The state-trait disjunction of anhedonia in schizophrenia: Potential affective, cognitive and social-based mechanisms. Clin. Psychol. Rev. 31, 440–448 (2011).
Gard, D. E., Kring, A. M., Gard, M. G., Horan, W. P. & Green, M. F. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schiz. Res. 93, 253–260 (2007).
Juckel, G. et al. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage 29, 409–416 (2006).
Blanchard, J. J., Mueser, K. T. & Bellack, A. S. Anhedonia, positive and negative affect and social functioning in schizophrenia. Schiz. Bull. 24, 413–424 (1998).
Knutson, B., Westdorp, A., Kaiser, E. & Hommer, D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage 12, 20–27 (2000).
Knutson, B., Fong, G. W., Bennett, S. M., Adams, C. M. & Hommer, D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage 18, 263–272 (2003).
Hardin, M. G. et al. Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Pers. Indiv. Differ. 40, 699–711 (2006).
Xie, W. et al. Anhedonia and pain avoidance in the suicidal mind: Behavioral evidence for motivational manifestations of suicidal ideation in patients with major depressive disorder. J. Clin. Psychol. (in press).
Smoski, M. J., Rittenberg, A. & Dichter, G. S. Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiat. Res: Neuroimaging 194, 263–270 (2011).
Lang, P. J., Bradley, M. M. & Cuthbert, B. N. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. (The Center for Research in Psychophysiology, University of Florida, 1997).
Kerns, J. G., Docherty, A. R. & Martin, E. A. Social and physical anhedonia and valence and arousal aspects of emotional experience. J. Abnorm. Psychol. 117, 735–746 (2008).
Zhou, X., Vohs, K. D. & Baumeister, R. F. The symbolic power of money: Reminders of money alter social distress and physical pain. Psychol. Sci. 20, 700–706 (2009).
Pechtel, P., Dutra, S. J., Goetz, E. L. & Pizzagalli, D. A. Blunted reward responsiveness in remitted depression. J. Psychiat. Res. 47, 1864–1869 (2013).
Granholm, E., Ben-Zeev, D. & Link, P. C. Social disinterest attitudes and group cognitive-behavioral social skills training for functional disability in schizophrenia. Schiz. Bull. 35, 874–883 (2009).
Chan, R. C. K. et al. A study of trait anhedonia in non-clinical Chinese samples: Evidence from the Chapman Scales for Physical and Social Anhedonia. PLoS ONE 7, e34275 (2012).
Gong, Y. X. Manual of Wechsler Adult Intelligence Scale - Chinese version. (Chinese Map Press, 1992).
Gold, J. M. et al. Intact attentional control of working memory encoding in schizophrenia. J. Abnorm. Psychol. 115, 658–673 (2006).
Broyd, S. J. et al. An electrophysiological monetary incentive delay (e-MID) task: A way to decompose the different components of neural response to positive and negative monetary reinforcement. J. Neurosci. Meth. 209, 40–49 (2012).
Raine, A. The SPQ: A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schiz. Bull. 17, 555–564 (1991).
Chan, R. C. K. et al. The Temporal Experience of Pleasure Scale (TEPS): Exploration and confirmation of factor structure in a healthy Chinese sample. PLoS ONE 7, e35352 (2012).
Acknowledgements
This study was supported by a grant from the “Strategic Priority Research Program (B)” of the Chinese Academy of Sciences (XDB02030002), the National Science Fund China (81088001, 91132701) and a grant from the Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-EW-J-8).
Author information
Authors and Affiliations
Contributions
W.Z.X. designed the study, analyzed the data and wrote the paper. Y.C. designed the study and interpreted the data. W.Z.X., X.Y.Y., S.Y.Z. and H.S.S., Y.W. collected the data. R.C.K.C. generated the idea, interpreted the data and wrote up the paper. E.F.C.C. commented and contributed to the paper writing. All authors read and commented the final version of the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Xie, Wz., Yan, C., Ying, Xy. et al. Domain-specific hedonic deficits towards social affective but not monetary incentives in social anhedonia. Sci Rep 4, 4056 (2014). https://doi.org/10.1038/srep04056
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04056
This article is cited by
-
Reward motivation adaptation in people with negative schizotypal features: development of a novel behavioural paradigm and identifying its neural correlates using resting-state functional connectivity analysis
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Relationships between schizotypal features, trait anticipatory and consummatory pleasure, and naturalistic hedonic States
Motivation and Emotion (2021)
-
The neural transfer effect of working memory training to enhance hedonic processing in individuals with social anhedonia
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.