Abstract
Sex in insects is determined by a cascade of regulators ultimately controlling sex-specific splicing of a transcription factor, Doublesex (Dsx). We recently identified homolog of dsx in the red flour beetle, Tribolium castaneum (Tcdsx). Here, we report on the identification and characterization of a regulator of Tcdsx splicing in T. castaneum. Two male-specific and one female-specific isoforms of T. castaneum transformer (Tctra) were identified. RNA interference-aided knockdown of Tctra in pupa or adults caused a change in sex from females to males by diverting the splicing of Tcdsx pre-mRNA to male-specific isoform. All the pupa and adults developed from Tctra dsRNA injected final instar larvae showed male-specific sexually dimorphic structures. Tctra parental RNAi caused an elimination of females from the progeny resulting in production of all male progeny. Transformer parental RNAi could be used to produce all male population for use in pest control though sterile male release methods.
Similar content being viewed by others
Introduction
Generation of two sexes (male and female) is an essential and universal phenomenon among animals to reproduce and maintain their existence1. In spite of being such a widespread developmental process, different strategies have been adopted by different organisms to accomplish the same goal2. Insects are no exception; a myriad of mechanisms are found in insects for their sex determination3,4,5. The homologue of doublesex (dsx), the bottom most gene of the sex determination cascade in Drosophila melanogaster, has been characterized in several insect species belonging to the orders Diptera, Lepidoptera and Hymenoptera6. In most of the insect species examined, the pre-mRNA of dsx sex-specifically splices to produce one female- and one male-specific RNAs, most likely generating one female- and one male-specific Dsx proteins. The dsx pre-mRNAs of Musca domestica7, Apis mellifera8, Bombyx mori9,10 and Aedes aegypti11 are spliced to produce more than two isoforms.
Although the information about the sex-specific splicing regulators of dsx pre-mRNA has increased during recent years3, not many studies have been performed to characterize the nature of sex-determining signals. An initial signal, at very early stage during the embryonic development, is required to initiate the sex determination cascade; in the absence of this signal the opposite sex develops in a default mode12. In D. melanogaster the initial sex-determining signal, provided by the X:A ratio13 or the dose of X-linked genes (X-Signaling Element, XSE) in females activates the transcription of an autosomal gene sex-lethal (sxl) from its early promoter14,15,16. Sxl, through an auto regulatory feedback mechanism regulates the sex-specific splicing of its pre-mRNA in turn leading to the production of functional Sxl protein throughout the life13,17. On the other hand, males which are deprived of the initial pulse of Sxl protein, due to insufficient level of XSEs, accomplish the splicing of sxl pre-mRNA in a default mode which produces nonfunctional truncated protein. Sxl protein in females, maintains the splicing of transformer (tra) pre-mRNA18,19, leading to the production of functional Tra protein which in association with Tra2 modulates the splicing of dsx pre-mRNA in female mode; dsx pre-mRNA splices in a default mode in males in the absence of functional Tra protein20,21. Therefore, in drosophilids XSEs are responsible for producing the initial sex-determining signal by inducing the production of Sxl protein. In medfly, Ceratitis capitata, the initial male sex determination signal comes from Y chromosome and in the absence of this signal, in XX embryos the maternal supply of CcTra, the Tra homologue of C. capitata initiates female sex determination pathway22,23. In A. mellifera, the initial sex-determining signal is provided, in females, by the heterozygous state of a gene “complementary sex-determining (csd) locus”24,25 but this female-determining signal is maintained by a protein produced by the feminizer (fem) gene26 which is an ortholog of tra25. C. capitata, Lucilia cuprina and Nasonia vitripennis has been reported to exploit the maternal supply of tra as a female-determining signal27,28,29.
Beetles belonging to order Coleoptera of class Insecta, include one fourth of all animal species described and many of them are major pests. Not much is known about the molecular mechanisms of sex determination in this group of insects. Recently, we identified dsx homologue in the red flour beetle, T. castaneum. Tcdsx gene codes for three female-specific and one male-specific isoforms. RNA interference-aided knockdown of Tcdsx isoforms revealed isoform-specific functions of Tcdsx in T. castaneum (Shukla and Palli, in preparation). Here, we report on the identification and characterization of the splicing regulator of Tcdsx pre-mRNA, Transformer (TcTra). Knockdown of Tctra induced a change in sex from females to males by diverting the splicing of Tcdsx pre-mRNA into male-specific isoform. We have also identified several putative Tra/Tra2 binding sites in the female-specific exon and adjacent introns of Tcdsx (Shukla and Palli, in preparation). Also, parental RNAi-aided knockdown of Tctra caused an elimination of females from the progeny. Tctra parental RNAi could be employed to produce all male populations for use in control of insect pests.
Results
Identification of T. castaneum Tra/Feminizer
To identify protein responsible for the sex-specific splicing of Tcdsx pre-mRNA, we searched (tblastn) the NCBI and the Beetlebase using the known splicing regulators of dsx pre-mRNA,Transformer (Tra) protein (P11596) of Drosophila melanogaster20,21, BmPSI (P-element somatic inhibitor) protein of Bombyx mori (BAF91871)30 and Feminizer protein of Apis mellifera (NP_001128300.1)26, as a query. Two homologous sequences from T. castaneum LOC660887 and LOC100142574 related to PSI and Feminizer respectively were identified. dsRNA specific to these genes were injected in male and female pupae and the knockdown efficiency and splicing status of Tcdsx and Tctra were assayed using RT-PCR at 5 days after dsRNA injections. Injection of dsRNA of TcPSI did not affect Tcdsx splicing pattern; the Tcdsx isoforms detected are identical to the isoforms detected in the control insects injected with malE dsRNA (data not shown). In contrast, injection of Tctra dsRNA affected Tcdsx splicing (details are shown in the next section). Based on this RNAi data, we selected Tctra as the candidate protein involved in regulation of Tcdsx splicing and characterized Tctra further.
Characterization of Tctra
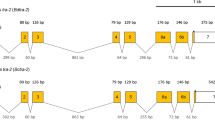
Comparison of deduced amino acid sequence of TcTra with the related sequences in the databases showed that this is similar to Tra/Fem identified from other insects therefore; T. castaneum homologue of Tra/Fem has been named as T. castaneum transformer (Tctra). RT-PCR was performed using sex-specific cDNAs and primers specific to the ends of Tctra ORF (Fig. 1A). A single band of 927 bp and two bands of 1033 and 1130 bp were amplified when female and male cDNAs respectively, were used as templates (Fig. 1B). Sequencing of these sex-specific PCR products and aligning of sequences with the corresponding genomic DNA sequence (AAJJ01000782) identified a complete Tctra ORF. Due to the sex-specific alternative splicing of Tctra pre-mRNA, male Tctra mRNAs contain several in-frame stop codons leading to the production of truncated non-functional protein (Fig. 1A). Putative auto regulation domain, Arg/Ser domain and proline rich region were identified in the deduced amino acid sequence of Tctra based on their similarity with those present in Tra/Fem homologs cloned from other insects (Fig. 1S). Sex-specific isoform DNA sequences and the deduced amino acid sequences of Tctra have been submitted to the GenBank (Accession no. for Tctra-f, Tctra-m1 and Tctra-m2 are JQ857102, JQ857104 and JQ857103, respectively). The transformer 2 (tra2) gene has been annotated in the T. castaneum (XM_963457.2) genome. As reported for other insects, tra2 of T. castaneum (Tctra2) expresses in a non sex-specific manner, but we have not performed the detail analysis of Tctra2.
(A) Schematic representation of isoforms of Tctra pre-mRNA showing the primer positions and region used for the preparation of dsRNA.Boxes show exons and lines show introns and the numbers in the exons show sizes (bp). Common regions are shown in blue and male-specific regions are shown in red color. Green horizontal bar shows the region used to prepare Tctra dsRNA. Black dots represent the presence of stop codons in male-specific exons. Arrows represent the primer locations. Location of putative Tra/Tra2 binding sites are marked using green and yellow vertical lines. RBP1 binding sites are shown using red vertical lines. ISS sites are shown by black triangles. Primers shown as F and female-specific R were used for quantifying female-specific isoform. Primers shown as male-specific F and R2 were used for quantifying male-specific isoforms. (B) Gel picture showing the amplicons generated by PCR using sex-specific c-DNA as templates and F and R1 primers of Tctra.
The tra genes of several insects have been reported to contain multiple putative Tra/Tra2 binding sites within the male specific exons and the flanking introns29,31,32,33. The tra of these species also contain sequences similar to intronic splicing suppressor sequence (ISS) and RBP1 binding sites. On the basis of sequence similarity of putative Tra/Tra2 binding sites from these insects, they were grouped into two types, ATCAA type and CAAT type where these nucleotides are found to be 100% conserved (Fig. 2S). The sequences belonging to ATCAA group have five nucleotides upstream and two nucleotides downstream to ATCAA sequence with different degeneracies, probably depending on the insect species (Fig. 2S). The sequences belonging to CAAT group contain four nucleotides upstream and five nucleotides downstream to CAAT sequence with different degeneracy. We searched for the presence of Tra/Tra2 binding sites, ISS sequence (CAAGG/A) and putative RBP1 binding sequences (Type A: DCADCTTTA and Type B: ATCYNNA) in the Tctra gene. Ten putative Tra/Tra2 binding sequences, four similar to ATCAA type and six similar to CAAT type were found in the male-specific exons and the adjacent intron sequences (Fig. 1A and Fig. 3S). Three putative RBP1 binding sites ATA(T)A(T)CTTTA and four ISS sites (Fig. 1A and Fig. 3S) were also detected in Tctra DNA. The presence of multiple putative Tra/Tra2 binding sites, ISS sequences and RBP1 binding sites in the male-specific exon and the adjacent intron of Tctra suggest the possibility of autoregulation of Tctra splicing.
Tctra RNAi experiments
To verify the predicted function of Tctra as a splice regulator of Tcdsx pre-mRNA, dsRNA targeting Tctra was injected into newly ecdysed adults and the total RNA isolated on the 5th day after injections were used to assay Tcdsx and Tctra mRNAs. In qRT-PCR analysis efficient knockdown of Tctra in both female and male insects tested was observed (data not shown). Interestingly, the male-specific Tcdsx isoform was detected in all the female insects injected with Tctra dsRNA (Fig. 2A). Also, both female- and male-specific isoforms of Tctra were detected in all the Tctra RNAi females (Fig. 2B). In contrast, all the male insects injected with Tctra dsRNA showed no change in the splicing pattern of Tcdsx and Tctra; only male isoform of Tcdsx and Tctra were detected (Fig. 2A & 2B). Similarly, male-specific isoform of Tcdsx and both male- and female-specific isoforms of Tctra were detected in Tctra dsRNA injected females and no change in splicing of Tcdsx and Tctra in males was observed when the Tctra dsRNA was injected into 48 hr-old adults (Figs. 2C&D) or newly ecdysed pupae (Figs. 2E&F).
Sex-specific splicing of Tcdsx (AC&E) and Tctra (BD&F) in Tctra or malE dsRNA injected insects.
Tctra or malE dsRNAs were injected into newly emerged (A&B), 48 hr-old adults (C&D) or newly ecdysed pupa (E&F). RNAs were isolated on 5th day after injections and the mRNA of Tcdsx and Tctra were detected by resolving RT-PCR products on agarose gel. Female and male adults were injected with Tctra or malE dsRNAs soon after adult emergence. The ovaries (G&H) or testes (I&J) from malE (G&I) or Tctra (H&J) dsRNA injected insects were dissected on 7th day after adult emergence, stained with acridine orange and photographed using a fluorescent microscope.
The ovaries dissected from the Tctra RNAi adults on the 7th day post-adult ecdysis (PAE) showed significantly different morphology compared to the ovaries dissected from the control females injected with malE dsRNA (Figs. 2G&H). The ovaries from RNAi insects showed lobes similar to the lobes present in testes and these ovaries showed no signs of oocyte development (Fig. 2H). Besides, Tctra RNAi females mated with normal males did not produce even a single egg (Table 1). Tctra RNAi males, on the other hand, developed normal testes (Fig. 2J) similar to the testis observed in males injected with malE dsRNA (Fig. 2I). Also, females mated with Tctra RNAi males produced equal number of eggs as that of females mated with males injected with malE dsRNA (Table 1). These data suggest that Tctra is required for production of female-specific isoforms of Tcdsx that regulate female reproduction.
Expression of Tctra during larval stage is required for development of pupal and adult sexually dimorphic structures
Pupal and adult stages of T. castaneum show sexually dimorphic structures which make it easy to separate males from females, unambiguously. Papillae, two finger-like structures just anterior to the urogomphi, can be used to separate female pupae (Fig. 3A) from the male pupae (Fig. 3B), since the male papillae are much smaller and look like fingertip rather than fingers (http://www.ars.usda.gov/Research/docs.htm?docid=12892). Males (Fig. 3D), during the adult stage show a small patch of short bristles on the inner side of the first pair of legs (1/3 distance from the base) whereas these bristles are absent in the females (Fig. 3C, http://www.ars.usda.gov/Research/docs.htm?docid=12892). In spite of the splicing of Tcdsx pre-mRNA to male isoform (Tcdsxm, Fig. 2E), splicing of Tctra to both male- and female-specific isoforms (Fig. 2F) and the change in the ovaries into testis-ike lobes (Fig. 2H) in female adults developed from the Tctra RNAi pupae, no changes in the sexually dimorphic structures was observed during the pupal or adult stages and these structures in Tctra RNAi insects are similar to the untreated insects (Fig. 3A–D). To determine whether the development of these sexually dimorphic structures depend on the presence of Tctra during larval stages, Tctra dsRNA was injected into newly molted 4th, 5th and 6th instar larvae. None of the pupa developed from Tctra dsRNA injected larvae showed the female-specific papillae and about 50% of the pupae developed from control larvae injected with malE dsRNA showed female-specific papillae (Table 2). In addition, all the adults developed from Tctra dsRNA injected larvae showed the male-specific bristles (Table 2, Fig. 3E & 3F). About half of the adults developed from Tctra dsRNA injected larvae were deformed (Fig. 3G&H) and the other half are normal (Fig. 3I&J).
Tctra RNAi induced changes in sexual dimorphism during pupal and adult stages.
Female (red arrow-A) and male (blue arrow-B) pupae show characteristic papillae. Control females (C) do not show bristle seen on the ventral side of 1st pair of legs in males (D). Enlargements of the region of leg showing the presence (in male) or absence (in female) of bristles are shown in the panel above each picture. All the adults developed from Tctra dsRNA injected larvae showed male-specific sex patch (E&F). About 50% of adults emerged from Tctra RNAi larvae did not develop properly (G & H dorsal and ventral view, respectively) and died day 2 PAE. The rest of the adults developed normally (I & J dorsal and ventral view, respectively). K. Multiplex PCR with Y-specific and non sex-specific primers34 amplifies two bands in males whereas only one band in females. About 50% of adults (deformed) developed from Tctra RNAi larvae that showed male phenotype amplified a single band similar to that detected in control females. The rest of the 50% adults showed two bands characteristic of males (normal). L. Genetic males, either from malE RNAi or from Tctra RNAi larvae showed male-specific Tcdsx isoform (Tcdsxm). The genetic females developed from Tctra RNAi larvae (deformed) also showed Tcdsxm isoform in RT–PCR analysis using sex-specific Tcdsx primers. M. Genetic males, either from malE RNAi or from Tctra RNAi larvae showed male-specific Tctra (Tctra-m). The genetic females (masculinized deformed adults), developed from Tctra RNAi larvae showed both Tctra-m and Tctra-f in RT–PCR analysis using sex-specific Tctra primers.
Multiplex PCR using Y-specific and non sex-specific primers34 that amplifies two bands in males and one band in females was employed to identify genetic males and females using the genomic DNA isolated from the Tctra dsRNA injected insects as well as two male and two female untreated control insects. Approximately, 50% of adults developed from Tctra RNAi larvae were genetic females (since there was no amplification of Y-specific region in genomic PCR, (Fig. 3K) but showed male phenotypes; absence of female papillae during the pupal stage whereas, the presence of male-specific bristles during the adult stage (Fig. 3E). Strikingly, these converted males (genetic females) did not develop normally and showed deformities in development of wing and other appendages (Fig. 3G&H) and died on day 2 PAE. Male-specific isoform of Tcdsx (Fig. 3L) and both female- and male-specific isoforms of Tctra (Fig. 3M) were detected in these genetic females that showed male phenotypes. The other half of the adults developed from Tctra RNAi larvae are genetic males (Fig. 3K), developed similar to the untreated control insects and showed male-specific isoforms of Tcdsx (Fig. 3L) and Tctra (Fig. 3M). These data showed that expression of Tctra during final instar larval stage is a prerequisite for development of pupal and adult sexually dimorphic structures.
Tctra is maternally transferred
The expression of sex-specific isoforms of Tctra during the embryonic development was analyzed using qRT-PCR. cDNAs prepared from the RNA isolated from the staged embryos were used as templates to perform PCR using primers specific to female or male isoforms of Tctra. As shown in Figure 4A, higher levels of female-specific Tctra mRNA when compared to the levels of male-specific isoform were detected in both fertilized and unfertilized eggs collected during early stages of embryonic development. Interestingly, the mRNA levels of male-specific isoform of Tctra are very low to undetectable in both fertilized and unfertilized eggs during the early stages of embryonic development (Fig. 4A). qRT-PCR analysis of Tctra mRNA levels in staged eggs showed that a peak of female-specific isoform of Tctra is detected at 12–13 hr after egg laying (Fig. 4B) while a peak of male-specific isoform of Tctra is detected at 18–23 hr after egg laying (Fig. 4C). The presence of female-specific isoform of Tctra in the unfertilized eggs suggests that this mRNA may be maternally transferred.
(A) Relative expression of female (Tctraf) and male-specific (Tctram) isoforms of Tctra in unfertilized (0–12 hr after egg laying (unmated females laid fewer eggs therefore, 12 hr collection was necessary to obtain enough eggs) and fertilized eggs (0–5 hr after egg laying).Eggs laid over 0–12 hr period by unmated females and 0–5 hr period by mated females were collected, total RNA was isolated and the mRNA levels of female- and male-specific isoforms of Tctra were quantified by qRT-PCR. Higher levels of female-specific Tctra mRNA were detected in both fertilized and unfertilized eggs. (B &C) Relative change in the expression of female-specific isoform (B) and male-specific isoform (C) of Tctra during the embryonic development. Eggs laid by mated females over an hour period were collected and incubated at 30°C. Samples were collected at 2 hr intervals until 12 hr after egg laying. Similarly, eggs laid by mated females over 5 hr period were collected and incubated at 30°C. Samples were collected at 12 hr intervals until hatching. Total RNA was isolated and the mRNA levels of female- and male-specific isoforms of Tctra were quantified by qRT-PCR. A peak of female-specific isoform of Tctra is detected at 12–13 hr after egg laying and a peak of male-specific isoform of Tctra is detected at 18–23 hr after egg laying.
Tctra dsRNA injected females produce only male progeny
We employed parental RNAi that works well in T. castaneum35,36 to target maternally transferred Tctra mRNA. Tctra or malE dsRNA were injected into female adults on the 5th day PAE; 24 hr after injections the females were mated with un-injected virgin males. Five malE or Tctra dsRNA injected females and five untreated males were placed in separate cups for mating and the larvae hatched from the eggs laid by malE or Tctra dsRNA injected females were counted on 20th day after initiation of mating. When compared to the malE dsRNA injected females, Tctra dsRNA injected females produced fewer larvae (Table 3). All the pupae and adults developed from the larvae hatched from the eggs laid by the Tctra dsRNA injected females developed into males (evident by the presence of sexually dimorphic structures during pupal and adult stages). When all these males were mated with un-injected virgin females, the number of eggs produced in a week period by each female mated with male developed from Tctra RNAi insects is similar to the eggs laid by females mated with normal males (shown in supplementary Table 1S). Analysis of the genetic sex of all the males developed from eggs laid by Tctra RNAi females showed that only three out of 34 tested are genetic females and the rest of them are genetic males (Fig. 5A, Table 3). These genetic females and genetic males (along with control females and males) were analyzed for the presence of sex-specific isoform of Tctra and Tcdsx. Genetic males showed usual male-specific isoforms of Tcdsx and Tctra (similar to untreated control males) but genetic females developed from eggs laid by Tctra RNAi females showed male isoform of Tcdsx (Fig. 5B) and both female and male isoforms of Tctra (Fig. 5C). The ovaries dissected from female adults injected with Tctra dsRNA showed smaller oocytes (Fig. 5E&F) when compared to the ovaries dissected from malE injected adults (Fig. 5G&H). Parental RNAi of Tctra showed the requirement of Tctra during early stages of embryonic development of XX females.
Parental RNAi of Tctra affects splicing of both Tcdsx and Tctra pre-mRNA.
(A) Multiplex PCR with Y-specific and non sex-specific primers amplifies two bands in males whereas only one band in females. About 9% of adults developed from eggs laid by females injected with Tctra dsRNA showed male phenotype during the pupal stage (the presence of male papillae) and the adult stage (the presence of male-specific bristles) amplified a single band similar to that detected in control females. All the other adults (~91%) were genetic males since two bands (as in control males) were detected. (B) Genetic males developed from eggs laid by either malE or Tctra dsRNA injected females showed male-specific Tcdsx isoform (Tcdsxm). The rare genetic females (escapers), developed from Tctra dsRNA injected females, also showed Tcdsxm isoforms. (C) Genetic males developed from eggs laid by either malE or Tctra dsRNA injected females showed male-specific Tctra (Tctra-m). The genetic females (masculinized) developed from eggs laid by Tctra RNAi females showed both Tctram and Tctraf isoforms. (D) Amplification product of rp49 shown as a control. (E)–(H) The ovaries from Tctra (E&F) or malE (G&H) dsRNA injected adults were dissected on 20th day after injection of dsRNA, stained with acridine orange and photographed using a fluorescent microscope. F&H show higher magnification image of a single ovariole.
Discussion
The order Coleoptera contains the largest group of insects37 and the red flour beetle, T. castaneum is an excellent model for this group because RNAi works efficiently and systemic and the genome of this insect has been sequenced38. We recently identified dsx homolog from T. castaneum (Tcdsx) which codes for three female-specific and one male-specific isoforms. The functions of Tcdsx isoforms in sex determination and maintenance were also investigated employing RNAi (Shukla and Palli, in preparation). In this paper, we report the identification and functional characterization of splicing regulator of the Tcdsx pre-mRNA, Tctra. Searches of Tribolium genome sequence using Tra/Fem sequences previously identified in dipteran and hymenopteran insects identified Tctra. Further analysis of Tctra sequences revealed that the pre-mRNA of Tctra is sex-specifically spliced into one female- and two male-specific isoforms. Only female isoform codes for protein containing autoregulation, Arg/Ser and proline rich domains conserved in Tra/Fem proteins identified in other insects (Fig. 1A, Supplementary Fig. 1S39). Interestingly, Tra/Tra2 binding sequences, RBP1 binding site and ISS are also present in Tctra (Supplementary Figs. 2S&3S).
Knockdown in the expression of gene coding for Tctra led to the conversion of genetic females to males. This change is seen in soma (disappearance of female papillae during the pupal stage and appearance of male-specific bristles in Tctra knockdown female adults) when Tctra dsRNA injections were performed during the larval stages. Changes in germ line tissues were observed when Tctra dsRNA was injected during the larval, pupal or adult stages. Therefore, Tctra appears to regulate both germ cell and soma sex determination and maintenance, the differences observed could be due to the temporal differences in development of structures studied. Similar case of female-specific regulation of germline and soma, by feminizer, has been reported in honey bees26.
When parental RNAi was used by injecting Tctra dsRNA into adult females to silence the expression of the gene coding for Tctra during early embryogenesis, all the larvae hatched from eggs laid by RNAi females were males. The number of eggs laid by Tctra RNAi females was significantly lower compared to the number of eggs laid by the malE RNAi control females. After Tctra dsRNA injections, oogenesis and fertilization may have proceeded in these females in a normal fashion until Tctra knockdown took place. Later, after Tctra knockdown, both oogenesis and fertilization would have been affected. Consistent with this, we found a reduction in size and arrest in maturation of oocytes in Tctra RNAi females compared to the control females at 20 days after injection of dsRNA (Fig. 5G&H). Since Tctra dsRNA was injected into 5th day PAE females, the oocytes that matured already40,41 would have been developed, fertilized and laid by RNAi females. These data show the requirement of TcTra (and TcDsxF) for maintenance of female sex even in the sexually mature adults. Testing the genetic sex of the adults developed from the eggs laid by the RNAi females showed only 3 out of 34 insects to be genetic females (Table. 3). The Tctra dsRNA injected into the females may have resulted in a reduction in the amount of Tctra mRNA transferred to egg. Alternatively, the Tctra dsRNA injected into the females may have been transferred to the eggs along with Tctra transcript and the dsRNA cleaved and degraded the maternally supplied Tctra transcript. It is also possible that the knockdown in the expression of Tctra may have occurred both in the mother as well as in the embryo after transfer of both mRNA and dsRNA. In either case, the end result is the presence of too low levels of Tctra during the initiation of sex determination cascade. Interestingly, the three genetic females (in the progenies of Tctra parental RNAi parents) were masculinized and produced sperm, successfully mated with females and the mated females produced approximately the same number of eggs as the females mated with normal males.
In D. melanogaster, through the process of dosage compensation, the genes in X-chromosome are hyper-activated, in males, by a group of proteins (forming “dosage compensation complex”) equalizing the overall amount of X-linked gene products in females (2X) and males (1X)42,43,44. The dosage compensation complex is not formed in females, deficient in Msl2 (Male-specific Lethal-2) protein (a key component of dosage compensation complex); Sxl protein (present only in females) suppresses the translation of msl2 mRNA by binding to its UTR sequences45. Highly skewed sex ratio in the adults developed from the eggs laid by Tctra RNAi females is likely due to the mis-regulation of dosage compensation gene(s). Over expression of X- linked genes in Tctra RNAi females may have caused lethality. The presence of Tcdsxm isoform was detected in all the 3 masculinized genetic females. These genetic females (converted to functional males due to parental Tctra RNAi) would have escaped the zygotic death probably because of the presence of TcTra (protein) above the threshold level to inhibit the activation of dosage compensation pathway (and hence no death of genetically masculinized females) but likely, below the levels required to execute the splicing of Tcdsx pre-mRNA into female mode. This argument is supported by the previous work on the D. melanogaster recessive mutants for the virlizer (vir) gene (regulator of sxl pre-mRNA splicing) which causes female-specific zygotic lethality in the embryonic stage and masculinizes the escapers46. Similarly, D. melanogaster mutant, sans-filee (snf16,21), a splicing regulator of sxl pre-mRNA, reduces the zygotic dose of Sxl to one copy number resulting into female-specific lethal maternal effect and a dominant masculinizing zygotic effect47. Consistent with this, we found the reduced amount of female-specific Tctra isoforms in masculinized genetic females developed from eggs laid by Tctra RNAi females, compared to control females (Fig. 5C). The escapers (genetic females) were converted to males that are fertile since virgin females mated with these males laid eggs that successfully developed into larvae. Detailed studies on the dosage compensation mechanisms in insects have only been done in dipterans48. Studies on the insects belonging to the orders Hymenoptera and Lepidoptera suggest the absence of global dosage compensation mechanism in these insects49,50. Further, the existence of dosage compensation in coleopterans is completely a black box since no study on this aspect has been performed in any insect belonging to this order. Tctra knockdown studies presented here, to our knowledge provides the first genetic evidence for the possible existence of dosage compensation in T. castaneum. Another likely explanation for female elimination from the Tctra RNAi progenies is an increase in non-disjunction in XX embryos, as seen in D. melanogaster51. In this insect, a partial reduction in sxl expression in the germline results in high levels of non-disjunction.
The nature of the inhibition of maternal Tctra, in males, is not known at this time. Though, different regulatory mechanisms are known to play role in the regulation of maternal mRNA52,53 we hypothesize the presence of a dominant male-determining gene (M factor) to be present on Y-chromosome of T. castaneum responsible for the inhibition of maternally transferred Tctra (Fig. 6). Similar prediction has been made for the male determination in L. cuprina also29. In D. melanogaster, msl2 is the direct target of Sxl; the primary gene of D. melanogaster sex determination cascade. The observed defects in Tctra RNAi insects suggest that msl2 (or some other dosage compensation component) may be direct or indirect target of Tctra. Also, RNAi studies on Tctra showed the requirement of Tctra throughout the life, in females, to maintain the splicing of Tctra and Tcdsx pre-mRNA in female mode. Data reported here provide the first steps towards understanding of sex determination pathway in a coleopteran insect which not only expands our understanding of sex determination mechanism in insects but also help in designing strategies for the control of harmful insect pests by employing parental RNAi of Tra/Fem and the sterile insect releases.
Model for sex determination in T. castaneum.
Maternally transferred TcTra is translated to make TcTra protein only in females. This TcTra protein splices the zygotically transcribed TcTra pre-mRNA into female mode in turn production of TcTra protein. Continuous production of TcTra, in females, is ensured by the positive autoregulatory feedback loop. TcTra splices the Tcdsx pre-mRNA to produce three female-specific isoforms (Tcdsxf1, Tcdsxf2 and Tcdsxf3). Also, TcTra inhibits some of the Dosage compensation components (DCC) to prevent the formation of active dosage compensation complex, in females. In males, an unknown dominant factor (M) inhibits the translation of maternally supplied Tctra transcript and/or degrades the maternal transcripts or inhibits its autoregulation. The lack of initial TcTra protein, in males, leads to the default splicing of Tctra, coding for truncated non-functional protein. In the absence of TcTra protein Tcdsx pre-mRNA splices in a male mode producing Tcdsxm. Absence of TcTra protein in males allows the formation of functional dosage compensation complex owing to the presence of all the DCC components (dark blue). Dark color represents active protein whereas corresponding light color represents truncated or inactive protein.
Methods
Tribolium castaneum strain, RNA isolation, PCR and RT-PCR
Young (0 day) Larvae, Pupae and adults of T. castaneum strain GA-1 were used in the experiments conducted. Pupa and adults were sexed based on the visualization of sex-specific structures. Dissected adults were frozen in liquid nitrogen and stored at −70°C until further use. Both RNA and DNA (simultaneously), from the same sample, was isolated using Trizol method (Invitrogen Corporation, USA). DNAse treated total RNA was denatured at 75°C for 5 min and immediately chilled on ice. First strand cDNA was synthesized with MMLV reverse transcriptase (Invitrogen, USA) using 17-mers polyT primer, according to the manufacturer’s instructions. Primers targeting male- and female-specific isoforms of Tctra were designed based on the sequence at the junctions of the first and second exons. PCR reaction conditions were as follows-Initial denaturation at 94°C for 2 min, 32 cycles of 94°C for 30 s, 60°C for 30 s, 72°C for 2 min and the final extension at 72°C for 10 min.
Sequence analysis
Exons and introns, of Tctra were identified by aligning sequences of RT-PCR products with their corresponding genomic DNA sequences obtained from the Beetlebase and the NCBI. Exon-intron boundaries were confirmed by aligning the sequences through Spidey (http://www.ncbi.nlm.nih.gov/spidey/).
Double stranded RNA (dsRNA) synthesis and injections
Using T. castaneum cDNA template and primers (shown in supplementary Table 2S) containing the T7 promoter sequence at their 5’ ends and sequence specific to common regions of Tctra was amplified by RT-PCR (Fig.1A). Purified amplicons were in-vitro transcribed to synthesize dsRNA using MEGAscript T7 kit (Ambion, Austin, TX). Amplicon from Escherichia coli malE gene was used to prepare control dsRNA. dsRNA injections were performed on the first day of final instar larvae, 0 h pupa or newly emerged male and female adults (~6 h PAE). The insects were either anesthetized with ether vapor (for larvae) or kept on ice (for pupa and adults) for 8–10 minutes prior to injections. dsRNAs (≈500–600 ng per insect) were injected on the dorsal side of larvae and pupae whereas on the ventral side in adults using an aspirator tube assembly (Sigma) fitted with 3.5″ glass capillary tube (Drummond) pulled by a needle puller (Model P-2000, Sutter Instrument Co.). Injected insects were allowed to recover for 8 h at room temperature (~22°C) and then transferred to standard conditions. Knockdown efficiencies of gene expression in the RNAi insects were calculated as the ratio of gene expression between target dsRNA injected and malE dsRNA injected beetles.
Collection and staging of embryos
For the collection of unfertilized eggs, newly emerged virgin females were sex-separated and allowed to grow. After 5 days, the females were transferred to fine flour and eggs laid over 12 hr period were collected by filtering the flour through sieve of 250 µm pore size. For the collection of fertilized eggs, mated females were allowed to lay eggs in fine flour either for 1 hr or for 5 hr. Eggs collected during one hour period were frozen at 2 hr intervals until 12 hr after egg laying and stored at −70°C until further use. Thus, seven samples at 0–1, 2–3, 4–5, 6–7, 8–9, 10–11 and 12–13 hr after egg laying were tested. Similarly, eggs laid over 5 hr period were staged at 6–11, 18–23, 30–35, 42–47, 54–59, 66–71 hr after egg laying and hatched first instar larvae.
Analysis of parental RNAi
To evaluate the effect of dsRNA mediated knockdown and depletion of Tctra from the early embryos in the next generation; newly emerged adults were sex-separated and allowed to grow for 5 days. dsRNA for Tctra or malE was injected to these adults and allowed to recover at room temperature for one day (24 hrs). Females and males were allowed to mate in a cup kept at standard rearing conditions. After 20 days, cups were checked for the number of larvae and the larvae were further reared to sex-separate them during pupal and adult stages.
Quantitative real time PCR
Quantitative PCR was performed using the SYBR Green kit (Roche, USA) according to the manufacturer’s instructions. Female-specific reverse primer was designed based on the sequences at the junction of first and second exons (Fig. 1A). Male-specific forward primer was designed based on the sequence of male-specific exon (Fig. 1A). RNA isolation and RT-PCR was done as mentioned above. Three independent biological replicates were analyzed for each treatment. Tribolium rp49 gene was used as an endogenous control to normalize the expression data and the gene expression level were analyzed by 2−ΔΔCt method54.
Imaging and documentation
The gonads from the dissected insects were stained with acridine orange and the images were taken by Olympus 1×71 Inverted Research Microscope fitted with reflected fluorescence system. Acridine orange was excited using 502 nm laser line. “Megna Fire software” (version 1.5) was used to control the microscope, image acquisition and exportation of TIFF files. Figures of all micrographs were assembled using Adobe Photoshop element 9.
References
West, S. A., Reece, S. E. & Sheldon, B. C. Sex ratios. Heredity 88, 117–124 (2002).
Zarkower, D. Establishing sexual dimorphism: conservation amidst diversity? Nature Rev Genet. 2, 175–185 (2001).
Bopp, D. About females and males: continuity and discontinuity in flies. J Genet. 89, 315–323 (2010).
Gempe, T. & Beye, M. Function and evolution of sex determination mechanisms, genes and pathways in insects. Bioessays 33, 52–60 (2011).
Sanchez, L. Sex-determining mechanisms in insects. Int J Dev Biol. 52, 837–856 (2008).
Shukla, J. N. & Nagaraju, J. Doublesex: a conserved downstream gene controlled by diverse upstream regulators. J Genet. 89, 341–356 (2010).
Hediger, M. et al. Sex determination in Drosophila melanogaster and Musca domestica converges at the level of the terminal regulator doublesex. Dev Genes Evol 214, 29–42 (2004).
Cho, S., Huang, Z. Y. & Zhang, J. Sex-specific splicing of the honeybee doublesex gene reveals 300 million years of evolution at the bottom of the insect sex-determination pathway. Genetics 177, 1733–1741 (2007).
Ohbayashi, F., Suzuki, M. G., Mita, K., Okano, K. & Shimada, T. A homologue of the Drosophila doublesex gene is transcribed into sex-specific mRNA isoforms in the silkworm, Bombyx mori. Comp Biochem Physiol B 128, 145–158 (2001).
Shukla, J. N., Jadhav, S. & Nagaraju, J. Novel female-specific splice form of dsx in the silkworm, Bombyx mori. Genetica 139, 23–31 (2011).
Salvemini, M. et al. Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti. BMC Evol Biol 11, 41 (2011).
Salz, H. K. Sex determination in insects: a binary decision based on alternative splicing. Curr Opin Genetics Dev 21, 395–400 (2011).
Penalva, L. O. & Sanchez, L. RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol Mol Biol Rev 67, 343–359 (2003).
Erickson, J. W. & Quintero, J. J. Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS biology 5, e332 (2007).
Cline, T. W. Autoregulatory functioning of a Drosophila gene product that establish es and maintains the sexually determined state. Genetics 107, 231–277 (1984).
Schutt, C. & Nothiger, R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development 127, 667–677 (2000).
Keyes, L. N., Cline, T. W. & Schedl, P. The primary sex determination signal of Drosophila acts at the level of transcription. Cell 68, 933–943 (1992).
Boggs, R. T., Gregor, P., Idriss, S., Belote, J. M. & McKeown, M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell 50, 739–747 (1987).
Inoue, K., Hoshijima, K., Sakamoto, H. & Shimura, Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature 344, 461–463 (1990).
Hoshijima, K., Inoue, K., Higuchi, I., Sakamoto, H. & Shimura, Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science 252, 833–836 (1991).
Tian, M. & Maniatis, T. Positive control of pre-mRNA splicing in vitro. Science 256, 237–240 (1992).
Pane, A., De Simone, A., Saccone, G. & Polito, C. Evolutionary conservation of Ceratitis capitata transformer gene function. Genetics 171, 615–624 (2005).
Salvemini, M. et al. Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int J Dev Biol 53, 109–120 (2009).
Beye, M., Hasselmann, M., Fondrk, M. K., Page, R. E. & Omholt, S. W. The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114, 419–429 (2003).
Hasselmann, M. et al. Evidence for the evolutionary nascence of a novel sex determination pathway in honeybees. Nature 454, 519–522 (2008).
Gempe, T. et al. Sex determination in honeybees: two separate mechanisms induce and maintain the female pathway. PLoS biology 7, e1000222 (2009).
Saccone, G., Salvemini, M. & Polito, L. C. The transformer gene of Ceratitis capitata: a paradigm for a conserved epigenetic master regulator of sex determination in insects. Genetica 139, 99–111 (2011).
Verhulst, E. C., Beukeboom, L. W. & van de Zande, L. Maternal control of haplodiploid sex determination in the wasp Nasonia. Science 328, 620–623 (2010).
Concha, C. & Scott, M. J. Sexual development in Lucilia cuprina (Diptera, Calliphoridae) is controlled by the transformer gene. Genetics 182, 785–798 (2009).
Suzuki, M. G. et al. Establishment of a novel in vivo sex-specific splicing assay system to identify a trans-acting factor that negatively regulates splicing of Bombyx mori dsx female exons. Mol Cell Biol 28, 333–343 (2008).
Pane, A., Salvemini, M., Delli Bovi, P., Polito, C. & Saccone, G. The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development 129, 3715–3725 (2002).
Lagos, D., Koukidou, M., Savakis, C. & Komitopoulou, K. The transformer gene in Bactrocera oleae: the genetic switch that determines its sex fate. Insect Mol Biol. 16, 221–30 (2007).
Ruiz, M. F., Milano, A., Salvemini, M., Eirín-López, J. M., Perondini, A. L. P. et al. The Gene Transformer of Anastrepha Fruit Flies (Diptera, Tephritidae) and Its Evolution in Insects. PLoS ONE. 2(11), e1239 (2007).
Lagisz, M., Wilde, K. E. & Wolff, K. The development of PCR-based markers for molecular sex identification in a model insect species Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Entomologia Experimentalis et Applicata 134, 50–59 (2010).
Tan, A. & Palli, S. R. Ecdysone [corrected] receptor isoforms play distinct roles in controlling molting and metamorphosis in the red flour beetle, Tribolium castaneum. Mol Cell Endocrinol 291, 42–49 (2008).
Bitra, K. & Palli, S. R. The members of bHLH transcription factor superfamily are required for female reproduction in the red flour beetle, Tribolium castaneum. J insect physiol. 56, 1481–1489 (2010).
Hunt, T. et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science 318, 1913–1916 (2007).
Richards, S. et al. The genome of the model beetle and pest Tribolium castaneum. Nature. 452, 949–955 (2008)
Verhulst, E. C., van de Zande, L. & Beukeboom, L. W. Insect sex determination: it all evolves around transformer. Current opinion in genet dev. 20, 376–383 (2010).
Parthasarathy, R. & Palli, S. R. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem Molec Biol. 41, 294–305 (2011).
Parthasarathy, R., Sheng, Z., Sun, Z. & Palli, S. R. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem Molec Biol. 40, 429–439 (2010).
Lucchesi, J. C. Dosage compensation in Drosophila. Annu Rev Genet. 7, 225–237 (1973).
Cline, T. W. & Meyer, B. J. Vive la difference: males vs females in flies vs worms. Annu Rev Genet. 30, 637–702 (1996).
Straub, T. & Becker, P. B. Dosage compensation: the beginning and end of generalization. Nature Rev Genet. 8, 47–57 (2007).
Bashaw, G. J. & Baker, B. S. The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell 89, 789–798 (1997).
Hilfiker, A., Amrein, H., Dubendorfer, A., Schneiter, R. & Nothiger, R. The gene virilizer is required for female-specific splicing controlled by Sxl, the master gene for sexual development in Drosophila. Development 121, 4017–4026 (1995).
Oliver, B., Perrimon, N. & Mahowald, A. P. Genetic evidence that the sans fille locus is involved in Drosophila sex determination. Genetics 120, 159–171 (1988).
Ruiz, M. F., Esteban, M. R., Donoro, C., Goday, C. & Sanchez, L. Evolution of dosage compensation in Diptera: the gene maleless implements dosage compensation in Drosophila (Brachycera suborder) but its homolog in Sciara (Nematocera suborder) appears to play no role in dosage compensation. Genetics 156, 1853–1865 (2000).
Wolf, J. B. & Bryk, J. General lack of global dosage compensation in ZZ/ZW systems? Broadening the perspective with RNA-seq. BMC genomics 12, 91 (2011).
Dearden, P. K. et al. Patterns of conservation and change in honey bee developmental genes. Genome Res. 16, 1376–1384 (2006).
Bopp, D., Schutt, C., Puro, J., Huang, H. & Nothiger, R. Recombination and disjunction in female germ cells of Drosophila depend on the germline activity of the gene sex-lethal. Development 126, 5785–5794 (1999).
Bettegowda, A. & Smith, G. W. Mechanismsof maternal mRNA regulation: implications for mammalian early embryonicdevelopment. Front Biosci 12, 3713–3726 (2007).
Zhang, D. X., Cuiand, X. S. &. Kim, N. H. Involvement ofpolyadenylation status on maternal gene expression during in vitro maturationof porcine oocytes. Mol Reprod Dev 76, 881–889 (2009).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Acknowledgements
This work was supported by grants from National Science Foundation (IBN-0421856), National Institutes of Health (GM070559-06) and USDA-CSREES (2007-04636). This is contribution number 09-08-045 from the Kentucky Agricultural Experimental Station.
Author information
Authors and Affiliations
Contributions
Both authors planned and conducted experiments and wrote manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary info
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Shukla, J., Palli, S. Sex determination in beetles: Production of all male progeny by Parental RNAi knockdown of transformer. Sci Rep 2, 602 (2012). https://doi.org/10.1038/srep00602
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00602
This article is cited by
-
Female-to-male sex conversion in Ceratitis capitata by CRISPR/Cas9 HDR-induced point mutations in the sex determination gene transformer-2
Scientific Reports (2020)
-
RNAi mediated knockdown of RpL11, RpS2, and tra-2 led to reduced reproduction of Phytoseiulus persimilis
Experimental and Applied Acarology (2019)
-
Effects of stable ectopic expression of the primary sex determination gene Yob in the mosquito Anopheles gambiae
Parasites & Vectors (2018)
-
Identification and functional characterization of the sex-determining gene doublesex in the sawfly, Athalia rosae (Hymenoptera: Tenthredinidae)
Applied Entomology and Zoology (2017)
-
Identification and functional analyses of sex determination genes in the sexually dimorphic stag beetle Cyclommatus metallifer
BMC Genomics (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.