Abstract
Parasites have a variety of mechanisms to be transmitted to new susceptible hosts, which can be largely grouped in two main modes: vertical (i.e., from parents to the offspring) and horizontal (i.e., between hosts regardless of descent). Because between-host dispersal is a key trait for parasite fitness, scientists studying host-parasite interactions have been long interested in understanding the evolution of their transmission mode(s). Most work in this regard has been theoretical, which resulted in the development of the so-called Continuum hypothesis. This theory states that because vertically transmitted parasites require the host to reproduce, the evolution of this mode of transmission will involve reduced virulence (i.e., the effect of infection on host fecundity) in order to allow maximal host viable progeny production. Conversely, the evolution of horizontal transmission does not have this limitation and parasites with this mode of transmission will evolve higher virulence. Therefore, a trade-off between both modes of transmission across a continuum of virulence values is predicted, with each transmission mode located at the extremes of the continuum. Using plant viruses as a focal parasite, here we review existing theory surrounding the Continuum hypothesis and the experimental work testing the predictions of the theory. Finally, we briefly discuss molecular mechanisms that may explain the existence of vertical-to-horizontal transmission trade-offs and potential implications for the management of virus epidemics.
Similar content being viewed by others
Introduction
The capacity to infect new susceptible individuals, that is, the between-host transmission rate, is arguably the most important determinant of parasite fitness, i.e., its capacity to produce a new generation of individuals1,2. Owing to its importance, parasites have evolved various mechanisms for between-host dispersal. The vector-borne and trans-mammary infections of plants, humans and other mammals, the egg-borne infections of poultry, the transmission by contact and seed-borne infections of plants, and the transovarial infections of invertebrates are some well (and long) known examples3,4. Based on common denominators of these transmission mechanisms, in the 1940s, Gross and others grouped them into two transmission modes: Vertical and horizontal5. Under vertical transmission (from here on, VT), parasites are transmitted across generations, and each host can infect only its own progeny. Under horizontal transmission (from here on, HT), parasites are transmitted to all susceptible hosts in the population regardless of descent6. Since then, scientists have devoted considerable effort to understanding how one, the other, or both modes of transmission are favored during parasite evolution, to explore the epidemiological consequences of this process, and to analyze the evolutionary forces controlling it. Focusing on plant viruses, here we review: (i) The theory on the evolution of the parasite transmission mode; and (ii) the experimental analyses of the predictions of this theory. Finally, the molecular mechanisms that may explain the relative importance of each mode of transmission through parasite evolution, and the potential implications for the management of epidemics, are also discussed. Although we place particular emphasis on plants viruses, most of what is discussed in this review can be applied to viruses (and to other parasites) at large.
Transmission modes of plant viruses
To understand how plant viruses evolve HT and/or VT, it is first necessary to summarize how they are transmitted.
Horizontal transmission by arthropods, particularly aphids, is the most frequent and widely studied plant-virus transmission mode, with at least 25 virus genera transmitted this way7. Based on the acquisition and inoculation timings, aphid-borne transmission can be divided into non-persistent and persistent. Aphids that transmit plant viruses in a non-persistent manner acquire them within a few seconds. Because of the stylet-dependent nature of non-persistently transmitted viruses such as cucumber mosaic virus (CMV) and turnip mosaic virus (TuMV), aphids remain viruliferous for only short periods and spread mainly over short distances7. In contrast, persistently transmitted viruses like begomoviruses or barley yellow dwarf virus (BYDV) require an acquisition period of several minutes/hours but can be retained, and in most cases, remain transmissible, for the vector lifetime8. Some persistently transmitted plant viruses can multiply in the vector cells (circulative replicative plant viruses), thus having cross-kingdom host ranges9. Soil-borne HT is also a frequent way of plant-virus dispersal. Viruses belonging to at least 17 genera are known to be transmitted by soil-inhabiting organisms10, which can be largely categorized into three groups, namely plasmodiophorids (Protista), Olpidium spp. (Fungi), and nematodes (Animalia). Some examples of soil-borne viruses are beet necrotic yellow vein virus (BNYVV)11, grapevine fanleaf virus (GFLV)12 or barley yellow mosaic virus (BYMV)13. Plant viruses can also spread by vegetative propagation invading tubers, which is relatively frequent for sweet potato leaf curl virus (SPLCV)14 and potato virus Y (PVY) in potato15. In addition, plant viruses transmitted by the grafting of infected tissue into a healthy host are not uncommon in grapevine, Prunus spp. and citrus tree orchards worldwide12,16,17. Finally, mechanical transmission by contact is the major way of dispersal during field epidemics of economically important viruses in the genera Tobamovirus, Potexvirus, and Hordeivirus (e.g., ref. 18).
Although less studied, parent-to-offspring VT through seeds has been described for >25% of all known plant viruses19 and for some of them, such as persistent (also known as cryptic) viruses, it is the only way to infect new hosts20. According to the distribution of the virus in the seed, there are two distinct and non-mutually exclusive mechanisms of seed infection: embryonic (infection of embryos) and non-embryonic (contamination of seeds)17,19,21,22,23. Viruses using an embryonic route are considered seed-transmitted, and most often, the seedlings growing from infected embryos harbor the virus, although not always. For instance, the presence of CMV in pepper seed embryos does not guarantee transmission24. Viruses that undergo embryonic seed transmission can infect the embryo by two routes: First, indirectly, by infection of plant gametes prior to fertilization, either the ovules or the pollen25. Second, directly from infected maternal tissue, which has been proposed to occur through the embryonic suspensor before its programmed cell death25. Plant viruses using a non-embryonic route are thought to be transported externally on the seed coat26,27. However, studies revealed that they can also invade the seed coat epidermis and parenchyma cells, and the endothelium that surrounds the endosperm28,29. Plant viruses with non-embryonic seed transmission are considered seed-borne because, although they are carried by seeds externally or internally, they do not infect the embryo, and transmission depends on their capacity to infect the seedling during germination17,30. The paradigmatic and most studied examples of this type of virus are the members of the genus Tobamovirus27.
This dichotomy in plant-virus transmission mode opens the question of how these parasites evolve in one and/or the other transmission mode. Moreover, the majority of seed-transmitted viruses can also achieve HT17. This is apparently a redundancy that may appear counterintuitive in terms of resource optimization for the virus. Then, why is it so common? These questions have been mostly approached from a theoretical perspective, which is the subject of the next section.
Theory on the evolution of parasite transmission mode
The importance of parasite (including plant viruses) transmission to understand the emergence and severity of epidemics31,32, and the increasing awareness of the role that VT may have in initiating outbreaks and in parasite long-term persistence in the host population33,34, has resulted in a well-developed theory on the evolution of parasite transmission modes.
Early mathematical models on the evolution of parasite transmission focused exclusively on HT and its consequences for infection prevalence (see references in ref. 35). It was not until the 1970s that Fine36 developed what he called the fundamental vertical transmission equation with the goal of addressing the contribution of VT to parasite prevalence. Under the assumption that such contribution would be determined by the effect of infection on host progeny production and survival, as well as by the VT rate, the equation predicted that VT parasites would persist in the host population only if infection were beneficial for the host; that is when parasites become mutualists. At any level of virulence (defined as the effect of infection on host fecundity37, which in the case of plants is quantified as the number of viable seeds), VT parasites would also require HT for persistence. Applied to virulent plant viruses, these would evolve VT only in co-existence with HT. As a paradigmatic example, this is the case of CMV which can be transmitted either through seeds at low-medium rates or horizontally via aphids19. Many other plant viruses follow the same pattern17, which would support the prediction of Fine´s model, and then provide a first explanation for why many plant viruses have both transmission modes. This author predicted one exception for this general rule: a (virulent) VT parasite would persist in the host population with no need for HT only at high rates of vertical transmission36.
Using this work as a basis, Ewald35,38 proposed that endosymbionts will move along a parasitism-mutualism continuum depending on the relative importance of VT and HT for infection prevalence. That is, parasites would be facultative mutualists depending on the transmission mode (thus the use of the more general term “endosymbiont”), which is referred to as the Continuum hypothesis (Fig. 1). According to this hypothesis, the fitness of VT endosymbionts would be highly dependent on host reproductive potential, as such endosymbionts need the hosts to reproduce in order to be transmitted. Thus, such organisms will evolve towards lower (or no) virulence to maximize viable progeny production and, therefore, the number of infected descendants. In contrast, HT endosymbionts will have no direct benefit from increased host fecundity. Following Anderson and May2 Trade-off hypothesis, Ewald assumed that higher HT is positively correlated with endosymbiont load, which in turn increases virulence. Accordingly, he predicted that HT parasites will tend towards higher virulence to maximize HT (Fig. 1). Let us again use a plant virus with both transmission modes, such as CMV, as an example. According to the Continuum hypothesis, a CMV strain in which VT and HT have the same relative importance for its fitness would have intermediate levels of virulence and multiplication. Evolution towards strict VT would reduce virulence to nearly zero. That way, infected plants would produce as many viable seeds as non-infected ones. Even if the percentage of seeds that are infected and can germinate (i.e., infected viable seeds) does not change, reduced virulence would increase the total number of infected propagules produced by the plant, and would therefore be evolutionarily advantageous for the CMV strain adapted to VT. If, additionally, the seed transmission rate evolves to be perfect (100% of infected viable seeds), the evolutionary advantage would be even higher (Fig. 1). This decrease in virulence would be associated with lower virus multiplication, thus coming at the cost of reduced HT. Conversely, for the same CMV isolate with intermediate rates of HT and VT to evolve towards strict HT, an increase in virus multiplication, and therefore in virulence, would be required. This would result in lower production of viable plant seeds and therefore reduced VT.
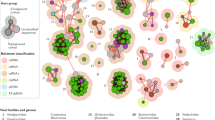
a Relationship between the virus transmission mode (triangles) and virulence (dark red line) leading to a parasitism-commensalism-mutualism continuum (light red line). The width of the triangles represents the relative importance of vertical and horizontal transmission for virus fitness. Mostly vertically transmitted viruses evolve lower virulence to maximize plant fitness and the production of virus-infected seeds. Therefore, these viruses establish more frequently commensalist or mutualistic relationships with the host. In contrast, mostly horizontally transmitted viruses do not require the host to produce progeny and will evolve higher virulence, thus being more often on the parasitism side of the continuum. b Illustration of the two extremes of the mutualism-commensalism-parasitism continuum. Left: a strictly vertically transmitted virus will evolve to be avirulent (commensalist) or to confer a benefit to the plant (mutualist), allowing greater production of viable seed and achieving perfect transmission to the plant progeny. Right, a strictly horizontally transmitted virus will maximize virulence (leading to plant castration in the most extreme cases) and, therefore within-host multiplication, which is positively associated with horizontal transmission rate (here represented by the aphid vector). Such viruses will establish a parasitic relationship with the host plant and will not be vertically transmitted.
Yet, virulent parasites with substantial degrees of VT are known to occur (e.g., refs. 39,40,41). Hence, Lipstich et al. 42,43 explored the conditions in which VT and high virulence might co-exist. These authors distinguished two epidemiological stages: invasion of the host population and equilibrium. In the former stage, their model predicted that 100% prevalence can only be attained by parasites with both modes of transmission, but not by strict VT or strict HT, providing another evolutionary outcome compatible with the observation that in many plant viruses, both modes of transmission co-exist. At equilibrium, strict VT can maintain 100% prevalence42. In this phase, whether VT parasites outcompete HT ones would depend on the level of virulence necessary for parent-to-offspring transmission: High VT attained at low virulence has low cost for host progeny production, which makes this mode of transmission highly efficient. In this context, HT is not favored even if it is highly efficient because as prevalence increases, the number of available hosts for HT decreases, a limitation that does not apply to VT parasites. When high VT requires high virulence, HT is favored as high virulence results in a low number of VT-infected progeny, and this mode of transmission becomes less efficient. Thus, Lipstich and coworkers´ model predicted that the Continuum hypothesis only holds if no constraint of high virulence for VT is imposed on the parasite43. Using a discrete-time model to consider the dynamics of three different kinds of hosts: uninfected, infected via HT and infected via VT, Lively44 reached a similar conclusion. Moreover, this author found that VT parasites could not only establish a mutualistic relationship with the host by evolving to lower virulence, but also by protecting the host from infection by highly virulent HT parasites (provided that VT and HT mixed infections cannot occur).
Interestingly, all these seminal models, although intended to be applicable to parasites at large, did not consider a recovery class of hosts such that they are suitable for plant viruses, as plants cannot clear the infection. Thus, using this theoretical work as a basis, plant virus-specific models have been subsequently developed. Indeed, many of them assume a trade-off between VT and HT such that both modes of transmission cannot be simultaneously maximized as posed by the Continuum hypothesis. For instance, Hamelin et al.45 predicted that, if VT is perfect and the trade-off between modes of transmission is convex (Fig. 2), evolutionary branching results in the appearance of genotypes with either VT or HT. If the trade-off is concave (Fig. 2), intermediate rates of both modes of transmission can co-exist. These predictions are in line with those reported by Bernhauerová and Berec46, who addressed the evolution of VT and HT in sexually transmitted parasites considering an additional trade-off with host mortality. In addition, the model developed by Hamelin et al.45 predicted that tolerance to virus infection, defined as the ability of the plant to reduce the effect of infection on plant fitness at a given parasite load47, selects for high VT. This makes sense as tolerant plants tend to produce more seeds under infection than non-tolerant ones, even at high virus multiplication rates, increasing the advantage of evolving VT. Later, the same authors expanded their model to include the possibility that virus infection increased plant fitness (i.e., the evolution of mutualism from parasitism) and trade-offs of VT and HT with infected host fecundity (i.e., with virulence). Their simulations predicted that, when a VT to virulence trade-off is included, evolution maximizes VT relative to virulence, except if initial virulence is too high, which leads to VT virus extinction. When an HT-to-virulence trade-off was included, both co-existence of parasitic and mutualistic viruses, and the extinction of the parasitic virus leading to strict VT, were possible. This latter outcome leads to mutualists outcompeting parasites48.
Left: linear trade-off, meaning that vertical transmission rate decreases monotonically as horizontal transmission increases and vice versa. Middle: convex trade-off, meaning that small increases of horizontal transmission lead to a rapid decrease of vertical transmission up to a certain point in which further increases of horizontal transmission have little effect on vertical transmission. Right: concave trade-off, meaning that only large increases in horizontal transmission led to reductions in vertical transmission. For convex and concave trade-offs, extreme cases are represented. Changes in the slope of the correlation would smooth the described effects.
The vast majority of the theoretical developments discussed above focused on how VT and HT evolve in relation to virulence, considering that all these traits are determined by the parasite. However, there is also a group of models aimed at understanding how host evolution, parasite-specific characteristics or environmental factors affect the evolution of the transmission mode. Yamamura49 allowed both the host and the parasite to control the evolution of VT. His simulations predicted that, below a threshold of VT, host and parasite interests are not aligned: the host will evolve towards lower parasite exploitation to reduce the effect of infection on its fitness, and the parasite would evolve towards the opposite as the most important transmission mode below the threshold is HT, which requires higher host exploitation (note that here exploitation might be considered a proxy of parasite multiplication/virulence). In this situation, if the host dominates the interaction, the resulting evolutionary outcome is parasitism, and therefore higher virulence, even if VT exists. If the parasite dominates the interaction during evolution, it is possible that, at some point, a mutation appears that increases VT above the threshold. Then, VT becomes more important than HT and, therefore central for parasite fitness. Consequently, the parasite benefits from suppressing host exploitation (lower virulence) to achieve higher VT. In this context, the interests of parasites and hosts align, leading to co-evolution towards mutualism. Similarly, Shillock et al.50 used a game-theory model of co-evolution between parasites and hosts but, at odds with Yamamura49, they showed that high VT does not always lead to more benign parasites, for instance, when achieving VT requires high virulence. Bergstrom et al.6 explored how transmission bottlenecks affect the evolution of virulence in VT and HT RNA viruses (which is the case for most plant viruses51). Their model predicted that stronger population bottlenecks reduce virulence faster in VT than in HT viruses because the stochastic loss (genetic drift) of the most virulent variants in the virus population is more likely in VT virus populations. Also, increasing replication time favours virulence in both VT and HT parasites because it increases the weight of selection and reduces that of bottleneck-associated genetic drift, therefore limiting the loss of virulent variants. The effect of the spatial structure of the host population on the evolution of the transmission mode has also been modeled, predicting that a spatial structure favors VT and reduces virulence due to the limited number of susceptible hosts for HT. In contrast, in well-mixed populations, HT is the preferred strategy as it is more likely to find susceptible hosts52. If we use again as an example a plant virus that has both non-persistent HT by vectors and VT via seed such as CMV, the virus would be more likely to be seed transmitted if the plant population is patched, as for HT the vector would need to travel relatively long distances to find another patch of susceptible plants with the risk of becoming non-viruliferous in the meantime. This limitation would not apply to a homogeneous host population. This prediction is in line with that of van den Bosch et al.53 using a model specifically developed for fungal plant parasites. Finally, the consequences for plant virus prevalence in the plant population of vertical transovarial transmission in insect vectors have also been modeled54,55. Note that these viruses are transmitted in a persistent replicative manner between plants, but generally are not seed transmitted56. The model predicted that transovarial transmission increases the number of viruliferous vectors but has little effect on virus prevalence in the plant population. The authors concluded that, despite such limited impact, transovarial virus transmission in the vector is still evolutionarily advantageous as it allows virus persistence when plant hosts are not available.
From the theoretical framework summarized above, two general predictions arise: (i) evolution of VT is generally associated with reduced virulence (although certain conditions may break such relationship), and (ii) maximization of VT or HT is possible, but rarely both transmission modes can be maximized at the same time (perhaps with the exception of infections in tolerant hosts). Both predictions generally fit with the Continuum hypothesis, and in the next section, we discuss the experimental evidence supporting or challenging them.
Experimental evidence of the theoretical predictions on the evolution of parasite transmission mode
For plant viruses, most of the evidence supporting the predictions of the Continuum hypothesis is indirect (Table 1). For instance, persistent (cryptic) viruses (Partitiviridae, Endornaviridae, Chrysoviridae, Totiviridae) cause asymptomatic infections and are highly prevalent in wild plant populations20,57. These cytoplasmic viruses are not transmitted mechanically or by grafting, and have no biological vectors known58,59. Persistent viruses apparently do not move from cell-to-cell60. Thus, it is thought that they undergo strict vertical transmission via meiosis61, generally at high rates62,63. Moreover, it has been suggested that the high prevalence of these viruses is explained because they confer a competitive advantage to the plant in certain situations (for instance, tolerance to abiotic stresses)63. Hence, they would represent a paradigmatic example of one extreme of the VT to HT continuum: Strict VT with a mutualistic relationship (Fig. 1). Data on plant viruses that cause acute infections are also compatible with the Continuum hypothesis. It has been reported that TuMV isolates with lower virulence in Arabidopsis thaliana have higher seed transmission than those causing more severe infections39,64. Also, CMV is seed transmitted in wild-type A. thaliana genotypes39, but not in mutants impaired in autophagy whereby virus multiplication and virulence are significantly higher65. In the opposite side of the continuum, BYDV, which is a virus restricted to plant phloem that is strictly HT, often induces severe symptoms in the ~100 wild grasses that it infects56. In addition, a relatively mild strain of BYDV (MAV) was displaced by a severe strain (PAV) over a 20-year period in New York state. This shift in strain prevalence appears to reflect differences in HT by aphids56. Similar dynamics have been reported for two other aphid-borne viruses: sugarcane mosaic virus (SCMV) and maize dwarf mosaic virus (MDMV)66. Indirect evidence suggests a positive association between HT and virulence in plant viruses transmitted by other vectors. In susceptible tomato plants, resistance-breaking (RB) isolates of thrip-transmitted tomato spotted wilt virus (TSWV) have HT rates above 60% and as high as 100%, whereas wild-type (WT) isolates have generally lower HT rates (30 to 80%)67,68. Because RB isolates induce symptoms in susceptible and resistant plants and WT strains only in susceptible hosts, RB isolates could be considered as more virulent than WT ones. However, when only susceptible hosts were considered, time to symptom development and severity did not differ between RB and WT isolates despite the increased HT rates of the former, which argues against the generality of the Continuum hypothesis. In the same line, though TSWV has not been reported to be seed transmitted (but see ref. 69), virus isolates causing asymptomatic infections have been repeatedly reported (e.g., refs. 70,71). Other examples challenging the predictions of the Continuum hypothesis do exist. Virulent strains of raspberry ringspot virus (RPRSV) multiply and induce systemic symptoms more rapidly, are more competitive, and are transmitted to seeds more frequently than less virulent strains72. Also, a comparison of seed transmission rates between CMV strains differing in virulence yielded no significant differences39 (Table 1).
Formal analyses of the predictions of the Continuum hypothesis are scant for plant viruses and generally involve the analysis of the effect of virus adaptation to HT and VT through serial passages (Table 1). Following this experimental evolution approach, Stewart et al.73 performed 3–4 serial passages of barley stripe mosaic virus through strict HT and strict VT in barley. Virus adaptation to higher VT resulted in a reduction of virulence, whereas adaptation to higher HT increased virulence, thus supporting the relationship between this trait and the transmission mode predicted by theory. Moreover, adaptation to HT resulted in a reduction in the VT rate, also supporting the prediction that both modes of transmission cannot be simultaneously optimized. In a similar experiment involving CMV and A. thaliana, serial passages of strict VT increased the efficiency of this transmission mode and reduced both virulence and within-host multiplication. In contrast, serial passages of HT did not alter any of these two traits74. These results again fitted one of the predictions of the Continuum hypothesis. However, the seed transmission rate of HT passaged viruses was not determined here, which prevented analyzing VT to HT trade-offs. These authors tested the performance of VT-passaged viruses in seeds obtained before and after serial passages of VT to understand the contribution of host-virus co-evolution to the transmission mode. Remarkably, VT passaged viruses had higher seed transmission in passaged than in ancestral plants, indicating that hosts may also adapt to the virus transmission mode, a possibility largely overlooked by theoretical work (see previous section)74. To our knowledge, no other experiment involving adaptation to virus seed transmission has been published to date (Table 1). However, several analyses of plant virus adaptation to HT are available in the literature. For example, serial passages of soybean dwarf virus (SbDV) through aphid transmission in pea resulted in a significant increase in HT rate and symptom severity, here used as a proxy of virulence75. Similarly, serial passages of plum pox virus (PPV) in pea via aphids resulted in higher HT and accelerated symptom development76. Some experiments have also mimicked HT through aphids using mechanical inoculation. One such work involved 60 serial passages of TuMV HT via mechanical inoculation in A. thaliana, which resulted in higher virulence (which should negatively affect VT) but reduced plant mortality (which enlarges the infectious period, thus theoretically favoring HT)77. Using the same experimental system, the group of Prof. Elena also observed that serial passages of mechanical inoculation generally increased symptom severity (e.g., refs. 78,79). It could be argued that the virus evolution towards higher virulence observed in these works is the consequence of the higher inoculum dose achieved through mechanical inoculation as compared to transmission via aphids, as inoculum dose has been linked to symptom severity51. To our knowledge, the only study in which serial passages of horizontal transmission through mechanical inoculation and aphid transmission were performed in parallel was reported by Wallis et al.76. These authors showed that, although slower in aphid-transmitted virus lineages, evolution towards faster symptom development and higher virus multiplication occurs regardless of the inoculation mode. Moreover, serial passages of mechanical inoculation do not always lead to increased virulence. For instance, Montes et al.80 performed HT serial passages of TuMV through mechanical inoculation in tolerant and non-tolerant A. thaliana plants. Evolved viruses reduced virulence per unit of parasite load in the former but not in the latter. Although these authors did not quantify VT of the passaged viruses, their observations were compatible with the Hamelin et al.45 model. In addition, serial passages of CMV in beans, cucumber, and tomato did not increase infectivity, which could be considered as a proxy of HT, even when evolution resulted in higher virulence81 (Table 1). Thus, it is reasonable to conclude that the results of the works referenced here are, at least in part, associated with adaptation to the transmission mode. Overall, 74% of the direct and indirect experimental analyses support the predictions of the Continuum hypothesis (Table 1). Although this may appear to be a high percentage, it is based only on ~25 works (many of which are not intended to test this hypothesis), and its generality must be taken with caution.
Although out of the scope of this review, it is worth mentioning that evidence supporting the predictions of the Continuum hypothesis also comes from other host-virus interactions. For instance, as early as the 1930s, in an experimental study of the epidemiology of lymphocytic choriomeningitis virus (LCMV) in laboratory mice, in which infected animals were placed in cages with initially uninfected ones, the prevalence of the infection reached 100%, at which point all transmission was vertical. Concurrently, the virulence characteristics of the virus changed, such that infections acquired vertically that had caused 100% morbidity at the beginning of the experiment were asymptomatic by the end82,83. The lower morbidity was not due to the development of a stronger immune response in the host because of recurrent exposure to the virus, as the author reported that the absence of disease occurred even if the virus load remained unchanged83. Insect viruses have also been shown to display similar patterns, for instance, nuclear polyhedrosis virus in fall armyworm (Spodoptera frugiperda)84 or acute paralysis virus and deformed wing virus in honeybees85. Finally, theoretical predictions on the role of host population spatial structure on the evolution of transmission mode have been supported by experimental analyses in bacteria-phage interactions52,86. However, trade-offs between transmission modes have been proven not to be universal: Positive correlations between VT and virus replication/virulence have also been reported for HIV or human papillomavirus87,88.
Determinants of vertical and horizontal transmission
Both theoretical models and experimental evidence seem to largely agree that VT to HT trade-offs mediated by virus multiplication and virulence are widespread in plant-virus (and host-virus) interactions. However, very little is known about the virus genetic determinants of seed transmission, which hampers addressing the molecular bases of the adaptation to the transmission mode19. We envision two possibilities that may explain the trade-off between transmission modes:
First, VT and HT genetic determinants are located in different viral proteins. It is commonly acknowledged that the rate of protein evolution is largely set by the fraction of sites that are involved in protein function (i.e., “functional density”)89,90. Thus, when mutations in one protein increase VT such that it becomes the main mode of transmission, HT determinants become less functionally relevant, and mutations reducing the HT rate are more likely to accumulate as they will have a lesser effect on virus fitness. Current information on the VT and HT determinants of CMV would fit with this possibility: CMV genetic determinants of seed transmission have been mapped in the viral replicase91, whereas those associated with aphid transmission are located in the coat protein92. Interestingly, these proteins modulate virus multiplication and symptom development93, perhaps explaining with these two traits are associated with the evolution of the transmission mode.
Second, VT and HT genetic determinants co-occur in the same protein or are even the same, and this protein mediates virus multiplication and/or virulence. Thus, mutations have one-way effects increasing or decreasing these traits, such that only one mode of transmission can be maximized, but not both. Examples that may be compatible with this possibility are the species in the genus Potyvirus, for which determinants of both HT and VT have been mapped in the helper component proteinase (HC-Pro)94,95. The HC-Pro is the viral suppressor of the RNA silencing plant defense response and interacts with the plant RNA silencing machinery and with other components of the plant defenses such as the proteasome96. Thus, mutations in protein domains affecting VT and HT likely affect virus multiplication and virulence. Indeed, the HC-Pro domain responsible for the interaction with the proteasome fully overlaps with the region of the HC-Pro where the HT determinant is located94,97. Moreover, components of the plant RNA silencing machinery mediate plant meristem invasion by TuMV98, which is thought to be key for VT99.
To our knowledge, none of these possibilities has been tested, neither others that may exist. Thus, how the VT to HT trade-off is genetically controlled remains unknown.
Implications of the evolution of parasite transmission mode for the management of virus outbreaks
As mentioned above, the main mode of plant virus transmission is horizontal. In previous sections, we summarized experimental evidence of how increased HT relates to higher per-plant virulence. In addition, from an epidemiological perspective, evolution towards higher HT increases virus prevalence and, therefore virulence at the population levels: The higher the prevalence, the more individuals are infected and the lower the sum of the number of seeds produced by all individuals in the populations100. Therefore, research on control methods for HT plant viruses is a hot topic in plant virology. Many of these are directed towards interfering with the vector transmission in different ways. Because several comprehensive reviews dealt with this subject in the past years (e.g., refs. 101,102) we will not enter in detail here. However, it is worth mentioning that, in the context of the Continuum hypothesis, if these control methods are successful, they will impose selection pressure on the virus for evolving VT, as this becomes the only mode for transmission available. However, to our knowledge, the consequences of control methods on vector transmission for the evolution of VT and virulence have not been experimentally analyzed.
On the other extreme of the HT to VT continuum, seed transmission has far-reaching consequences for plant virus epidemiology. First, seed infection provides the virus with a means to persist for long periods of time when hosts and/or vectors are not available. Second, seed transmission allows for long-distance dissemination of plant viruses, even at a transcontinental scale. Finally, seed transmission is an important source of primary inoculum for many viruses with vertical transmission, which are horizontally disseminated afterwards via vectors19. Therefore, virus evolution towards higher VT may cause devastating epidemics. For instance, seed-borne CMV epidemics in pepper, a host in which virus seed infection is high24, resulted in yield losses of over 80%103. Also, seed-borne alfalfa mosaic virus (AMV) epidemics in Australian pulse crops resulted in yield losses of up to 100%104. These epidemics are more devastating at higher VT rates19,104. Despite the importance of seed-borne virus outbreaks, control measures are largely limited to seed health tests19. The use of certified virus-free seeds or of varieties resistant to seed transmission has also been proposed in a series of theoretical works on Cassava mosaic disease (CMD) and Maize lethal necrosis (MLN), which are caused by a combination of plant viruses105,106,107. These models predict that the use of clean seeds may result in virus eradication, provided that the economic cost of these seeds is not high. Interestingly, combining resistance to seed transmission and the use of clean seeds may be counterintuitive, reverting the beneficial effect of using clean seeds in controlling disease epidemics. This is because, in this context the use of clean seeds becomes not profitable as reduced VT due to plant resistance does not justify the seed cost. Indeed, currently, the use of clean seeds, but not of resistance to seed transmission, is advised in countries where CMD and MLN are endemic108. Again, the consequences of reducing VT for the evolution of HT remain unexplored.
Concluding remarks
The capacity of a parasite to be transmitted to new susceptible hosts determines the severity and persistence of epidemics. Therefore, understanding how parasites evolve and optimize different modes of transmission has received considerable attention in the past decades from a theoretical perspective. The Continuum hypothesis results from such efforts and provides a conceptual framework to explore how VT and HT are optimized and the factors affecting this process. Theoretical developments have not been accompanied by a similar experimental effort to test the predictions of the Continuum hypothesis (Table 1). Consequently, the generality of the predictions of the Continuum hypothesis remains debatable, and various aspects of the conditions promoting one or the other mode of transmission, as well as the molecular basis controlling the evolution of VT and HT, remain poorly understood. We think that future studies would pay special attention to aspects that include, but are not restricted to:
-
Despite the ample use of mathematical modelling to explore the conditions in which HT and/or VT evolve in plant-virus interactions, most of these models only let the parasite evolve. However, experimental evidence points to a relevant role of host evolution in determining the mode of transmission, such that parasite evolution is not the only force at play74. Considering how co-evolutionary processes may influence the optimization of VT and HT will yield valuable information on the evolution of parasite transmission mode. Indeed, the few theoretical works allowing for host-parasite co-evolution expand the array of evolutionary outcomes regarding the parasite transmission mode as compared with models exclusively considering virus evolution. In addition, plant viruses are, in general, multi-host parasites. For instance, CMV can infect more than 1000 plant species93. However, most mathematical models on the evolution of the transmission mode consider one parasite in a single host. Between-host adaptation trade-offs have been described for plant viruses109, such that changes that allow plant virus adaptation to VT in a given host would not be universal to others. Therefore, an effort should be made to develop a theoretical framework to include the evolution of parasites in more than one host. For this purpose, models that explore the prevalence of plant viruses considering more than one host genotype and both VT and HT exist110, and could be adapted by incorporating the modelling of between-host fitness trade-offs.
-
The Continuum hypothesis was formulated more than 30 years ago. Still, the experimental evidence supporting or challenging this hypothesis is scant, at least for plant-virus interactions. Addressing its generality requires expanding the number of pathosystems currently analyzed. Experimental evolution experiments are a suitable approach for this purpose. Certainly, serial passages of VT may be time-consuming if long-lived plant hosts are utilized. Using short-lived hosts, such as A. thaliana, maybe a way of overcoming this limitation. It is also worth mentioning that, to date, most experimental analyses on the evolution of the transmission mode utilized serial passages of mechanical inoculation as a proxy of HT. Considering that in nature, many plant viruses are transmitted through aphids, using the actual vector in serial passage experiments would be a more realistic way of testing the Continuum hypothesis.
-
A main premise of many mathematical models on the evolution of the parasite mode of transmission is that there is a trade-off between VT and HT. In general, the few experimental analyses of the Continuum hypothesis that involved parallel evolution of the same virus strain by strict VT and strict HT quantified VT only in the viruses evolved through seed transmission (but see ref. 73). Moreover, none considered analyzing HT rate of the isolates passaged by VT. Therefore, future studies should address whether the VT to HT trade-off holds in plant-virus interactions. This would require performing serial passages of strict HT and strict VT of the same virus isolate in parallel, and further analyses of the efficiency of both modes of transmission in the evolved viruses.
-
There is very little information on the molecular bases of plant virus adaptation to a given transmission mode. For instance, none of the serial passage experiments described in this review mapped the genomic changes associated with modifications of VT. Only some of the experiments in which HT passages were performed did so. With the development of next-generation sequencing techniques, obtaining sequence information has become easier and more affordable. Incorporating this information into experimental evolution approaches will provide a more comprehensive picture of how viruses evolve, one of the main traits that control their fitness.
-
Finally, there is a lack of information on how the evolution of plant virus transmission mode relates to the management of viral epidemics in field conditions. Approaching this problem would require understanding the relative contribution of VT and HT to virus fitness before and after applying control measures. This information is currently lacking for virtually every plant virus19. In addition, in nature mixed infections by more than one plant virus in the same plant are commonplace111. Co-existing viruses may also share vectors and seeds as vehicles for transmission. Thus, how virus-virus antagonistic and synergistic interactions affect the evolution of the transmission mode in the presence and absence of control methods should also be addressed.
References
Anderson, R. M. & May, R. M. The population dynamics of microparasites and their invertebrate hosts. Philos. Trans. R. Soc. B: Biol. Sci. 291, 451–524 (1981).
Anderson, R. M. & May, R. M. Coevolution of hosts and parasites. Parasitology 85, 411–426 (1982).
Ross, R. Peculiar pigmented cells found in two mosquitoes fed on malarial blood. Ind. Med. Gaz. 32, 357–358 (1897).
Doolittle, S. P. The mosaic disease of cucurbits. US Dep. Agric. Bull. 879, 1–69 (1920).
Gross, L. The vertical epidemic of mammary carcinoma in mice - its possible implications for the problem of cancer in general. Surg. Gynecol. Obstet. 88, 295–308 (1949).
Bergstrom, C. T., McElhany, P. & Real, L. A. Transmission bottlenecks as determinants of virulence in rapidly evolving pathogens. Proc. Natl Acad Sci. USA 96, 5095–5100 (1999).
Fereres, A. & Raccah, B. Plant virus transmission by insects. In: Encyclopedia of life sciences; https://doi.org/10.1002/9780470015902.a0000760.pub3 (John Wiley & Sons Ltd., 2015).
Makkouk, K. M. & Kumari, S. G. Epidemiology and integrated management of persistently transmitted aphid-borne viruses of legume and cereal crops in West Asia and North Africa. Virus Res. 141, 209–218 (2009).
Bonnamy, M., Blanc, S. & Michalakis, Y. Replication mechanisms of circular ssDNA plant viruses and their potential implication in viral gene expression regulation. mBio 14, e0169223 (2023).
Andika, I. B., Kondo, H. & Sun, L. Interplays between soil-borne plant viruses and RNA silencing-mediated antiviral defense in roots. Front. Microbiol. 7, 1458 (2016).
Tamada, T. General features of beet necrotic yellow vein virus. In: Rhizomania (ed. Biancardi, E. & Tamada, T.) 55–83 (Springer, 2016).
Andret-Link, P. et al. Grapevine fanleaf virus: still a major threat to the grapevine industry. J. Plant Pathol. 86, 183–195, (2004) https://www.jstor.org/stable/41992424.
Kühne, T. Soil-borne viruses affecting cereals: known for long but still a threat. Virus Res. 141, 174–183 (2009).
Kim, J. et al. Seed transmission of Sweet potato leaf curl virus in sweet potato (Ipomoea batatas). Plant Pathol. 64, 1284–1291 (2015).
da Silva, W. et al. Transmission modes affect the population structure of potato virus Y in potato. PLoS Pathog. 16, e1008608 (2020).
Cambra, M. et al. Incidence and epidemiology of Citrus tristeza virus in the Valencian Community of Spain. Virus Res. 71, 85–95 (2000).
Sastry, K. S. Seed-borne plant virus diseases (Springer, 2013).
Sacristán, S., Díaz, M., Fraile, A. & García-Arenal, F. Contact transmission of Tobacco mosaic virus: a quantitative analysis of parameters relevant for virus evolution. J. Virol. 85, 4974–4981 (2011).
Pagán, I. Transmission through seeds: the unknown life of plant viruses. PLoS Pathog. 18, e1010707 (2022).
Roossinck, M. J. Plants, viruses and the environment: ecology and mutualism. Virology 479– 480, 271–277 (2015).
Carroll, T. W. Seedborne viruses: virus-host interactions. In: Plant diseases and vectors: ecology and epidemiology (ed. Maramorosch, K. & Harris, K. F.) 293–317 (Academic Press, 1981).
Johansen, E., Edwards, M. C. & Hampton, R. O. Seed transmission of viruses: Current perspectives. Annu. Rev. Phytopathol. 32, 363–386 (1994).
Card, S. D., Pearson, M. N. & Clover, G. R. G. Plant pathogens transmitted by pollen. Australas. Plant Pathol. 36, 455–461 (2007).
Ali, A. & Kobayashi, M. Seed transmission of Cucumber mosaic virus in pepper. J. Virol. Methods 163, 234–237 (2010).
Maule, A. J. & Wang, D. Seed transmission of plant viruses: a lesson in biological complexity. Trends Microbiol. 4, 153–158 (1996).
Mink, G. I. Pollen and seed-transmitted viruses and viroids. Annu. Rev. Phytopathol. 31, 375–402 (1993).
Dombrovsky, A. & Smith, E. Seed transmission of tobamoviruses: aspects of global disease distribution. In: Advances in seed bioloigy (ed. Jiménez-López, J. C.) Available from: https://doi.org/10.5772/intechopen.70244 (IntechOpen, 2017).
Genda, Y. et al. Immunolocalization of Pepper mild mottle virus in Capsicum annuum seeds. J. Gen. Plant Pathol. 71, 238–242 (2005).
Genda, Y. et al. Immunolocalization of Pepper mild mottle virus in developing seeds and seedlings of Capsicum annuum. J. Gen. Plant Pathol. 77, 201–208 (2011).
Pagán, I. Movement between plants: vertical transmission. In: Cucumber mosaic virus (eds. Palukaitis, P. & García-Arenal, F.) 185–198 (APS Press, 2019).
Thines, M. An evolutionary framework for host shifts - jumping ships for survival. New Phytol. 224, 605–617 (2019).
Holmes, E. C. The ecology of viral emergence. Annu. Rev. Virol. 9, 173–192 (2022).
Adams, B. & Boots, M. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics 2, 1–10 (2010).
Montes, N. & Pagán, I. Challenges and opportunities for plant viruses under a climate change scenario. Adv. Virus Res. 114, 1–66 (2022).
Ewald, P. W. Host-parasite relations, vectors, and the evolution of disease severity. Annu. Rev. Ecol. Syst. 14, 465–485 (1983).
Fine, P. E. Vectors and vertical transmission: an epidemiologic perspective. Ann. N. Y. Acad. Sci. 266, 173–194 (1975).
Pagán, I. & García-Arenal, F. Tolerance of plants to pathogens: a unifying view. Annu. Rev. Phytopathol. 58, 77–96 (2020).
Ewald, P. W. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. N. Y. Acad. Sci. 503, 295–306 (1987).
Cobos, A. et al. Within-host multiplication and speed of colonization as infection traits associated with plant virus vertical transmission. J. Virol. 93, e01078–19 (2019).
Amin, O., Powers, J., Bricker, K. M. & Chahroudi, A. Understanding viral and immune interplay during vertical transmission of HIV: implications for cure. Front. Immunol. 12, 757400 (2021).
Itabashi, K. & Miyazawa, T. Mother-to-child transmission of human T-cell leukemia virus type 1: mechanisms and nutritional strategies for prevention. Cancers 13, 4100 (2021).
Lipsitch, M., Nowak, M. A., Ebert, D. & May, R. M. The population dynamics of vertically and horizontally transmitted parasites. Proc. R. Soc. Lond. B 260, 321–327 (1995).
Lipsitch, M., Siller, S. & Nowak, M. A. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 50, 1729–1741 (1996).
Lively, C. M., Clay, K., Wade, M. J. & Fuqua, C. Competitive coexistence of vertically and horizontally transmitted parasites. Evol. Ecol. Res. 7, 1183–1190 (2005).
Hamelin, F. M. et al. The evolution of plant virus transmission pathways. J. Theor. Biol. 396, 75–89 (2016).
Bernhauerová, V. & Berec, L. Role of trade-off between sexual and vertical routes for evolution of pathogen transmission. Theor. Ecol. 8, 23–36 (2015).
Little, T. J. et al. The coevolution of virulence: t in perspective. PLoS Pathog. 6, e1001006 (2010).
Hamelin, F. M. et al. The evolution of parasitic and mutualistic plant–virus symbioses through transmission-virulence trade-offs. Virus Res. 241, 77–87 (2017).
Yamamura, N. Vertical transmission and evolution of mutualism from parasitism. Theor. Popul. Biol. 44, 95–109 (1993).
Shillcock, G., Úbeda, F. & Wild, G. Vertical transmission does not always lead to benign pathogen-host associations. Evol. Lett. 7, 305–314 (2023).
Hull, R. Plant virology, 5th edn. (Academic Press, 2014).
Berngruber, T. W., Lion, S. & Gandon, S. Spatial structure, transmission modes and the evolution of viral exploitation strategies. PLoS Pathog. 11, e1004810 (2015).
van den Bosch, F., Fraaije, B. A., van den Berg, F. & Shaw, M. W. Evolutionary bi-stability in pathogen transmission mode. Proc. Biol. Sci. 277, 1735–1742 (2010).
Jeger, M. J., Madden, L. V. & van den Bosch, F. The effect of transmission route on plant virus epidemic development and disease control. J. Theor. Biol. 258, 198–207 (2009).
Jeger, M. J., van den Bosch, F., Madden, L. V. & Holt, J. A model for analysing plant-virus transmission characteristics and epidemic development. Math. Med. Biol. J. IMA 15, 1–18 (1998).
Power, A. G. Patterns of virulence and benevolence in insect‐borne pathogens of plants. Crit. Rev. Plant Sci. 11, 351–372 (1992).
Valverde, R. A. & Gutierrez, D. L. Molecular and biological properties of a putative partitivirus from jalapeño pepper (Capsicum annuum L.). Rev. Mex. Fitopatol. 26, 1–6 (2008).
Okada, R. et al. Bell pepper endornavirus, molecular and biological properties, and occurrence in the genus Capsicum. J. Gen. Virol. 92, 2664–2673 (2011).
Sabanadzovic, S. & Valverde, R. A. Properties and detection of two cryptoviruses from pepper (Capsicum annuum). Virus Genes 43, 307–312 (2011).
Boccardo, G., Lisa, V., Luisoni, E. & Milne, R. G. Cryptic plant viruses. Adv. Virus Res. 32, 171–214 (1987).
Roossinck, M. J. Persistent plant viruses: molecular hitchhikers or epigenetic elements? In: Viruses: essential agents of life (ed. Witzany, G.) 177–186 (Springer, 2012).
Sáez, C. & Pagán, I. When plants are Trojan horses for viruses. New Phytol. 237, 1071–1073 (2023).
Verhoeven, A. et al. Arabidopsis latent virus 1, a comovirus widely spread in Arabidopsis thaliana collections. New Phytol. 237, 1146–1153 (2023).
Montes, N. & Pagán, I. Light intensity modulates the efficiency of virus seed transmission through modifications of plant tolerance. Plants 8, 304 (2019).
Shukla, A. et al. Salicylic acid and the viral virulence factor 2b regulate the divergent roles of autophagy during cucumber mosaic virus infection. Autophagy 18, 1450–1462 (2022).
Watson, L. & Gibbs, A. J. Taxonomic patterns in the host ranges of viruses among grasses, and suggestions on generic sampling for host-range studies. Ann. Appl. Biol. 77, 23–32 (1974).
Debreczeni, D. E. et al. Transmission of Tomato spotted wilt virus isolates able and unable to overcome tomato or pepper resistance by its vector Frankliniella occidentalis. Ann. Appl. Biol. 164, 182–189 (2014).
Chinnaiah, S. et al. Novel strains of a pandemic plant virus, tomato spotted wilt orthotospovirus, increase vector fitness and modulate virus transmission in a resistant host. Front. Microbiol. 14, 1257724 (2023).
Wang, H. et al. Seed transmission of tomato spotted wilt orthotospovirus in peppers. Viruses 14, 1873 (2022).
Culbreath, A. K. & Srinivasan, R. Epidemiology of spotted wilt disease of peanut caused by Tomato spotted wilt virus in the southeastern U.S. Virus Res. 159, 101–109 (2011).
Gautam, S. et al. First report of a resistance-breaking strain of tomato spotted wilt orthotospovirus infecting Capsicum annuum with the Tsw resistance gene in Texas. Plant Dis. 107, 6 (2023).
Hanada, K. & Harrison, B. D. Effects of virus genotype and temperature on seed transmission of nepovirus. Ann. Appl. Biol. 85, 79–92 (1977).
Stewart, A. D., Logsdon, J. M. & Kelley, S. E. An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 59, 730–739 (2005).
Pagán, I., Montes, N., Milgroom, M. G. & García-Arenal, F. Vertical transmission selects for reduced virulence in a plant virus and for increased resistance in the host. PLoS Pathog. 10, e1004293 (2014).
Tian, B. et al. Host Adaptation of Soybean dwarf virus following serial passages on pea (Pisum sativum) and soybean (Glycine max). Viruses 9, 155 (2017).
Wallis, C. M. et al. Adaptation of Plum pox virus to a herbaceous host (Pisum sativum) following serial passages. J. Gen. Virol. 88, 2839–2845 (2007).
Vijayan, V. et al. Virulence evolution of a sterilizing plant virus: tuning multiplication and resource exploitation. Virus Evol. 3, vex033 (2017).
González, R., Butković, A. & Elena, S. F. Role of host genetic diversity for susceptibility-to-infection in the evolution of virulence of a plant virus. Virus Evol. 5, vez024 (2019).
Ambrós, S., Olmo-Uceda, M. J., Corrêa, R. L. & Elena, S. F. Phenotypic and genomic changes during Turnip mosaic virus adaptation to Arabidopsis thaliana mutants lacking epigenetic regulatory factors. Evolution 78, 69–85 (2024).
Montes, N., Vijayan, V. & Pagán, I. Host population structure for tolerance determines the evolution of plant-virus interactions. New Phytol. 231, 1570–1585 (2021).
Sacristán, S., Fraile, A., Malpica, J. M. & García-Arenal, F. An analysis of host adaptation and its relationship with virulence in Cucumber mosaic virus. Phytopathology 95, 827–833 (2005).
Traub, E. The epidemiology of lymphocytic choriomeningitis in white mice. J. Exp. Med. 64, 183–200 (1936).
Traub, E. Epidemiology of lymphocytic choriomeningitis in a mouse stock observed for four years. J. Exp. Med. 69, 801–817 (1939).
Fuxa, J. R. & Richter, A. R. Selection for an increased rate of vertical transmission of Spodoptera frugiperda (Lepidoptera: Noctuidae) nuclear polyhedrosis virus. Environ. Entomol. 20, 603–609 (1991).
Fries, I. & Camazine, S. Implications of horizontal and vertical pathogen transmission for honeybee epidemiology. Apidologie 32, 199–214 (2001).
Ebert, D. The epidemiology and evolution of symbionts with mixed-mode transmission. Annu. Rev. Ecol. Evol. Syst. 44, 623–643 (2013).
Kaye, J. N. et al. Viral load as a determinant for transmission of human papillomavirus type 16 from mother to child. J. Med. Virol. 44, 415–421 (1994).
McCarthy, M. HIV levels in mother’s blood predicts risk of perinatal transmission. Lancet 354, 573–573 (1999).
Zuckerkandl, E. Evolutionary processes and evolutionary noise at the molecular level. I. Functional density in proteins. J. Mol. Evol. 7, 167–183 (1976).
Brookfield, J. F. Y. Evolution: what determines the rate of sequence evolution? Curr. Biol. 10, R410–R411 (2000).
Hampton, R. O. & Francki, R. I. B. RNA-1 dependent seed transmissibility of cucumber mosaic virus in Phaseolus vulgaris. Phytopathology 82, 127–130 (1992).
Ng, J. C., Liu, S. & Perry, K. L. Cucumber mosaic virus mutants with altered physical properties and defective in aphid vector transmission. Virology 276, 395–403 (2000).
Palukaitis, P. & García-Arenal, F. Cucumber mosaic virus (APS Press, 2019).
Johansen, I. E. et al. Multiple viral determinants affect seed transmission of pea seedborne mosaic virus in Pisum sativum. J. Gen. Virol. 77, 3149–3154 (1996).
Llave, C., Martínez, B., Díaz-Ruíz, J. R. & López-Abella, D. Amino acid substitutions within the Cys-rich domain of the tobacco etch potyvirus HC-Pro result in loss of transmissibility by aphids. Arch. Virol. 147, 2365–2375 (2002).
Valli, A. A. et al. The HCPro from the Potyviridae family: an enviable multitasking Helper Component that every virus would like to have. Mol. Plant Pathol. 19, 744–763 (2018).
Jin, Y. et al. HC-Pro protein of Potato virus Y can interact with three Arabidopsis 20S proteasome subunits in planta. J. Virol. 81, 12881–12888 (2007).
Incarbone, M. et al. Salicylic acid and RNA interference mediate antiviral immunity of plant stem cells. Proc. Natl. Acad. Sci. USA 120, e2302069120 (2023).
Bradamante, G., Mittelsten Scheid, O. & Incarbone, M. Under siege: virus control in plant meristems and progeny. Plant Cell 33, 2523–2537 (2021).
Agrios, G. N. Plant Pathology. 5th Edn. (Elsevier, 2005).
Bragard, C. et al. Status and prospects of plant virus control through interference with vector transmission. Annu. Rev. Phytopathol. 51, 177–201 (2013).
Heck, M. & Brault, V. Targeted disruption of aphid transmission: a vision for the management of crop diseases caused by Luteoviridae members. Curr. Opin. Virol. 33, 24–32 (2018).
Martín-Rivilla, H. & Pagán, I. Virus seed transmission in Capsicum species. In: Pepper virome. Molecular biology, diagnostics and management (ed. Ali, A. & Gaur, R. K.) (Elsevier, 2024).
Jones, R. A. C. & Congdon, B. S. Australian cool-season pulse seed-borne virus research: 1. Alfalfa and cucumber mosaic viruses and less important viruses. Viruses 16, 144 (2024).
van den Bosch, F., Jeger, M. J. & Gilligan, C. A. Disease control and its selection for damaging plant virus strains in vegetatively propagated staple food crops; a theoretical assessment. Proc. Biol. Sci. 274, 11–18 (2007).
Hilker, F. M. et al. Modeling virus coinfection to inform management of Maize lethal necrosis in Kenya. Phytopathology 107, 1095–1108 (2017).
Hamelin, F. M., Bowen, B., Bernhard, P. & Bokil, V. A. Optimal control of plant disease epidemics with clean seed usage. Bull. Math. Biol. 83, 46 (2021).
Zhan, B., Yang, X., Lommel, S. A. & Zhou, X. Recent progress in Maize lethal necrosis disease: from pathogens to integrated pest management. J. Integr. Agric. 21, 3445 (2022).
Pagán, I., Fraile, A. & García-Arenal, F. Evolution of the interactions of viruses with their plant hosts. In: virus evolution: current research and future directions (eds. Weaver, S. C.; Denison, M.; Roossinck, M. & Vignuzzi, M.) (Caister Academic Press, 2016).
Gutiérrez-Sánchez, Á., Cobos, A., López-Herranz, M., Canto, T. & Pagán, I. Environmental conditions modulate plant virus vertical transmission and survival of infected seeds. Phytopathology 113, 1773–1787 (2023).
Alcaide, C., Rabadán, M. P., Moreno-Pérez, M. G. & Gómez, P. Implications of mixed viral infections on plant disease ecology and evolution. Adv. Virus Res. 106, 145–169 (2020).
Acknowledgements
This work was funded by Plan Nacional I + D + i, Ministerio de Ciencia, innovación y Universidades (Agencia Estatal de Investigación), Spain (grant number: PID2022-141836OB-I00). The funder played no role in the study design, data collection, analysis, and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
L.G.-O. and I.P. collected the information and wrote the manuscript. L.G.-O. prepared the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Ordóñez, L., Pagán, I. Vertical and horizontal transmission of plant viruses: two extremes of a continuum?. npj Viruses 2, 18 (2024). https://doi.org/10.1038/s44298-024-00030-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44298-024-00030-8