Abstract
Direct human-caused mortality accounts for about half of all large mammal mortality in North America. For social species like gray wolves (Canis lupus), the death of pack members can disrupt pack structure and cause pack dissolution, and mortality of breeding adults or wolves during reproduction and pup-rearing can decrease pup recruitment. We estimated minimum and maximum probability of wolf pack persistence in Wisconsin, USA, during biological years (15 April–14 April) 2011–2019 and evaluated the influence of pack size and legal harvest mortality on pack persistence during 2012–2014. Harvests comprised 75–161 mortalities within 194 monitored packs during 2012–2014, with 56–74% of packs having no wolves harvested each year. As an index of reproduction during 2013–2019, we also estimated the proportion of packs where pups responded to howl surveys. We evaluated the influence of pack size, legal harvest, and agency removal on reproduction during 2013–2015. Annual maximum pack persistence probability was uniformly high (0.95–1.00), and annual minimum pack persistence probability ranged from 0.86–0.98 with a possible decline during years of harvest. Reproduction was similar in years following harvest and agency removal (2013–2015, pup response = 0.27–0.40), and years without harvest or agency removal the year prior (2016–2019, pup response = 0.28–0.66). Pack size had a positive effect on pack persistence and reproduction. Total number of wolf mortalities and number of adult male and females removed did not influence pack persistence or reproduction. We suggest that low per-pack mortality, timing of harvest and agency removal, and harvest characteristics during 2012–2014 supported stable pack persistence and reproduction.
Similar content being viewed by others
Introduction
About half of large-vertebrate mortality can be directly attributed to human causes which include vehicle collisions, poaching, agency removal, and legal harvest1. Harvest typically occurs during designated periods in fall or winter and can represent over 70% of anthropogenic mortality for mammals1, yet some species can sustain high rates of harvest with no effects on population abundance. For example, an increase in immigration and recruitment of cougars (Puma concolor) to areas < 1000 km2 offset harvest mortality resulting in stable populations2. Harvest rates ≤ 29% are sustainable for gray wolves3,4, though higher rates can be sustained in some cases5. For large carnivores, other sources of anthropogenic mortality account for most deaths where harvest is not allowed6,7. Although natural deaths can decrease when anthropogenic mortality increases5, human-related mortality can differ from natural death in timing8,9 and across sex-age groups10,11. Management actions (e.g., harvest quotas and timing of hunting), hunter selection, or individual variation in animal vulnerability can result in anthropogenic mortalities being demographically selective. For example, hunter selection of larger bobcats (Lynx rufus) in Wisconsin, USA, resulted in a higher proportion of older, male bobcats harvested12. In Sweden, grizzly bears (Ursus arctos) with lower activity and that spent more time closer to roads were more susceptible to harvest13.
In social species, the loss of one member can reduce the group’s ability to secure resources and reproduce. African elephants (Loxodonta africana) exhibited higher physiological stress and reduced reproductive performance in groups when the matriarch was killed14. Lion (Panthera leo) populations where management removals are common due to livestock depredations experienced reduced reproductive success15. However, reduced group size can also lead to greater food availability for remaining members5 and more breeding opportunities for subordinates16. Group-level responses to mortality can vary with group size and population density17 and the demographics of mortality18.
Anthropogenic mortality accounted for 61% of gray wolf deaths in studies across North America during 1968–201419. Wolf harvest seasons can overlap with important life history events such as mating20, which can affect reproduction and exacerbate the effects of harvest mortality21. Gray wolves have complex social dynamics and a hierarchical group structure usually comprising a breeding pair, pups, and possibly non-breeding adults and subadults5. The loss of one wolf can lead to pack dissolution, particularly when breeding individuals are removed and pack size is small18,22. In stable populations and for larger packs, remaining or immigrating individuals can occupy the role of the removed member and maintain social functions22. Breeder loss can also decrease reproduction and recruitment when removal coincides with mating or pup-rearing21,22. The loss of adult members or subadults involved in pup rearing can lead to decreased pup survival if fewer members are available to secure food and care for pups23. However, multiple subordinate females could breed and increase recruitment the following year if the loss of adult females occurs early in the mating period16.
We first examined wolf pack persistence in Wisconsin during the 2011–2019 biological years (15 April–14 April;24) by estimating annual maximum and minimum probability of wolf pack persistence. Wolf harvest was allowed during a subset of those years (2012–2014), and we expected probability of pack persistence would be lower during this period due to increased mortality. We then evaluated the effect of pack size and harvest mortality on wolf pack persistence during biological years 2012–2014, predicting that smaller pack size and higher mortality, particularly of adult females, would result in lower probability of pack persistence.
We also examined reproduction in Wisconsin during biological years 2013–2019 by estimating the minimum proportion of wolf packs that reproduced (hereafter ‘reproduction’). We expected that reproduction would be lower in years following harvest and non-harvest lethal management (e.g., depredation control and removal due to safety concerns, disease, or injury; hereafter ‘agency removal’) which occurred during 2012–2015. We evaluated the effect of harvest and agency removal on reproduction in the following springs (i.e., spring 2013–2015) predicting that smaller pack size and higher mortality, particularly of adult females, would result in lower reproduction.
Methods
Study area
We studied the gray wolf population in northern and central Wisconsin, USA, where wolves occupy about 91,000 km2;25. The Wisconsin wolf population is part of the Western Great Lakes Distinct Population Segment which comprises about 4400 wolves in Minnesota, Wisconsin, and Michigan and is connected to larger populations in Canada26. The primary prey of wolves in the western Great Lakes Region are white-tailed deer (Odocoileus virginianus), which comprise up to 90% of consumed prey biomass, but wolves also feed on beaver (Castor canadensis), elk (Cervus canadensis), moose (Alces alces), and snowshoe hare (Lepus americanus;27). Other large predators in the area include black bear (U. americanus) and coyote (C. latrans).
Bounties and unregulated hunting and trapping in Wisconsin since 1839 led to the extirpation of gray wolves by 196028. Gray wolves were first listed as endangered in 1967 under the Endangered Species Preservation Act (ESPA) and were federally protected in 1974 under the Endangered Species Act (ESA;29). Wolves began recolonizing Wisconsin in 197528, and by 1999 the Wisconsin–Michigan population had exceeded the federal recovery goal of 100 individuals for 5 consecutive years30. Consequently, gray wolves were reclassified as threatened under the ESA in 2003 which allowed Wisconsin to implement lethal removal of wolves involved in conflicts with humans31. Gray wolf protection status has since changed ten times through 202229. During our study period, gray wolves within the western Great Lakes were delisted, and hunting, trapping, and lethal agency removal were consequently allowed in Wisconsin, during 28 December 2011–20 February 2015, after which legal actions required reinstatement of ESA protections26,32. Western Great Lakes wolves were again delisted on 4 January 2021, but protection under the ESA was reinstated on 10 February 202233. Under ESA protection, lethal control can be used if human safety is a concern (50 CFR 17.21(c)(3)(iv)) but is uncommon in Wisconsin.
Data
We used data collected annually by the Wisconsin Department of Natural Resources (WDNR) on pack territory boundaries, minimum pack size, and pup responses to howl surveys to estimate pack persistence and index reproduction. We also used mortality data collected by the WDNR and U.S. Department of Agriculture Wildlife Services to assess the impact of legal harvest and agency removal on pack persistence and reproduction (Table A1).
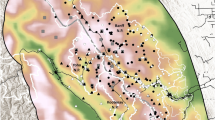
The WDNR monitors wolves annually to estimate population size, pack sizes, and pack territory boundaries within six wolf management zones and tribal reservations (Fig. 1;24). Wolves are captured and radio-collared annually by the WDNR. Telemetry locations were used to estimate annual territory boundaries using minimum convex polygons28,34, and radio-collared wolves were tracked via aerial surveys each winter to estimate pack size. Aerial and snow track surveys, conducted each winter by WDNR staff, tribal biologists, and volunteers who record locations of all wolf sightings and signs (e.g., tracks, scat) observed, were used to estimate minimum pack size and territory boundaries of packs without collared individuals and to supplement data for packs with monitored individuals24,28. Snow track surveys targeted areas with historical wolf presence, reported wolf observations, and highly suitable habitat28,35 and consistently covered over 90% of survey blocks20,24,36,37,38,39,40,41,42. Minimum pack size was estimated using the maximum number of individual track sets located together during a single observation across all surveys and data obtained while tracking radio-collared wolves. Approximate territory boundaries were generated by creating polygons containing telemetry locations, when available, and all locations of sign, tracks, and public sighting reports of wolves within assumed packs and territory boundaries from the prior year if the pack was collared and field sign did not indicate a territory shift28. Individual packs were distinguished based on spatial distribution, timing, and directionality of track and sign observations, locations of radiocollared packs, historical pack use, and information on den and rendezvous sites28. Annual wolf territory maps and pack counts generated by the WDNR represented pack status during winter of the respective biological year.
Howl surveys to monitor general pack location, group size, and pup presence were conducted primarily from dusk to 0100–0200 h during July–October 2013–201943. Howl surveys comprised surveyors howling from locations within or near delineated pack territories to elicit howl responses. Surveyors attempted to elicit responses about every 2.5 km but sometimes deviated to improve likelihood of response43. When a response was heard, the number of responding adults and pups and the estimated distance to and compass azimuth of the response was recorded43,44. We used wolf territory boundaries from the previous biological year and distance and direction of howl responses to determine which pack was associated with each response.
We used data from reported wolf mortalities from harvest or agency removal collected by the WDNR and U.S. Department of Agriculture Wildlife Services during 2012–2015. Though natural mortality and poaching can be difficult to detect45, WDNR obtains coordinates of each harvested wolf from hunters and agency removal is only performed by government agencies which record the location of mortalities. Wolves were identified as male or female and as pup (< 1 year old) or adult (≥ 1 year old). Using coordinates of mortalities, we assigned each wolf mortality to a pack when the mortality occurred within a mapped pack territory during the same or subsequent biological year, or if the mortality was within 3 km of a single pack territory. We selected this 3-km distance as 89% of mortalities were within a territory (60%) or within 3 km of a single pack boundary (29%). We removed 11% of mortalities, of which 2% were within 3 km of two or more territories and we were unable to assign a territory, and 9% were 3–86 km from the nearest known territory. We considered these individuals not associated with a pack and excluded them from analyses. Assessing mortalities within 3 km of a single pack territory can account for extra-territorial movements and seasonal territory shifts46 between data collection (winter) and harvest or agency removal (spring and fall of the following year). Wolf mortalities may also occur away from mapped pack territories if hunters used hounds and wolves were pursued from their territory. However, hounds were not used for hunting in 201247 and aided in < 14% and < 4% of harvests in 2013 and 2014, respectively48,49, suggesting this occurred infrequently.
Pack persistence
We evaluated changes in pack persistence during the 2011–2019 biological years. Our data included annual estimates of the number of wolves within each territory as determined by WDNR, which could be zero. We defined pack formation as the first year when at least two wolves were considered to be maintaining a territory. A count of zero wolves for a given territory during years following pack formation could indicate a pack was not detected, not surveyed, or had dissolved, and we were unable to distinguish among these outcomes. Therefore, we estimated two metrics, maximum and minimum pack persistence, which require different assumptions about the interpretation of no wolves observed in a territory during a given year and represent extreme values of estimated pack persistence (Table A1).
To estimate maximum persistence, we assumed that a count of zero wolves in a given territory and year represented a non-detection of a persisting pack, provided wolves were observed in that territory at least one year before and after the year wolves were not detected. This estimate of maximum persistence likely overestimates persistence given that observations of zero wolves in one year, followed by observations > 0 in the following years, could represent a dissolution and new pack formation. However, pack dissolution and territory replacement by a new pack does not usually occur within the same year22. To estimate minimum persistence, we assumed packs had dissolved when the annual pack count was zero and considered all counts ≥ 2 in subsequent years to belong to a newly formed pack, following our criteria for pack formation. This estimate is likely an underestimate of pack persistence as some zero counts could be due to methodological issues such as non-detection or lack of survey effort.
We defined the year of pack dissolution as the year following the last non-zero count for maximum and minimum persistence. We estimated probability of maximum and minimum overall annual pack persistence for eight biological years (2011–2019) using a Kaplan–Meier estimator50 in package survival51 for program R52 considering persistence equal to survival in a traditional survival analysis. We calculated the change in probability of pack persistence between consecutive years, which we considered similar if 95% confidence intervals overlapped (Table A2).
We conducted an additional analysis of data from the 2012–2014 biological years when wolves in Wisconsin were not listed under the ESA and harvest and agency removal were allowed26. We evaluated the influence of pack size, total number of harvest mortalities, and number of adult males and adult females harvested on maximum and minimum pack persistence using Cox proportional hazard regression models53 (Table A2). Because Cox models typically consider death as the event of interest53, we considered pack dissolution (i.e., a pack does not persist) as the event of interest, but for consistency we report results in the context of pack persistence (i.e., the opposite of dissolution). Pack size corresponded to the minimum count estimate for each pack at the end of the previous biological year. We calculated total number of harvest mortalities and number of adult females and adult males harvested within each wolf biological year for each pack. We did not analyze agency removals due to insufficient sample size. We used pairwise correlations to test for multicollinearity of variables54 and found no correlation (|r|≤ 0.6).
We fit two sets of candidate Cox models, one for minimum persistence and one for maximum persistence. Each set included five models: (1) null model, (2) pack size only, (3) pack size and total number of mortalities, (4) pack size and number of adult male and female mortalities, and (5) pack size, total number of mortalities, and number of adult male and female mortalities. We included random intercepts by pack in all models to account for variation in baseline hazards21,45. We used Akaike Information Criterion corrected for small sample sizes (AICc) to rank models and considered models with ∆AICc ≤ 2 of the top-performing model to have substantial support unless additional covariates resulted in the same log-likelihood with no net reduction in AICc55,56. We also report coefficient estimates and 95% confidence intervals which we considered non-significant if they overlapped 0. The response variable for these models is pack dissociation but we interpret coefficient estimates in the context of pack persistence, where β < 0 represents a positive and β > 0 a negative relationship between the covariate and pack persistence. We conducted all analyses using package coxme57 for program R52.
Reproduction
We used pup response to howl surveys (1 = pups detected, 0 = pups not detected) during biological years 2013–2019 as an index of reproduction. For all packs monitored during howl surveys, we determined whether pups were detected each year regardless of adult detection and calculated the proportion of packs where pups were detected (Table A1).
We further analyzed a subset of data (2013–2015) from biological years following harvest and agency removal when wolves were not listed under the ESA (2012–2014). We used logistic regression to evaluate the influence of pack size, total number of harvest mortalities, and number of adult males and adult females harvested in the prior biological year on probability of reproduction. We fit the same set of five candidate models described above for persistence (Table A2). Agency removal was infrequent compared to harvest, so we assessed its effect on reproduction during biological years 2013–2015 using three models: (1) null model, (2) pack size, and (3) pack size and total number of agency removals. We included random intercepts by pack and biological year in each model. The response variable for these models is pup response where β > 0 represents a positive and β < 0 a negative relationship between the covariate and pup response. We used package lme458 in program R52 for analyses and evaluated models as described for pack persistence.
Results
Pack persistence
We evaluated maximum persistence for 236 packs during biological years 2011–2019 with median pack size of 3 wolves (range = 1–12). Annual maximum pack persistence probability was 0.95–1.00 (mean = 0.98, SD = 0.02) and was stable across years (Fig. 2). Our estimates of minimum pack persistence included the formation of putative new packs; therefore, we evaluated minimum pack persistence for 290 packs during 2011–2019. Annual minimum pack persistence probability was 0.86–0.98 (mean = 0.93, SD = 0.03; Fig. 2) and was similar across years except 2014 (0.86, 95% CI = 0.78–0.95), which was 0.12 less than the 2011 minimum persistence probability estimate (0.98, 95% CI = 0.96–1.00). Minimum pack persistence probability appeared to decline across years of harvest but stabilized beginning 2015. However, estimates of minimum pack persistence probability were relatively uncertain (Fig. 2).
Harvests comprised 75, 161, and 104 mortalities within 194 monitored packs in the 2012–2014 biological years, respectively. Annual wolf harvest mortalities were zero for 56–74% of packs, one for 14–22% of packs, and two or more for 12–22% of packs (Fig. 3). The top-ranked Cox model for maximum persistence during 2012–2014 included pack size a covariate, but the null model had equivalent support (∆AICc = 0.86, Table 1) and the 95% CI of the coefficient associated with pack size overlapped 0 indicating no effect (β = − 0.34, CI = − 0.76–0.08; Table A3). Three maximum pack persistence models failed to converge (Table 1).
The top-ranked model of minimum pack persistence during 2012–2014 included pack size as a covariate and indicated pack persistence increased with increasing pack size (β = − 0.48, CI = − 0.72–−0.25; Table A3). The model including both pack size and total number of mortalities had similar support to the top model (Table 1), but the model log-likelihood was only marginally reduced, indicating the additional covariate was uninformative. Furthermore, the effect of total number of mortalities on pack persistence was uncertain (Table A3).
Reproduction
Howl surveys included 240 wolf packs representing 637 pack-years during 2013–2019 with 1–17 (median = 2) visits per pack annually. For monitored packs, harvest accounted for 50, 92, and 62 mortalities and agency removal accounted for 15, 33, and 26 mortalities in the 2012–2014 biological years, respectively. Reproduction decreased slightly during 2013–2014 (reproduction = 0.31–0.40), was stable during 2015–2017 (reproduction = 0.26–0.28), then increased in 2018–2019 (reproduction = 0.59–0.66; Fig. 4).
Analysis of reproduction including only biological years 2013–2015 comprised 174 packs and 297 pack-years of data. The top–ranked logistic regression model evaluating the effect of harvest on reproduction included pack size only and indicated that reproduction increased with increasing pack size (β = 0.28, 95% CI = 0.08–0.49; Table A4). The model with pack size and total number of mortalities and the model with pack size, number of adult males removed, and number of adult females removed had ∆AICc < 2 (Table 2) but 95% confidence intervals for beta coefficients for total number of mortalities, number of adult males removed, and number of adult females within respective models overlapped 0 indicating uncertain effects on reproduction (Table A4).
Discussion
We found no evidence of decreased maximum pack persistence probability during 2012–2014 in the Wisconsin wolf population, while minimum pack persistence probability estimates followed an apparent decreasing trend during those years but were highly uncertain. Our approach to determining pack persistence estimated two extremes. We expect that not all packs with a count of zero in a given year dissolved, nor did all counts of zero represent a non-detection or lack of survey effort. We conclude that actual wolf pack persistence in Wisconsin was likely between our maximum and minimum estimates, and that while the potential decline in minimum pack persistence probability during 2012–2014 could suggest that harvest reduced persistence, high uncertainty in our persistence estimates limited our inference. Further, maximum pack persistence was stable throughout our study period.
Wolf mortality can reduce pack persistence, particularly when breeders are removed from small packs and the population is small (i.e., < 75 wolves22). Larger wolf populations as in Wisconsin likely have a larger number of dispersing wolves22 that can replace individuals removed from a pack5. Although wolf pack sizes in Wisconsin are small (mean minimum pack size in our study = 3.36), per-pack harvest mortalities during our study were low, with more than half of packs each year having no known harvest mortalities. We were unable to account for natural and other causes of human-caused mortality but assumed these were similar across our treatments. It is possible that per-pack harvest rates in Wisconsin were low enough to preclude any negative impacts of mortality or that lost wolves were replaced by dispersers before pack dissolution.
Minimum pack persistence probability in Wisconsin increased with increasing pack size but was not influenced by total number of wolves harvested or number of adults removed. Pack size did not influence maximum probability of persistence, which was uniformly high. Our results showing a positive relationship between pack size and minimum persistence probability support previous documented effects of pack size on pack dynamics, such as larger packs having more individuals available to defend territories, secure food, and aid in pup-rearing5 and being less likely to dissolve following wolf mortality18,22. However, even larger packs are more likely to dissolve when breeding wolves or adults involved in pup-rearing are removed, with breeder loss preceding 85% of pack dissolutions22. We were unable to differentiate breeding from non-breeding adults but expect at least some adults removed were breeders, and assumed all adults contributed to hunting or pup-rearing given wolf eusociality5. Pack dissolution is more likely when both breeders are lost18,22, which was unlikely in our study since per-pack mortality was low and harvests were pup-biased in 2013 and 2014 (but not 2012; Table A5), suggesting the proportion of breeding individuals lost also was low.
We found no evidence of decreased reproduction in years following harvests (2013–2015) in the Wisconsin wolf population. Harvest may affect reproduction differently if it occurs during the wolf mating period, such as the season in Wisconsin when 218 wolves were harvested during February 22–24 202159. Harvests in Wisconsin during 2012–2014 occurred in October–December and 98% of agency removals occurred from 15 April to November. Only one agency removal occurred during the mating period (late January–early March31), and seven mortalities occurred during the gestation period (early March–early April31). Reproduction can decline the following year if harvest occurs during the mating period21, particularly if mortalities are within three months before mating and breeders are lost22. Although agency removal during our study was adult-biased (Table A5), breeder replacement in large populations occurs quickly leading to reproduction within one year22. We suggest the low per-pack mortality that did not overlap the mating period and the large population size contributed to stable reproduction rates in years following harvest and agency removal in Wisconsin. However, we did not consider the effects of mortality on litter size or pup survival though harvest can reduce pack size and pup survival due to the loss of individuals contributing to hunting and territory maintenance23.
Reproduction was more likely for larger packs which contain more adult wolves that contribute to reproduction success22 but was not influenced by harvest mortality or agency removal. Breeder turnover can decrease reproductive success22, but within-pack shifts in social hierarchies can increase reproduction16. Harvests in Wisconsin during 2012–2014 could have resulted in similar or increased reproduction if the loss of a breeding female allowed for multiple non-breeding females to reproduce23.
We presume that the minimum size of the Wisconsin wolf population during years with harvest (660–834 individuals) and connectivity with wolf populations in Minnesota, Michigan, and Ontario60 helped maintain pack dynamics through replacement of removed individuals with dispersers. We were unable to consider the spatial distribution of wolf harvests though harvest quotas and harvest rates in Wisconsin differ among management zones20,21,40,41. We infer pack persistence and reproduction would remain stable if similar harvest rates were implemented outside of the mating period and harvest demographics are similar to the 2012–2014 harvest seasons. However, wolf responses to human-caused mortality are complex and demonstrate the importance of considering population size and demographics, timing, and magnitude of mortalities when implementing harvests and agency removal.
Data availability
Data are held by the Wisconsin Department of Natural Resources and represent a protected data set. For data permissions, please contact the Wisconsin Department of Natural Resources.
References
Hill, J. E., DeVault, T. L., Wang, G. & Belant, J. L. Anthropogenic mortality in mammals increases with the human footprint. Front. Ecol. Environ. 18, 13–18 (2020).
Robinson, H. S., Wielgus, R. B., Cooley, H. S. & Cooley, S. W. Sink populations in carnivore management: Cougar demography and immigration in a hunted population. Ecol. Appl. 18, 1028–1037 (2008).
Webb, N. F., Allen, J. R. & Merrill, E. H. Demography of a harvested population of wolves (Canis lupus) in west-central Alberta, Canada. Can. J. Zool. 89, 744–752 (2011).
Adams, L. G., Stephenson, R. O., Dale, B. W., Ahgook, R. T. & Demma, D. J. Population dynamics and harvest characteristics of wolves in the Central Brooks Range, Alaska. Wildl. Monogr. 170, 1–25 (2008).
Fuller, T. K., Mech, D. L. & Cochrane, J. F. Wolf Population Dynamics. In Wolves: Behaivor Ecology, and Conservation (eds Mech, L. D. & Boitani, L.) 161–191 (University of Chicago Press, 2003).
Gantchoff, M. G. et al. Mortality of a large wide-ranging mammal largely caused by anthropogenic activities. Sci. Rep. 10, 1–11 (2020).
Benson, J. F., Sikich, J. A. & Riley, S. P. D. Survival and competing mortality risks of mountain lions in a major metropolitan area. Biol. Conserv. 241, 108294 (2020).
Gunson, K. E., Chruszcz, B. & Clevenger, A. P. Large animal-vehicle collisions in the central Canadian rocky mountains: Patterns and characteristics. In International Conference on Ecology and Transportation (2003).
Barber-Meyer, S. M., Wheeldon, T. J. & Mech, L. D. The importance of wilderness to wolf (Canis lupus) survival and cause-specific mortality over 50 years. Biol. Conserv. 258, 109145 (2021).
Wright, G. J., Peterson, R. O., Smith, D. W. & Lemke, T. O. Selection of northern Yellowstone elk by gray wolves and hunters. J. Wildl. Manag. 70, 1070–1078 (2006).
Coltman, D. W. et al. Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658 (2003).
Allen, M. L., Roberts, N. M. & Van Deelen, T. R. Hunter selection for larger and older male bobcats affects annual harvest demography. R. Soc. Open Sci. 5, 180668 (2018).
Leclerc, M., Zedrosser, A., Swenson, J. E. & Pelletier, F. Hunters select for behavioral traits in a large carnivore. Sci. Rep. 9, 12371 (2019).
Gobush, K. S., Mutayoba, B. M. & Wasser, S. K. Long-term impacts of poaching on relatedness, stress physiology, and reproductive output of adult female African elephants. Conserv. Biol. 22, 1590–1599 (2008).
Woodroffe, R. & Frank, L. G. Lethal control of African lions (Panthera leo): Local and regional population impacts. Anim. Conserv. 8, 91–98 (2005).
Ausband, D. E., Mitchell, M. S. & Waits, L. P. Effects of breeder turnover and harvest on group composition and recruitment in a social carnivore. J. Anim. Ecol. 86, 1094–1101 (2017).
Ausband, D. E. & Mitchell, M. S. The effect of group size on reproduction in cooperatively breeding gray wolves depends on density. Anim. Conserv. 24, 994–1000 (2021).
Borg, B. L., Brainerd, S. M., Meier, T. J. & Prugh, L. R. Impacts of breeder loss on social structure, reproduction and population growth in a social canid. J. Anim. Ecol. 84, 177–187 (2015).
Hill, J. E. et al. Quantifying anthropogenic wolf mortality in relation to hunting regulations and landscape attributes across North America. Ecol. Evol. 12, 1–12 (2022).
MacFarland, D. M. & Wiedenhoeft, J. E. Wisconsin gray wolf post-delisting monitoring report 27 January 2012 through 14 April 2013. 18 (2013).
Kolash, J. et al. Changes in reproductive success and pack occupancy for Wisconsin wolves from 2020 to 2021. Technical report. pp 26. Northland College, Ashland, Wisconsin, USA. https://doi.org/10.13140/RG.2.2.29974.83527 (2023).
Brainerd, S. M. et al. The effects of breeder loss on wolves. J. Wildl. Manag. 72, 89–98 (2008).
Ausband, D. E., Mitchell, M. S., Stansbury, C. R., Stenglein, J. L. & Waits, L. P. Harvest and group effects on pup survival in a cooperative breeder. Proc. R. Soc. B Biol. Sci. 284, 1855 (2017).
Wiedenhoeft, J. E., Walter, S., Kluge, N. & Ericksen-Pilch, M. Wisconsin gray wolf monitoring report 15 April 2018 through 14 April 2019. 18pp. (2019).
Stauffer, G. E., Roberts, N. M., Macfarland, D. M. & Van Deelen, T. R. Scaling occupancy estimates up to abundance for wolves. J. Wildl. Manag. 85, 1410–1422 (2021).
USFWS (U.S. Fish and Wildlife Service). Gray wolf biological report: Information on the species in the lower 48 United States. (2018).
DelGiudice, G. D., McCaffery, K. R., Beyer, D. E. & Nelson, M. E. Prey of Wolves in the Great Lakes Region. In Recovery of Gray Wolves in the Great Lakes Region of the United States (eds Wydeven, A. P. et al.) 155–173 (Springer, 2009).
Wydeven, A. P. et al. History, Population Growth, and Management of Wolves in Wisconsin. In Recovery of Gray Wolves in the Great Lakes Region of the United States: An Endangered Species Success Story (eds Wydeven, A. P. et al.) 87–105 (Springer, 2009).
U.S. Congressional Research Service. The gray wolf under the Endangered Species Act (ESA): A case study in listing and delisting challenges (R46184; Nov 25, 2020), by Erin H. Ward. https://crsreports.congress.gov/product/details?prodcode=R46184. At https://crsreports.congress.gov (2020).
USFWS (U.S. Fish and Wildlife Service). Post-delisting monitoring plan for the Western Great Lakes Distinct Population Segment of the gray wolf. U.S. Fish and Wildlife Service, Twin Cities Field Office and Midwest Region. Bloomington, MN and Ft. Snelling, MN. 13pp. https://www.fws.gov/midwest/wolf/monitoring/pdf/FinalWGLDPSPDMPlan.pdf (2008).
Wisconsin Department of Natural Resources. Wisconsin wolf management plan. Wisconsin Department of Natural Resources, Bureau of Wildlife Management. Madison, WI. (2022).
Olson, E. R. et al. Pendulum swings in wolf management led to conflict, illegal kills, and a legislated wolf hunt. Conserv. Lett. 8, 351–360 (2015).
Defenders of Wildlife et al. U.S. Fish and Wildlife Service et al. Case 4:21-cv- 00344-JSW, document 138. https://biologicaldiversity.org/species/mammals/pdfs/Wolf-Order-2022-02-10.pdf. (2022).
Fuller, T. K., York, E. C., Powell, S. M., Decker, T. A. & DeGraaf, R. M. An evaluation of territory mapping to estimate fisher density. Can. J. Zool. 79, 1691–1696 (2001).
Mladenoff, D. J., Sickley, T. A., Haight, R. G. & Wydeven, A. P. A regional landscape analysis and prediction of favorable gray wolf habitat in the northern Great Lakes region. Conserv. Biol. 9, 279–294 (1995).
Wiedenhoeft, J. E., Macfarland, D. M., Libal, N. S. & Bruner, J. Wisconsin gray wolf monitoring report 15 April 2015 through 14 April 2016. 14pp. (2016).
Wiedenhoeft, J. E. et al. Wisconsin Gray Wolf Monitoring Report 15 April 2019 through 14 April 2020. 18pp. (2020).
Wiedenhoeft, J. E., Macfarland, D. M., Libal, N. S. & Bruner, J. Wisconsin Gray Wolf Monitoring Report 15 April 2016 through 14 April 2017. 15pp. (2017).
Wydeven, A. P., Wiedenhoeft, J. E., Schultz, R. N., Bruner, J. E. & Boles, S. R. Status of the timber wolf in Wisconsin, performance report 1 July 2011 through 30 June 2012. Wisconsin Endangered Resources Report #143, Wisconsin Department of Natural Resources, Madison, WI. 37 pp. http://images.library.wisc.edu/WI/EFacs/transactions/WT1978/reference/wi.wt1978.rpthiel.pdf (2012).
Wiedenhoeft, J. E., Macfarland, D. M. & Libal, N. S. Wisconsin gray wolf post-delisting monitoring report 15 April 2013 through 14 April 2014. 16pp. (2014).
Wiedenhoeft, J. E., Macfarland, D. M., Libal, N. S. & Bruner, J. Wisconsin gray wolf monitoring report 15 April 2014 through 14 April 2015. 17pp. (2015).
Wiedenhoeft, J. E., Walter, S., Libal, N. S. & Ericksen-Pilch, M. Wisconsin Gray Wolf Monitoring Report 15 April 2017 through 14 April 2018. 17pp. (2018).
Wydeven, A. P. Howl surveys for wolves in Wisconsin. http://eeinwisconsin.org/Files/eewi/2010/HOWLSURVEYSFORWOLVESINWISCONSIN.doc (2010).
Harrington, F. H. & Mech, L. D. Wolf howling and its role in territory maintenance. Behaviour 68, 207–249 (1979).
Stenglein, J. L. et al. Mortality patterns and detection bias from carcass data: An example from wolf recovery in Wisconsin. J. Wildl. Manag. 79, 1173–1184 (2015).
Mech, D. L. & Boitani, L. Wolf Social Ecology. In Wolves Behavior, Ecology, and Conservation 1–35 (The University of Chicago Press, 2003).
Johnson, R. & Schneider, A. Wisconsin Wolf Season Report 2012. Wisconsin Department of Natural Resources. https://widnr.widen.net/s/k8vtcgjwkf/wolf-season-report-february-2021 (2012).
Wisconsin Department of Natural Resources. Wisconsin Wolf Season Report 2014–15. (2015).
Wisconsin Department of Natural Resources. Wisconsin Wolf Season Report 2013–14. (2014).
Kaplan, E. L. & Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 457–481 (1958).
Therneau, T. M. A package for survival analysis in R. R package version 3.3-1. https://CRAN.R-project.org/package=survival (2022).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2022).
Cox, D. R. Regression models and life-tables. J. R. Stat. Soc. 34, 187–220 (1972).
Dormann, C. F. et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography (Cop.) 36, 27–46 (2013).
Arnold, T. W. Uninformative parameters and model selection using Akaike’s information criterion. J. Wildl. Manag. 74, 1175–1178 (2010).
Burnham, K. P. & Anderson, D. R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304 (2004).
Therneau, T. M. Coxme: Mixed effects cox models. R package version 2.2-18.1. https://cran.r-project.org/web/packages/coxme/index.html (2022).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Johnson, R. & Schneider, A. Wisconsin Wolf Season Report. Wisconsin Department of Natural Resources. 9pp. https://widnr.widen.net/s/k8vtcgjwkf/wolf-season-report-february-2021 (2021).
van den Bosch, M. et al. Identifying potential gray wolf habitat and connectivity in the eastern USA. Biol. Conserv. 273, 109708 (2022).
Acknowledgements
We thank the Wisconsin Department of Natural Resources and United States Department of Agriculture biologists and technicians for data collection. Funding for manuscript preparation was provided by the U.S. Fish and Wildlife Service through the Great Lakes Fish and Wildlife Restoration Act and the Boone and Crockett Program at Michigan State University.
Author information
Authors and Affiliations
Contributions
All authors were involved in conceiving the idea, designing methodology, and curating data. Alejandra Zubiria Perez led data analysis and writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zubiria Perez, A., Kellner, K.F., MacFarland, D.M. et al. Effects of lethal management on gray wolf pack persistence and reproduction in Wisconsin, USA. Sci Rep 14, 9895 (2024). https://doi.org/10.1038/s41598-024-60764-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60764-6
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.