Abstract
Similarities in morphology and in glandular and squamous differentiation patterns amongst syringomas of the breast nipple and of the skin suggest a common nature, but the origin of nipple syringoma remains undefined. Using triple immunofluorescence analysis, we found that cells immunopositive for basal keratins K5 and 14 undergo differentiation into glandular and squamous cell lineages. Both tumour types expressed K10, indicative of squamous lineage, but there were specific differences in their glandular lineage. In contrast to the breast nipple syringoma, which expressed glandular keratins K8/18/19, syringoma of the skin only expressed the glandular keratin K19. Therefore, syringomas of the breast nipple and of the skin resemble glandular lineages of the breast nipple duct or eccrine duct epithelium, respectively. From these results we conclude that K5/14-positive cells of the breast nipple ducts are the putative cells of origin for syringomas of the nipple, which highlights the organotypic glandular differentiation potential.
Similar content being viewed by others
Introduction
Syringomas of the nipple (or “syringomatous adenomas of nipple” as a formal entity) are rare tumours characterised by well-developed glands intimately admixed with solid nests of squamous differentiation that are immersed in a collagenous stroma1,2,3,4,5. Since the first description of syringomas of the nipple, their morphological similarities to syringomatous tumours of the skin6,7,8,9 have led researchers to propose a common histogenesis for syringomatous adenoma of the nipple and the skin4. Given their differentiation towards structures of the eccrine sweat gland duct10, the histogenetic relationship between syringoma of the skin to eccrine sweat gland ducts has been clearly demonstrated. As eccrine sweat glands are occasionally found in the dermis of the nipple, it was theorised that syringoma of the nipple might also have its origins in sweat gland ducts.
To test this hypothesis, we investigated syringoma of the breast nipple and the skin using multiple immunofluorescence staining methods11,12. Through simultaneous mapping of basal (K5/14), glandular (K7/8/18/19) and squamous (K10) keratins along with discriminatory myoepithelial markers in situ, we determined various immunophenotypes and outlined different cell lineages. These results were used to construct lineage trees in a novel manner compared to ordinary immunohistology. With this approach, we concluded that syringomas of the nipple derive from K5/14-positive breast epithelial cells from large ducts rather than nipple sweat gland ducts, which reflects the organotypic glandular differentiation potential.
Results
In this study, we analysed 10 cases of syringoma of the nipple and 16 cases of typical syringoma of the skin with conventional immunohistological and triple immunofluorescence staining methods. Tumour cell subtypes from both syringomas were compared with their normal counterparts based on the epithelial hierarchy of normal human breast epithelium13,14,15 and eccrine gland ducts10. Glandular cell phenotype was identified with antibodies against keratins K8/18/1910,16. Epidermal differentiation-specific antibody to keratin K10 was regarded as a surrogate marker for squamous differentiation17. Myoepithelial cells were probed with antibodies to smooth muscle actin (SMA)18 and antibodies to basal keratins K5/14 were used as references.
Syringoma of the breast nipple contains K5/14-positive cells with K8/18-positive glandular and K10–positive squamous differentiation
Breast nipple syringomas were composed of open tubules with rounded, ovoid or elongated lumens. These syringomas also had cord-like structures (trabecular and solid), which were often angulated, comma-like or tadpole-like in shape ( Fig. 1a ). These structures were composed of cuboidal to polygonal cells with bland nuclei ( Fig. 1b ). The epithelial structures regularly contained lumens with flattened and more eosinophilic epithelium; occasionally luminal cuticles were observed. The percentage of epithelial structures with epidermoid or squamous differentiations ranged from moderate to abundant. Formation of keratinous microcysts or horn pearls was observed. A myoepithelial layer was not found in HE stained sections. Abundant stroma consisted of densely collagenous to spindle-cell cellular fibromatosis-like stroma.
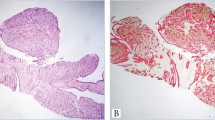
Syringoma of the nipple.
(a) and (b) HE staining shows the typical pattern of this tumour. (a) Solid cord-like epithelial proliferation with angulated or comma-like shape with infiltration of the nipple smooth muscle cells. The tumour contained keratinous cysts in the upper part. (b) Comma-like epithelial proliferation of monotonous cells with bland nuclei at a higher magnification. (c) Nearly all tumour cells are K5-positive. (d) Typical expression of the lineage specific keratins, K5 (pink, asterisks) K8/18 (red) and K10 (green) in the tumour cells. Scale bars, 100 µm.
Tracing cell lineages in syringomatous adenoma of the nipple
The most characteristic feature of these tumours was the extensive expression of basal keratins K5/14 in nearly all tumour cells ( Fig. 1c ). We also observed varying focal expression of glandular keratins K8/18, which were regarded in this context as a surrogate marker for glandular differentiation ( Fig. 1d ). Keratin 19 was variably expressed in these lesions and showed a similar expression pattern as K8/18, whereas syringoma of the skin expressed only K19. Epidermal differentiation-specific keratin K10, which is regarded as a surrogate marker for squamous differentiation, was moderately to abundantly expressed in all cases. Myoepithelial differentiation, as defined by the co-expression of basal keratins K5 and K14 with the myoepithelial marker SMA18, was found only in two cases.
To gain better insight into the nature and state of differentiation of these lesions, we performed triple staining with antibodies against basal (K5/14), glandular (K8/18) and squamous (K10) keratins along with antibodies against the myoepithelial marker SMA. Basal keratins K5 and/or K14 were used as references. A number of cords and bilayered tubules contained tumour cells that only expressed the basal keratins K5/14 ( Figs 1 , 2 ); in one tumour, more than 50% of the tumour cells were K5/14-positive cells. Two lineages of differentiation were observed in these lesions: (i) glandular and (ii) squamous differentiation with the typical distribution of cell subtypes. At the periphery of many epithelial structures, one or several layers of K5/14-positive cells ( Fig. 2 , asterisk) were found, which blended with the inner layers of the K10-positive squamous cells or the K8/18-positive glandular cells ( Figs 1d and 2a,b ).
Syringomatous adenoma of the nipple.
(a) and (b) Triple immunofluorescence labelling for basal K5 (pink), glandular K8/18 (red) and squamous K10 (green) keratins in tumour cells. The cord-like structures reveal many cells that only expressed K5, which are observed in the periphery (asterisks). The inner cells display a gradual differentiation to either glandular (8/18; red) or squamous (K10; green) differentiation. This lineage differentiation is characterised by a gradual transition from K5-positive cells to the lineage marker (in this case K8/18) with sequential expression of K5 and K8/18 with intermediary glandular cells (K5+; K8/18+; arrows) and finally glandular cells (only K8/18+; tailed arrows). A similar gradual evolution is shown in these figures with a sequential expression of K5 and K10. These figures provide evidence that glandular and squamous cell differentiation evolved from K5-positive cells.
Differentiation occurred gradually with the sequential expression of K5/14, then K5/14 and K8/18 (intermediary glandular cells) and finally K8/18-positive glandular cells (tailed arrow). In contrast, squamous differentiation presented with sequential alternative expression of K5/14 and squamous keratin K10 ( Fig. 2 , arrow). Thus, glandular and squamous differentiation could be assumed to result from K5/14-positive progenitor cells. During this process, basal keratins K5 and/or K14 were eventually downregulated and the lineage specific marker was solely expressed. These findings excluded an origin of the squamous lineage from myoepithelial or glandular cells, in contrast to suggestions from the literature19,20,21,22. Interestingly, hybrid glandular/squamous cells with co-expression of these two lineage markers or with additional basal keratins in the same cells were occasionally observed. Orderly lineage differentiation appeared to be lost in these cells. Earlier reports described myoepithelial differentiation as a constitutive feature of syringomas of the nipple23,24,25,26. However, our study showed that myoepithelial differentiation was not a constitutive feature. Likewise, myofibroblasts of the desmoplastic stroma potentially contained numerous SMA-positive myofibroblasts that were intermittently observed near the tumour sheets, which could be misinterpreted as myoepithelial cells (not shown). These cells did not express K5/14, which was in accord with a previous study on myofbroblasts18.
Three syringomas of the nipple in our series were associated with sclerosing lesions (adenomatous adenoma, sclerosing adenosis and sclerosing papilloma). In these cases, transitions from sclerosing adenosis and adenomatous adenoma to a classical pattern of syringoma were observed (not shown).
Syringoma of the skin derives from K5/14 target cells of the eccrine gland duct
In this study, 16 cases of syringomas of the skin were analysed and classified according to the WHO criteria27. They represented well-circumscribed nodules in the upper dermis that ranged from 1.5 mm to 8 mm in diameter. All these cases consisted of aggregates of epithelial structures (round to oval or tadpole-like) that were dispersed in the fibrous stroma ( Fig. 3a, b ). The epithelial structures consisted of monomorphic cells with bland nuclei. Some epithelial structures contained small cystic spaces that were outlined by two layers of cuboidal and occasionally flattened cells. The flattening of the inner cell lining was due to keratinisation of these cells. An eosinophilic cuticle lining the lumen was occasionally observed. Some microcysts contained horn pearls.
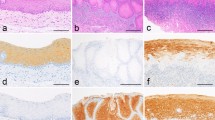
Syringoma of the skin.
(a) and (b) HE staining shows the typical patterns with round to oval or tadpole- like epithelial structures dispersed in a fibrous stroma. Many epithelial structures contain small cystic spaces lined by two layers of cuboidal and occasionally flattened cells. The epithelial structures consist of monomorphic cells with bland-looking nuclei. (c) Prototypic immunohistochemical results for syringoma of the skin for basal keratin K5, squamous keratin K10 and keratin K19. Nearly all the tumour cells stain for K5. Keratin K19 is specifically expressed in luminal cuticles of the tumour cells. (d) Triple immunofluorescence studies for the location of basal (K5, pink), squamous (K10, green) and (K19, red) keratins in the tumour cells. The lower row shows a section of this composite image decomposed into single colours for K5, K10 and K19. This figure demonstrates the extensive expression of basal keratin 5 in tumour structures. The cells in the periphery are intensively immunostained with K5 antibody. The antibody against K10 (green channel) displays focal staining in most epithelial structures. Keratin 19 is expressed in the cytoplasm of luminal cells of a variety of epithelial structures (red channel). These tumours display complete negativity of the tumour cells for K8/18. Furthermore, these structures lack a myoepithelial cell layer. Scale bars, 100 µm.
Tracing cell lineages in syringoma of the skin
Using K77 as a novel specific marker for eccrine duct lineage, a previous study10 recently demonstrated that this tumour differentiated towards duct epithelium of eccrine glands. We observed extensive staining of the epithelial structures with antibodies to basal keratins K5 and K14 without exception ( Fig. 3c ). With the squamous keratin K10 antibody, variable numbers of epithelial structures were stained ( Fig. 3c . We observed keratin K19 staining in the inner lining of the epithelial structures ( Fig. 3c ). K19 immunostaining was positive due to an accumulation of these keratin intermediate filaments in the cytoplasm of glandular cells. However in agreement with previous data10, glandular keratins K8 and K18 were not found in these tumours.
Triple immunostaining ( Fig. 3d ) revealed K5- or K14-positive progenitor cells in most epithelial structures with either focal epidermoid or glandular differentiation, which was revealed by sequential staining for K5/14 with K10 or K19. SMA-immunostaining of syringoma sections only stained the vascular structures (not shown).
Discussion
In this study, we used triple immunofluorescence to reveal the cellular composition of these tumours11,12. All cases from the total 10 cases of syringomatous adenoma of nipple as well as all cases from the total 16 cases of syringoma of the skin showed the identical triple immunofluorescence patterns specifically for each entity. We developed a cell model of syringomas of the nipple and the skin ( Fig. 4 ) using the epithelial cell models of human breast epithelium13,14,15, eccrine gland ducts10 and squamous epithelium28 as a reference. We demonstrated that syringomas of the breast nipple and the skin consist of epithelial structures containing tumour cells that express the following key intermediate keratin filaments: basal keratins K5/14 and probably keratin K1729. These K5/14-positive cells appeared to be the key players in these lesions as they provided the progeny cells for the two lineages observed in these tumours: the epidermal differentiation-specific K10-positive cell lineage and the glandular/luminal K8/18- and K19-positive cell lineage.
(a) Characteristic staining patterns of K5 and K10 in the epidermis.
(b) Schematic drawings of the differentiation lineages of syringoma of the skin (left) and syringoma of the nipple (right). The squamous differentiation resembles that of the differentiation of squamous cells from K5/14-progenitor cells in the basal layer of the epidermis. Glandular differentiation is different in both tumour types and resembles the skin sweat gland duct in syringoma of the skin (c) and resembles the breast nipple duct in the syringoma of the nipple (d). (c) and (d) Red channel (Cy3) for K19, green channel (Alexa Fluor-488) for K8/18 and pink (Alexa Fluor-647) for K5/14. Scale bar, 100 µm.
Interestingly, K5/14-positive tumour cells corresponded phenotypically to K5/14-positive normal counterpart cells of large nipple ducts, the outer layer of the sweat gland duct10 and the basal layer of squamous epithelium16 ( Fig. 4 ). All the nipple tumours in this study expressed basal keratins K5 and K14. Furthermore, these tumours had a triple-negative phenotype29,30,31,32,33,34,35,36. Although triple-negative and of basal phenotype, all these lesions are known to have an excellent prognosis and thus belong to recently described entities of basal type tumours of the breast with a good outcome29,30,31,32,33,34,35,36.
Both syringomas surprisingly displayed striking differences in differentiation in terms of the expression of glandular/luminal keratins K8/18/19. Thus, syringomas of the skin displayed a lineage differentiation characterised by the expression of keratin K19. This lineage demonstrated sequential expression of basal keratin K5/14 and glandular keratin K19, which provided evidence that K19-positive luminal cells were derived from K5/14-positive progenitor cells. Most importantly, these tumours completely lacked glandular keratins K8/18. This expression pattern resembled cells that constitute the innermost layers of eccrine gland ducts ( Fig. 4c ). These data were similar to previous results10, which suggested the histogenesis of syringoma of the skin from eccrine duct cells, based on the expression of a novel keratin K77.
Our findings on syringoma of the skin were in stark contrast to our findings on syringoma of the nipple. All the tumours of the nipple studied here expressed glandular keratins K8/18. Triple immunostaining unravelled the gradual transition of K5/14-positive progenitor cells to K8/18-positive glandular cells in these tumours. As previously suggested by other groups, these keratins were the typical intermediate keratin filaments expressed in normal glandular cells of breast epithelium ( Fig. 4d )16. We previously demonstrated that these cells are derived from K5/14-positive progenitor cells13. Given the similarities in both tumours between glandular cell lineage differentiation and their putative counterpart lineage differentiation of breast nipple duct and sweat gland duct, we propose that both tumours originated from K5/14-positive cells from either sweat ducts or breast epithelium, having retained their organotypic glandular differentiation.
A common feature of nipple and skin syringomas was the epidermal differentiation-specific keratin K10 lineage in a variable amount of tumour tissue. We have characterised the K10-positive lineage from its initiation. We demonstrated that the developmental process of K10-positive differentiation in syringomas from both sites mirrored the transformation of K5/14-positive cells to K10-positive cells. We rationalised with others10 that this transformation emulated the developmental process of the intradermal portion of the eccrine sweat duct. Given that syringoma of the nipple originated in the nipple duct epithelium, we propose that squamous differentiation must be regarded as a metaplastic change caused by the ability of emerging K5/14-positive tumour cells to differentiate towards ontogenetically related epithelial tissue, i.e., nipple epidermis37 ( Fig. 4 ).
This hypothesis explains the similarities and differences of the cell lineages found in these tumours.
In conclusion, we report that K5- and K14-positive tumour cells represent the key players in syringoma of the nipple and the skin. Furthermore, both tumour types showed an epidermal differentiation-specific keratin 10-positive differentiation. In skin syringomas, this phenomenon was akin to acrosyringial lineage differentiation of the intra-epidermal eccrine duct10. In syringomas of the nipple, this phenomenon reflected the differentiation potential of the embryonic rudiment breast epithelium derives from. Therefore, squamous cell lineage differentiation of syringoma of the nipple can be regarded as a true metaplasia and its glandular differentiation appears to be restricted to its organ specific developmental potential. Luminal sweat duct differentiation was observed in syringoma of the skin and breast duct epithelial differentiation in syringoma of the nipple. Our results identified K5/14-positive cells of the breast nipple ducts as the putative origin cells for syringomas of the nipple with the glandular ddifferentiation of this tumour reflecting the organotypic differentiation potential of this gland.
Methods
Human tissue source
Tissue probes were retrieved from the files of the Institute of Pathology of the University of Münster, the Reference and Consultation Centre of Gynaecology and Breast Pathology as well as the Institute of Dermatopathology. The patients were from 43 to 70 years of age. The cases of syringoma of the nipple had been seen in consultation at the University of Muenster. The tumours were routinely fixed in neutral formalin and embedded in paraffin. Histopathology of the nipple tumours was reviewed independently by four pathologists (WB, ThL, HB, TJ), while the skin syringomas were reviewed by three pathologists (WB, MR, TJ) with the criteria described in the WHO breast tumour classification from 200338 and the WHO classification of skin tumours27. Normal human breast tissues obtained from plastic surgery and normal skin with eccrine glands were used as controls.
Tissue probe processing and primary antibodies
For paraffin embedding, tissue probes were routinely fixed with 4% formaldehyde in PBS, pH 7.4. PBS was used for all washes and dilutions. Paraffin tissue sections (4 mµ thick) were deparaffinised with xylene and graded ethanol, while antigen retrieval was achieved by heating the sections in 10 mM sodium citrate buffer, pH 6.0, at 95°C for 30 min in a domestic vegetable steamer. Immunostaining was performed according to the standard protocol routinely used for immunohistopathology11. We recently reported that endogenous Fc receptors in routinely fixed cells and tissue probes do not retain their ability to bind Fc fragments of antibodies39; therefore, blocking the endogenous Fc receptors prior to incubation with primary antibodies was omitted. Antibodies were applied according to manufacturers’ recommendations. The final concentration of primary antibodies was 1 to 5 µg/ml in PBS and the final concentration of secondary antibodies was 5 to 10 µg/ml in PBS. For immunostaining, we used primary antibodies raised against keratin 5 (rabbit monoclonal, MEDAC Diagnostica), keratins K5/6, 7, 10, 14, 18, 18/19 and 8/18/19 (mouse monoclonal, DAKO, Sigma and Dianova), α-smooth muscle actin (SMA; rabbit polyclonal, Abcam), vimentin (Abcam and DAKO), Bcl2 (DAKO). The exclusion of the primary antibody from the immunohistochemical reaction or substitution of primary antibodies with normal IgG (mouse or rabbit) at the same final concentration as that of primary antibodies resulted in a lack of immunostaining.
Bright-field microscopy
After immunoreactions with primary antibodies, the sections were treated for 10 min with methanol containing 0.6% H2O2 to quench endogenous peroxidase. For bright-field microscopy, bound primary antibodies were detected with Dako LSAB REAL Detection System (Naphthol phosphate/Fast Red, #K5005, Dako Corporation, Hamburg, Germany) or with AmpliStain™ Horse Radish Peroxidase (HRP) conjugate (SDT GmbH, Baesweiler, Germany), according to manufacturers’ instructions. The HRP label was visualised using NovaRed substrate kit (Vector Laboratories, Burlingame, CA, USA). The sections were counterstained with haematoxylin. All steps were preceded by rinsing with PBS (pH 7.4).
Multiple fluorescence immunolabelling
For fluorescence microscopy, we used secondary antibodies (purchased from Dianova and Molecular Probes) conjugated with Cy3, Alexa Fluor-488, Alexa Fluor-647 or biotin. For double and triple immunostaining, we used secondary antibodies raised against corresponding IgG species or corresponding IgG isotype11,12. For simultaneous visualisation of primary antibodies of the same IgG isotype, primary antibodies were non-covalently labelled with a reporter molecule in vitro, which employed monovalent IgG Fc-specific Fab fragments according to previous literature40. The reporter molecule was either fluorophore Cy3 or biotin. The latter was visualised using fluorophore-labelled streptavidin. Nuclei were counterstained with DAPI (5 µg/ml PBS, 15 s) and sections were mounted with VectaShield (Vector Laboratories, Burlingame, USA).
Image acquisition
Immunostained sections were examined on a Zeiss microscope “Axio Imager Z1”. Microscopy images were captured using AxioCam digital microscope cameras and AxioVision image processing (Carl Zeiss Vision, Germany). The images were acquired at 96 DPI and submitted with the final revision of the manuscript at 300 DPI.
References
Carter, E. & Dyess, D. L. Infiltrating syringomatous adenoma of the nipple: a case report and 20-year retrospective review. Breast J 10, 443–447 (2004).
Oo, K. Z. & Xiao, P. Q. Infiltrating syringomatous adenoma of the nipple: clinical presentation and literature review. Arch Pathol Lab Med 133, 1487–1489 (2009).
Rosen, P. P. Syringomatous adenoma of the nipple. in Rosen's Breast Pathology 111–114 (Lippincott, Williams & Wilkins, Philadelphia, 2001).
Rosen, P. P. & Ernsberger, D. Low-grade adenosquamous carcinoma. A variant of metaplastic mammary carcinoma. Am J Surg Pathol 11, 351–358 (1987).
Suster, S., Moran, C. A. & Hurt, M. A. Syringomatous squamous tumors of the breast. Cancer 67, 2350–2355 (1991).
Banks, E. R. & Cooper, P. H. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol 18, 227–234 (1991).
Clement, P. B., Young, R. H. & Azzopardi, J. G. Collagenous Spherulosis of the Breast. Am J Surg Pathol 11, 411–417 (1987).
Cooper, P. H. et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol 9, 422–433 (1985).
Goldstein, D. J., Barr, R. J. & Santa Cruz, D. J. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer 50, 566–572 (1982).
Langbein, L. et al. New concepts on the histogenesis of eccrine neoplasia from keratin expression in the normal eccrine gland, syringoma and poroma. Br J Dermatol 159, 633–645 (2008).
Buchwalow, I. B. & Boecker, W. Immunohistochemistry: Basics and Methods, (Springer, Heidelberg, Dordrecht, London, New York, 2010).
Buchwalow, I. B., Minin, E. A. & Boecker, W. A multicolor fluorescence immunostaining technique for simultaneous antigen targeting. Acta Histochem. 107, 143–148 (2005).
Boecker, W. et al. Common Adult Stem Cells in the Human Breast Give Rise to Glandular and Myoepithelial Cell Lineages: A New Cell Biological Concept. Lab Invest 82, 737–746 (2002).
Dontu, G., Al Hajj, M., Abdallah, W. M., Clarke, M. F. & Wicha, M. S. Stem cells in normal breast development and breast cancer. Cell Prolif. 36 Suppl 1, 59–72 (2003).
Prat, A. & Perou, C. M. Mammary development meets cancer genomics. Nat Med 15, 842–844 (2009).
Moll, R., Divo, M. & Langbein, L. The human keratins: biology and pathology. Histochem Cell Biol 129, 705–733 (2008).
Moll, R. Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell.Biochem. 31205–62, 62 (1998).
Di Tommaso, L., Pasquinelli, G. & Damiani, S. Smooth muscle cell differentiation in mammary stromo-epithelial lesions with evidence of a dual origin: stromal myofibroblasts and myoepithelial cells. Histopathology 42, 448–456 (2003).
Palmer, J. O., Ghiselli, R. W. & McDivitt, R. W. Immunohistochemistry in the differential diagnosis of breast diseases. Pathol.Annu. 25 Pt 2, 287–315 (1990).
Raju, U., Zarbo, R. J., Kubus, J. & Schultz, D. S. The Histologic Spectrum of Apocrine Breast Proliferations: A Comparative Study of Morphology and DNA Content by Image Analysis. Hum Pathol 24, 173–181 (1993).
Tavassoli, F. A. Myoepithelial Lesions of the Breast. Myoepitheliosis, Adenomyoepithelioma and Myoepithelial Carcinoma. Am J Surg Pathol 15, 554–568 (1991).
Rosen, P. P. Chapter 16. Carcinoma with metaplasia. in Rosen's Breast Pathology 470–505 (Wolters Kluwer, Lippincott Wiliams & Wilkins, Philadelphie, PA, 2009).
Chen, K. T. Pleomorphic adenoma of the breast. Am J Clin Pathol 93, 792–794 (1990).
Diaz, N. M., McDivitt, R. & Wick, M. R. Pleomorphic Adenoma of the Breast: A Clinicopathologic and Immunohistochemical Study of 10 Cases. Hum Pathol 22, 1206–1214 (1991).
Jones, C. et al. CGH analysis of ductal carcinoma of the breast with basaloid/myoepithelial cell differentiation. Br J Cancer 85, 422–427 (2001).
Moran, C. A., Suster, S. & Carter, D. Benign mixed tumors (pleomorphic adenomas) of the breast. Am J Surg Pathol 14, 913–921 (1990).
LeBoit, P. A., Burg, G., Weedon, D., Sarasin, A. Syringoma. in WHO Classification of Tumours: Skin Tumours (ed. LeBoit P. A., Burg G., Weedon D., Sarasin A.) 140–148 (IARC Press, Lyon, 2006).
Moll, R., Franke, W. W., Schiller, D. L., Geiger, B. & Krepler, R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24 (1982).
Geyer, F. C. et al. Genomic and immunohistochemical analysis of adenosquamous carcinoma of the breast. Mod Pathol 23, 951–960 (2010).
Fadare, O. & Tavassoli, F. A. The phenotypic spectrum of basal-like breast cancers: a critical appraisal. Adv Anat Pathol 14, 358–373 (2007).
Gusterson, B. Do 'basal-like' breast cancers really exist? Nat Rev Cancer 9, 128–134 (2009).
Rakha, E. A., Reis-Filho, J. S. & Ellis, I. O. Basal-like breast cancer: a critical review. J Clin Oncol 26, 2568–2581 (2008).
Reis-Filho, J. S. & Tutt, A. N. Triple negative tumours: a critical review. Histopathology 52, 108–118 (2008).
Van Hoeven, K. H., Drudis, T., Cranor, M. L., Erlandson, R. A. & Rosen, P. P. Low-grade adenosquamous carcinoma of the breast. A clinocopathologic study of 32 cases with ultrastructural analysis. Am J Surg Pathol 17, 248–258 (1993).
Weigelt, B., Baehner, F. L. & Reis-Filho, J. S. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J Pathol 220, 263–280 (2010).
Weigelt, B., Kreike, B. & Reis-Filho, J. S. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat 117, 273–280 (2009).
Tosh, D. & Slack, J. M. How cells change their phenotype. Nat Rev Mol Cell Biol 3, 187–194 (2002).
Tavassoli, F. A., Devilee, P. WHO Classification of Tumours: Tumours of the Breast and Female Genital Organs. (ed. Tavassoli F. A., Devilee P.) (IARC Press, Lyon, 2003).
Buchwalow, I., Samoilova, V., Boecker, W. & Tiemann, M. Non-specific binding of antibodies in immunohistochemistry: fallacies and facts. Sci. Rep. 1, 28; DOI:10.1038/srep00028 (2011) (2011).
Brown, J. K., Pemberton, A. D., Wright, S. H. & Miller, H. R. Primary antibody-Fab fragment complexes: a flexible alternative to traditional direct and indirect immunolabeling techniques. J Histochem Cytochem 52, 1219–1230 (2004).
Acknowledgements
We thank our German colleagues for sharing the reagents and tissue samples as well as sending us the material as consultation cases. We also thank Dr. Markus Tiemann for critical reading of the manuscript and for financial support of the research.
Author information
Authors and Affiliations
Contributions
W.B. and I.B conceived the study and wrote the manuscript. T.L., M.R., H.B., E.K., T.J., D.M. and T.D. were involved in experimental design, data analysis and in the writing of the manuscript. V.S. and A.L. did the immunohistochemistry. All the authors reviewed and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Boecker, W., Junkers, T., Reusch, M. et al. Origin and differentiation of breast nipple syringoma. Sci Rep 2, 226 (2012). https://doi.org/10.1038/srep00226
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00226
This article is cited by
-
Identification of autofluorescent cells in human angioimmunoblastic T-cell lymphoma
Histochemistry and Cell Biology (2018)
-
Squamous/epidermoid differentiation in normal breast and salivary gland tissues and their corresponding tumors originate from p63/K5/14-positive progenitor cells
Virchows Archiv (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.