Abstract
Homocysteine (Hcy) and Hcy-thiolactone (HTL) affect fibrin clot properties and are linked to cardiovascular disease. Factors that influence fibrin clot properties and stroke are not fully understood. To study sulfur-containing amino acid metabolites, fibrin clot lysis time (CLT) and maximum absorbance (Absmax) in relation to stroke, we analyzed plasma and urine from 191 stroke patients (45.0% women, age 68 ± 12 years) and 291 healthy individuals (59.7% women, age 50 ± 17 years). Plasma and urinary levels of sulfur-containing amino acid metabolites and fibrin clot properties were significantly different in stroke patients compared to healthy individuals. Fibrin CLT correlated with fibrin Absmax in healthy males (R2 = 0.439, P = 0.000), females (R2 = 0.245, P = 0.000), female stroke patients (R2 = 0.187, P = 0.000), but not in male stroke patients (R2 = 0.008, P = ns). Fibrin CLT correlated with age in healthy females but not males while fibrin Absmax correlated with age in both sexes; these correlations were absent in stroke patients. In multiple regression analysis in stroke patients, plasma (p)CysGly, pMet, and MTHFR A1298C polymorphism were associated with fibrin Absmax, while urinary (u)HTL, uCysGly, and pCysGly were significantly associated with fibrin CLT. In healthy individuals, uHTL and uGSH were significantly associated with fibrin Absmax, while pGSH, and CBS T833C 844ins68 polymorphism were associated with fibrin CLT. In logistic regression, uHTL, uHcy, pCysGly, pGSH, MTHFR C677T polymorphism, and Absmax were independently associated with stroke. Our findings suggest that HTL and other sulfur-containing amino acid metabolites influence fibrin clot properties and the risk of stroke.
Similar content being viewed by others
Introduction
Stroke is one of the leading causes of morbidity and mortality in the world1. Overall stroke burden increased across the globe in both men and women of all ages2. Many traditional risk factors for stroke are known and include hypertension, diabetes, obesity, dyslipidemia, glycemia, etc.3. Ischemic stroke causes an injury in the brain that is mediated by thrombotic or embolic events originating from a cardiac source or periphery4. Thrombus formation involves the generation of a fibrin mesh scaffold that involves complex interactions between components of the coagulation cascade5. Accumulating evidence suggests that fibrin clot properties are associated with the development and progression of venous and thromboembolic disease6. For example, altered fibrin clot structure and function, reflected in increased maximum absorbance (Absmax), and longer clot lysis time (CLT), have been seen in cryptogenic stroke patients7. Another study reported that prolonged CLT did not affect the risk of stroke in young women whereas decreased CLT increases the risk8. Finding factors that affect fibrin clot properties is important in assessing the risk of stroke and in the development of preventive and treatment strategies9.
Elevated plasma (p) total homocysteine (pHcy) is a non-traditional risk factor for stroke10. Changes in pHcy levels are known to affect levels of other sulfur-containing amino acid metabolites11. However, how the sulfur-containing amino acid metabolites are related to stroke and how they affect fibrin clot structure and function in stroke patients was not known. In an earlier study with a large cohort of coronary artery disease (CAD) patients (n ~ 2000), we found that urinary Hcy-thiolactone (uHTL)12,13 and plasma cysteine (pCys)13 were associated with fibrin clot properties and predicted myocardial infarction (MI). These findings have led to a hypothesis that an increased risk of stroke is mediated by the unfavorable effects of sulfur-containing amino acid metabolites on fibrin clot properties. The present study was designed to test this hypothesis by examining the associations of sulfur-containing amino acid metabolites with fibrin clot properties and stroke. We also studied determinants of CLT and Absmax in stroke patients and healthy individuals.
Results
Sex-specific effects of stroke on fibrin clot properties
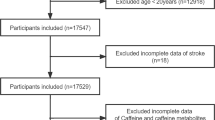
The study population included 191 ischemic stroke patients, 45.0% were female, and the mean age was 68 ± 12 years. Healthy controls included 291 individuals, 59.4% were female, and the mean age was 50 ± 17 years. Descriptive statistics of all variables analyzed in the present study are shown in Supplementary Table S2 online.
Analysis of fibrin clot formation and lysis (see Supplementary Fig. S1) showed that the kinetics of these processes were changed and the correlations between the involved variables were attenuated in stroke patients compared to healthy controls (Supplementary Table S2). For example, the correlation between fibrin CLT and Absmax was reduced to 0.29 in stroke patients from 0.55 in healthy individuals (Supplementary Table S2). This reduction can be traced to different effects of stroke on CLT and Absmax in men and women. Specifically, fibrin CLT and Absmax were strongly correlated in female stroke patients (R2 = 0.187, P = 0.000; Fig. 1A). In contrast, in male stroke patients, there was no correlation between CLT and Absmax (R2 = 0.006, P = ns; Fig. 1B). However, CLT and Absmax were strongly correlated both in healthy females (R2 = 0.236, P = 0.000; Fig. 1C) and healthy males (R2 = 0.437, P = 0.000; Fig. 1D).
Fibrin CLT was significantly longer in female stroke patients compared to healthy women (510 ± 264 vs. 400 ± 161 s, P = 0.000). In contrast, fibrin CLT was not significantly changed in male stroke patients compared to healthy males (405 ± 177 vs. 377 ± 143 s, P = 0.181 (Table 1). Sex significantly affected fibrin CLT in stroke patients (longer in women than in men: 510 ± 264 vs. 405 ± 177 s, P = 0.001) but not in healthy individuals (401 ± 161 vs. 377 ± 143 s, P = 0.199) (Table 1).
Fibrin Absmax was significantly elevated both in female (0.124 ± 0.074 vs. 0.100 ± 0.035 A340, P = 0.000) and male stroke patients (0.122 ± 0.056 vs. 0.103 ± 0.037 A340, P = 0.003) (Table 1). Sex did not affect Absmax in stroke patients (0.124 ± 0.074 A340 in females vs. 0.122 ± 0.056 A340 in males, P = 0.844) nor in healthy individuals (0.100 ± 0.035 A340 in females vs. 0.103 ± 0.037 A340 in males, P = 0. 399) (Table 1).
Sex-specific effects of stroke on levels of sulfur-containing metabolites
Levels of uHTL were significantly reduced in male (but not female, P = 0.729) stroke patients compared to healthy controls (24 ± 71 vs. 66 ± 77 nM, P = 0.000), while levels of uCys and uCysGly were elevated in female (but not male, P = 0.099 and P = 0.495, respectively) stroke patients (uCys: 265 ± 207 vs. 181 ± 124 μM, P = 000; uCysGly: 10.2 ± 7.2 vs. 8.4 ± 5.5 μM, P = 0.024). Levels of uHcy were significantly elevated in stroke patients compared to healthy controls (female: 9.2 ± 9.2 vs. 4.8 ± 3.7 μM, P = 0.000; male: 11.4 ± 10.9 vs. 8.9 ± 5.7 μM, P = 0.031). Levels of uGSH were significantly reduced in stroke patients (female: 3.5 ± 3.4 vs. 5.0 ± 5.5 μM, P = 0.021; male: 3.3 ± 3.2 vs. 5.6 ± 5.4 μM, P = 0.000) (Table 1).
Female stroke patients (but not male) had significantly elevated pHcy compared to healthy controls (7.5 ± 3.6 vs. 5.4 ± 1.7 μM, P = 0.000) while pCys was elevated both in female (272 ± 63 vs. 222 ± 63 μM, P = 0.000) and male (256 ± 58 vs. 235 ± 74 μM, P = 0.020) stroke patients. Levels of pCysGly (17 ± 9 vs. 22 ± 13 μM, P = 0.002) and pGSH (3.3 ± 1.8 vs. 4.6 ± 3.0 μM, P = 0.000) were significantly reduced in male (but not in female, P = 0.551 and P = 0.073, respectively) stroke patients. However, plasma methionine (pMet) was not changed in female (P = 0.608) or male (P = 0.316) stroke patients compared to healthy individuals (Table 1).
Changes in creatinine levels associated with ischemic stroke were also sex specific. For example, urinary creatinine was significantly reduced in male stroke patients compared to healthy males (12.8 ± 7.1 vs. 17 ± 9 mM, P = 0.000) but was not changed in female stroke patients compared to healthy females (9.8 ± 6.9 vs. 10.1 ± 6.7 mM, P = 0.682). However, plasma creatinine was significantly elevated in ischemic stroke patients, both female and male (female: 76 ± 21 vs. 64 ± 9 μM, P = 0.000; male: 94 ± 40 vs. 81 ± 12 μM, P = 0.001) (Table 1).
Sex-specific effects of age on fibrin clot properties
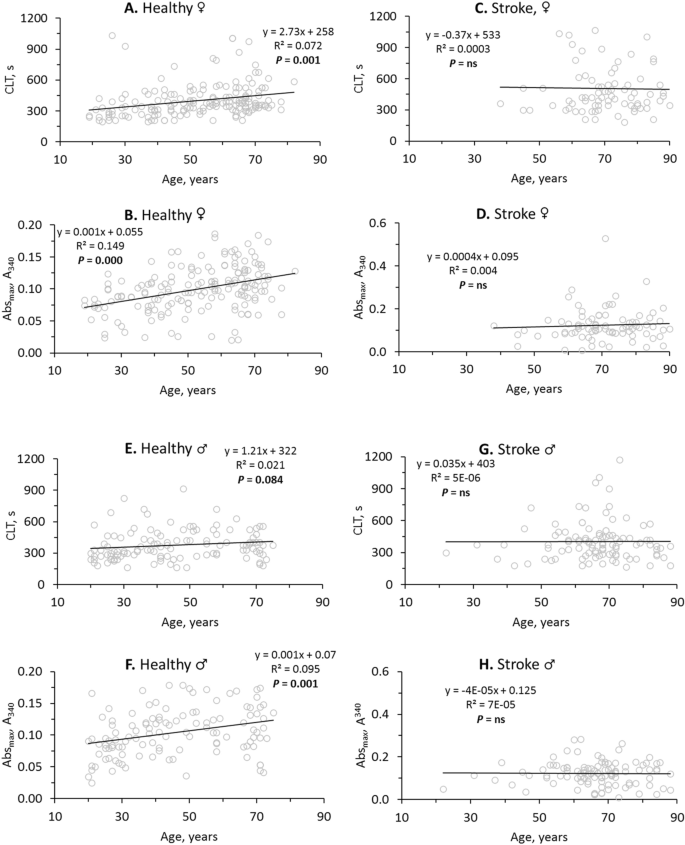
In healthy females, fibrin CLT (R2 = 0.072, P = 0.001; Fig. 2A) and fibrin Absmax (R2 = 0.149, P = 0.000; Fig. 2B) significantly increased with age. In contrast, age did not affect CLT (R2 = 0.0003, P = ns; Fig. 2C) nor Absmax (R2 = 0.004, P = ns; Fig. 2D) in female stroke patients.
Stroke abrogates the influence of age on fibrin clot properties. Pearsons’s correlations between fibrin CLT (A,C,E,G), Absmax (B,D,F,H) and age in healthy individuals (A,B,E,F) and ischemic stroke patients (C,D,G,H) are shown. Higher values of CLT and Absmax indicate lower susceptibility to lysis and worse clot structure, respectively.
In contrast to healthy females, CLT was not affected by age in healthy males (R2 = 0.021, P = 0.084; Fig. 2E). However, like in healthy females, significant increases of Absmax with age were seen in healthy males (R2 = 0.095, P = 0.001; Fig. 2F). Like in female stroke patients, age did not affect CLT (R2 = 5E−06, P = ns; Fig. 2G) and Absmax (R2 = 7E−05, P = ns; Fig. 2H) in male stroke patients.
In healthy females the intercept of the trendline at the CLT axis was 258 s and the CLT was increasing at a rate of 2.73 s/year (Fig. 2A) while in female stroke patients the intercept was elevated to 533 s and there was no significant change in CLT with age (Fig. 2C). This shows that in young female stroke patients CLT has already reached the maximal high value (Fig. 2C), characteristic of CLT in older healthy females (Fig. 2A). Similarly, in young female (Fig. 2D) and male (Fig. 2H) stroke patients Absmax has already reached the maximal high value, characteristic of CLT in older healthy females (Fig. 2B) and males (Fig. 2F). These findings suggest prothrombotic fibrin clot properties in young stroke patients like those of older healthy individuals.
Sulfur-containing amino acid metabolite levels affect fibrin clot properties in healthy individuals but not in ischemic stroke patients
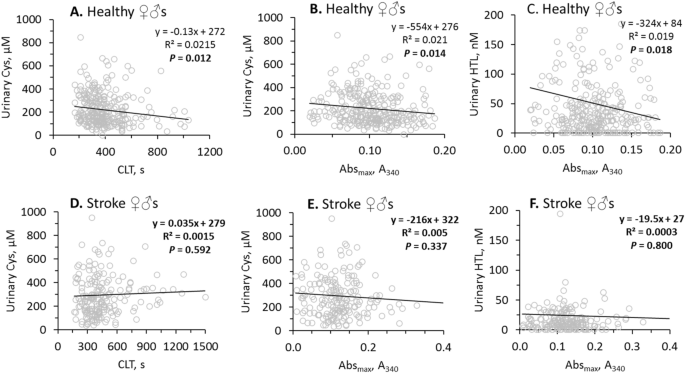
In healthy individuals, urinary sulfur-containing amino acid metabolites were negatively correlated with fibrin CLT and Absmax (Table 2). Specifically, uCys was significantly correlated with CLT (R2 = 0.0215, P = 0.012; Fig. 3A) and Absmax (R2 = 0.021, P = 0.014; Fig. 3B) while uHTL was significantly correlated only with Absmax (R2 = 0.019, P = 0.018; Fig. 3C). Weaker correlations were observed for uHcy with CLT (R2 = 0.013, P = 0.054) and Absmax (R2 = 0.016, P = 0.043), for uCysGly with CLT (R2 = 0.018, P = 0.023) and Absmax (R2 = 0.015, P = 0.038), and for uGSH with Absmax (R2 = 0.013, P = 0.055) (see Supplementary Figure S2).
In contrast, in ischemic stroke patients there were no correlations of uCys with CLT (Fig. 3D) and Absmax (Fig. 3E), and uHTL with Absmax (Fig. 3F). There were also no correlations of uHcy, uCysGly and uGSH with CLT and/or Absmax in ischemic stroke patients (see Supplementary Figure S2). Furthermore, correlations between uHTL and other urinary sulfur-containing amino acid metabolites seen in healthy individuals were also absent in ischemic stroke patients (Table 2). However, correlations between urinary creatinine and other urinary sulfur-containing amino acid metabolites seen in healthy individuals were also seen in ischemic stroke patients (Table 2).
These findings suggest that efficient urinary excretion of sulfur-containing amino acid metabolites in healthy individuals can contribute to favorable clot properties, i.e. structure that is less compact and more susceptible to lysis.
In contrast to urinary sulfur-containing amino acid metabolites, plasma sulfur-containing amino acid metabolites (pHcy, pCys, pCysGly, pMet, and pGSH) were not associated with fibrin CLT nor Absmax in healthy individuals. These plasma metabolites were also not associated with fibrin CLT nor Absmax in ischemic stroke patients (see Supplementary Table S3). These findings suggest that the urinary sulfur-containing amino acid metabolites might be better predictors of ischemic stroke risk than the plasma metabolites.
While urinary creatinine was associated with CLT and Absmax, plasma creatinine was associated with neither (see Supplementary Table S3).
Relationships of sulfur-containing amino acid metabolites with age and GFR in ischemic stroke patients and healthy individuals
Age
In healthy individuals, urinary sulfur-containing amino acid metabolites, uHcy (R2 = 0.101, P = 0.000), uCys (R2 = 0.143, P = 0.000), uCysGly (R2 = 0.118, P = 0.000), and uHTL (R2 = 0.018, P = 0.021) significantly decreased with age while uGSH levels were not affected by age (see Supplementary Figure S3).
In contrast, in ischemic stroke patients age did not significantly affect levels of uCysGly (R2 = 0.030, P = 0.092) and uHTL (R2 = 0.010, P = 0.171). However, uHcy (R2 = 0.013, P = 0.002) and uCys (R2 = 0.045, P = 0.005) remained negatively associated with age in ischemic stroke patients. Like in healthy individuals, levels of uGSH were not affected by age in stroke patients (see Supplementary Figure S3).
In ischemic stroke patients, the influence of age on plasma sulfur-containing amino acid metabolites was also changed in a metabolite specific manner. For example, pHcy (R2 = 0.041, P = 0.004) and pCys (R2 = 0.124, P = 0.000) were positively correlated with age in ischemic stroke patients but not in healthy individuals (see Supplementary Figure S4). While pCysGly (R2 = 0.0487, P = 0.000) and pGSH (R2 = 0.0585, P = 0.000) significantly decreased with age in healthy individuals, these metabolites increased with age in ischemic stroke patients (pCysGly: R2 = 0.016, P = 0.073; pGSH: R2 = 0.040, P = 0.005) (see Supplementary Figure S4).
GFR
Relationships between GFR and urinary sulfur-containing amino acid metabolites were also different in ischemic stroke patients compared to healthy individuals. Specifically, in healthy individuals, GFR was significantly associated with uHcy (R2 = 0.037, P = 0.001), uCys (R2 = 0.055, P = 0.000), and uCysGly (R2 = 0.047, P = 0.000) (see Supplementary Figure S5). In ischemic stroke patients, these associations were attenuated (uHcy: R2 = 0.0099, P = 0.075; uCys: R2 = 0.0114, P = 0.075) or absent (uCysGly: R2 = 0.0002, P = ns) (see Supplementary Figure S5). However, GFR did not significantly influence uHTL and uGSH in healthy controls and ischemic stroke patients (see Supplementary Figure S5; Table 3).
Determinants of fibrin CLT and Absmax in ischemic stroke patients and healthy individuals
Bivariate correlations
In healthy individuals, bivariate correlation analysis showed that fibrin CLT was associated with uCys, uCysGly, and ten other variables: CBS T833C 844ins68 polymotphism (β = 0.12, P = 0.035), uCreatinine (β = − 0.18, P = 0.001), total cholesterol (β = 0.17, P = 0.003), LDL-cholesterol (β = 0.14, P = 0.022), triglycerides (β = 0.11, P = 0.062), BMI (β = 0.19, P = 0.001), hypertension (β = 0.17, P = 0.003), earlier CVD (β = 0.12, P = 0.043), age (β = 0.23, P = 0.000), and Absmax (β = 0.55, P = 0.000) (Table 4).
In ischemic stroke patients, fibrin CLT was associated with a different set of variables that included pGSH (β = − 0.17, P = 0.021), pMet (β = − 0.17, P = 0.021), and five other variables: MTHFR C1298A polymorphism (β = 0.15, P = 0.033), glucose (β = 0.16, P = 0.031), sex (β = − 0.23, P = 0.001), earlier CVD (β = 0.16, P = 0.031), and Absmax (β = 0.29, P = 0.000) (Table 4).
Fibrin Absmax was associated with different sets of variables. In healthy individuals, fibrin Absmax was associated with uHTL (β = − 0.14, P = 0.018), uHcy (β = − 0.13, P = 0.043), uCys (β = − 0.14, P = 0.014), uCysGly (β = − 0.12, P = 0.038), and ten other variables: uCreatinine (β = − 0.14, P = 0.014), anti-N-Hcy-protein autoantibody (β = 0.13, P = 0.028), total cholesterol (β = 0.22, P = 0.000), LDL-cholesterol (β = 0.22, P = 0.000), triglycerides (β = 0.13, P = 0.024), BMI (β = 0.29, P = 0.000), hypertension (β = 0.13, P = 0.024), other heart disease (β = 0.14, P = 0.019), age (β = 0.33, P = 0.000), and fibrin CLT (β = 0.55, P = 0.000) (Table 4). In stroke patients, fibrin Absmax was associated with a different set of variables that included pMet (β = 0.19, P = 0.009) and three other variables: MTHFR A1298C polymorphism (β = 0.21, P = 0.004) and fibrin CLT (β = 0.29, P = 0.000) (Table 4).
Multiple regression analysis
In healthy individuals, multiple regression analysis showed that fibrin CLT was positively associated with pGSH (β = 0.11, P = 0.019) and four other variables: CBS T833C 844ins68 polymorphism (β = 0.13, P = 0.008), earlier CVD (β = 0.13, P = 0.009), sex (β = − 0.12, P = 0.018), and fibrin Absmax (β = 0.55, P = 0.000); adjusted R2 was 0.34, P = 0.000 (Table 4).
In ischemic stroke patients, fibrin CLT was associated with a different set of variables that included uHTL (β = 0.13, P = 0.048), uCysGly (β = 0.20, P = 0.003), pCysGly (β = − 0.22, P = 0.001) and four other variables: GFR (β = 0.19, P = 0.007), diabetes (β = 0.19, P = 0.004), sex (β = − 0.21, P = 0.001), and fibrin Absmax (β = 0.35, P = 0.000); adjusted R2 was 0.24, P = 0.000 (Table 4).
In healthy individuals, fibrin Absmax was associated with a different set of variables: uHTL (β = − 0.10, P = 0.025), uGSH (β = − 0.12, P = 0.012), and four other variables: BMI (β = 0.13, P = 0.013), age (β = 0.19, P = 0.000), sex (β = 0.12, P = 0.032), and fibrin CLT (β = 0.48, P = 0.000); adjusted R2 was 0.38, P = 0.000 (Table 4).
In stroke patients, fibrin Absmax was associated with a different set of variables that included pCysGly (β = 0.17, P = 0.017), pMet (β = − 0.18, P = 0.009), and three other variables: MTHFR A1298C (β = 0.18, P = 0.009) and CBS T833C 844ins68 (β = 0.13, P = 0.049) polymorphisms, and fibrin CLT (β = 0.31, P = 0.000); adjusted R2 was 0.18, P = 0.000 (Table 4).
These findings show that different sets of sulfur-containing amino acid metabolites and other variables contribute to fibrin clot properties in healthy individuals and stroke patients.
Associations of sulfur-containing amino acid metabolites, fibrin CLT and Absmax with ischemic stroke
Bivariate correlations
In bivariate correlation analysis, urinary (uHTL, uHcy, uCys, uGSH) and plasma (pHcy, pCys, pCysGly, pGSH) sulfur-containing amino acid metabolites, but not uCysGly, were significantly associated with ischemic stroke (Table 5) as were fibrin CLT and Absmax. Other variables such as pCreatinine, glucose, lipid measures, GFR, age, sex, and prior CAD, MI, hypertension, diabetes, and other heart diseases were also associated with ischemic stroke (Table 5).
Logistic regression
In logistic regression analysis, adjusting for anti-N-Hcy autoantibodies14, age, and sex, uHTL, uHcy, uCys, uGSH, pCys, and pCysGly remained significantly associated with ischemic stroke while pHcy and pGSH did not (Model 1, Table 5). MTHFR C677T polymorphism that affects the MTHFR enzyme, important for B-vitamin-dependent recycling of Hcy to methionine15, was also associated with ischemic stroke in Model 1 (Table 5), consistent with earlier studies16.
Adjustments for earlier diseases did not affect these associations (Model 2, Table 5). Other adjustments for GFR, glucose, LDL cholesterol, HDL cholesterol, and triglycerides, also did not affect these associations (Model 3, Table 5), except for uHcy which was not significantly associated with stroke in Model 3. The associations of sulfur-containing amino acid metabolites and MTHFR C677T polymorphism with ischemic stroke were independent of other metabolites and traditional stroke risk factors such as lipid measures, GFR, glucose, age, sex, early CAD, MI, hypertension, diabetes, and other heart diseases.
Logistic regression analysis in a model adjusted for anti-N-Hcy autoantibodies14, age, and sex, also showed that fibrin Absmax, but not CLT, was significantly associated with ischemic stroke (P = 0.049, Model 1, Table 5). The association of fibrin Absmax, with stroke became stronger in models adjusted for earlier diseases (P = 0.007, Model 2, Table 5) and for GFR, glucose, LDL cholesterol, HDL cholesterol, and triglycerides (Model 3, Table 5). These findings show that the association of fibrin Absmax with ischemic stroke was independent of sulfur-containing amino acid metabolites and traditional stroke risk factors such as lipid measures, glucose, GFR, age, sex, and earlier CAD, MI, hypertension, diabetes, and other heart diseases (Table 5).
Contribution of individual sulfur-containing amino acid metabolites to the risk of ischemic stroke
To estimate the contribution of individual sulfur-containing amino acid metabolites to the risk of ischemic stroke, we examined effects of each of these metabolites on R2 values in the logistic regression models described in Table 5. We found that uHTL, uHcy, uCys, and uGSH explained individually 1, 0.5, 0.5, and 1.5%, respectively, of the ischemic stroke risk, with all four urinary metabolites explaining up to 6.5% of the risk (Model 3, Table S4). pCys and pCysGly individually explained 1.5 and 0.5%, respectively, of the ischemic stroke risk, with all four plasma metabolites explaining 2% of the risk. Notably, pHcy and pGSH did not contribute to the risk of stroke. Similar values were obtained using Model 1 (Table S4). Urinary sulfur-containing amino acid metabolites explained 6.5–9% stroke risk compared to just 2–2.5% explained by the plasma metabolites. Urinary and plasma sulfur-containing amino acid metabolites together explained 9–14% to the ischemic stroke risk, with the greatest contribution from pCys (1.5–2%) and uGSH (1–1.5%) (Table S4).
Discussion
We found that (i) sulfur-containing amino acid metabolites such as uHTL, uHcy, uCys, uGSH, pCys, and pCysGly were independently associated with stroke; (ii) fibrin Absmax was associated with stroke; (iii) uHTL and uGSH were associated with fibrin Absmax while pGSH was associated with fibrin CLT in healthy individuals. (iv) pCysGly, pGSH, and pMet were associated with fibrin Absmax while uHTL, uCysGly and pCysGly were associated with fibrin CLT in stroke patients.
In addition to stroke-associated changes in levels of sulfur-containing amino acid metabolites, we found profound changes in the relationships of these metabolites to fibrin clot properties, age, and GFR in stroke patients. For example, negative associations of fibrin clot properties (CLT, Absmax) with uCys (Fig. 3A,B) and uHTL (Fig. 3C) observed in healthy individuals were not seen in stroke patients (Fig. 3D–F). Further, negative associations of fibrin clot properties (CLT, Absmax) with uHcy, uCysGly, and uGSH in healthy individuals were not seen in stroke patients (Figure S2). Negative associations between urinary sulfur-containing amino acid metabolites (uHTL, uCysGly) and age in healthy individuals were also not seen in stroke patients (see Supplementary Figure S3). Moreover, negative associations between plasma sulfur-containing amino acid metabolites (pCysGly, pGSH) and age in healthy individuals were either not seen in stroke patients (pCysGly) or were changed to positive association (pGSH) (see Supplementary Figure S3). Two other plasma sulfur-containing amino acid metabolites (pHcy, pCys) were positively associated with age in stroke patients but were not affected by age in healthy individuals (see Supplementary Figure S4). Positive associations between urinary sulfur-containing amino acid metabolites (uHcy, uCys, uCysGly) and GFR in healthy individuals were not seen in stroke patients (see Supplementary Figure S5). These changes suggest profound metabolic rewiring that affects sulfur amino acid metabolism associated with stroke. Whether these changes were causally related to stroke is still to be figured out in future studies.
Accumulating evidence suggests that impaired fibrin clot properties are associated with stroke7,8. In the present study we found that CLT and Absmax were significantly correlated with each other in healthy individuals (β = 0.55, P = 0.000) and that this correlation was attenuated in stroke patients (β = 0.29, P = 0.000). Notably, we found that the CLT vs. Absmax correlation was sex-specific in stroke patients and was seen in women (Fig. 1D) but not in men (Fig. 1B). The size of this correlation in stroke patients was like that previously reported in CAD patients (β = 0.23, P = 0.000)13. Other studies reported similar correlations between CLT and Absmax in patients with diabetes17,18 and in healthy individuals9.
In the present study we showed that sulfur-containing amino acid metabolites were associated with fibrin clot properties in a disease status-dependent manner. Specifically, in healthy individuals uHTL and uGSH were associated with Absmax while pGSH was associated with CLT (Table 4). A different set of metabolites was associated with fibrin clot properties in stroke patients: pCysGly and pMet were associated fibrin Absmax while uHTL, uCysGly, and pCysGly were associated fibrin CLT. These associations have not been reported before.
Our present finding that uHTL was associated with fibrin CLT in ischemic stroke patients together with an earlier finding that uHTL was associated with CLT in CAD patients13 suggests that uHTL can affect fibrin clot properties in heart and brain diseases. These findings in conjunction with the present finding that uHTL was associated with Absmax but not CLT in healthy individuals suggest that associations of uHTL with specific fibrin clot properties such as CLT and Absmax can be disease status specific. This suggestion is supported by previous findings showing that other metabolites such as HDL cholesterol, triglycerides, pCreatinine (which predicted Absmax in CAD patients13), and other variables such as GFR (which predicted CLT in CAD patients13), were not associated with Absmax nor CLT in stroke patients in the present study (Table 4).
The disparate influences of uHTL (in stroke patients and healthy controls), uGSH and pGSH (in healthy controls), pCysGly (in stroke patients), uCysGly and pMet (in stroke patients) on CLT and Absmax (Table 4) suggest that each metabolite can affect fibrin clot properties via metabolite-specific mechanisms. For example, modification of protein lysine residues by HTL produces N-Hcy-protein in a process called N-homocysteinylation, which results in protein damage11 and generation anti-N-Hcy-protein antibodies14. Under normal circumstances in healthy individuals, HTL is efficiently cleared by excretion into urine by the kidney19. However, in stroke patients, kidney function is impaired as shown by significantly reduced GFR and uCreatinine, and significantly elevated pCreatinine (see Supplementary Table S2). Notably, we found that uHTL levels were lower in stroke patients and were accompanied by higher plasma levels of anti-N-Hcy-protein antibodies compared to healthy individuals (Table S2). These findings suggest that the increase in anti-N-Hcy-protein antibody levels in stroke patients is caused by HTL accumulation in the plasma due to its impaired urinary clearance (see Supplementary Table S2). The accumulation of HTL in the human body elevates N-Hcy-protein levels20, including N-Hcy-fibrinogen and N-Hcy-albumin21, which stimulate the generation of anti-N-Hcy-protein antibodies14. Apart from their ability to generate an autoimmune response14, N-Hcy-proteins that are present in the human plasma22 can be prothrombotic as demonstrated for N-Hcy-fibrinogen, which formed fibrin clots that were more resistant to lysis by plasmin23.
Alternatively, low-molecular weight plasma thiols such as pCysGly and pGSH can affect fibrin clot properties by binding via disulfide bonds to plasma proteins that become components of the fibrin clot. In fact, S-CysGly- and S-GSH-bound forms of albumin and globulin have been identified in human plasma with S-CysGly-globulin and S-CysGly-albumin representing 40–50% of the total thiolated proteins24. Although S-Cys-globulin and S-pCys-albumin levels are comparable to the levels of S-pCysGly-globulin and S-pCysGly-albumin, plasma Cys was not associated with fibrin clot properties, suggesting a specific influence of plasma CysGly and possibly S-CysGly-modified proteins on fibrin clot properties.
We found that sulfur-containing amino acid metabolites, uHTL, uGSH, and pCysGly, were associated both with fibrin clot properties (Table 4) and stroke (Table 5). Of these, uHTL has been previously shown to affect fibrin clot properties13 and predict MI in CAD patients12, suggesting that HTL can contribute to stroke and CAD via similar mechanisms involving protein modification by N-homocysteinylation11.
MTHFR A1298C and MTHFR C677T polymorphisms affect the MTHFR enzyme, important for B-vitamin-dependent recycling of Hcy to methionine15. In the present study, MTHFR A1298C polymorphism was associated with fibrin clot properties (Table 4) while MTHFR C677T polymorphism was associated with stroke (Table 5). The association of MTHFR A1298C polymorphism with fibrin clot properties has been reported before25. Although MTHFR A1298C polymorphism has been reported to be associated with stroke26, in the present study there was no association either in bivariate or logistic regression analyses (Table 5). However, in the present study we found that MTHFR C677T polymorphism was associated with stroke (Table 5), consistent with earlier studies16.
Of the sulfur-containing amino acid metabolites associated with stroke (uHTL, uHcy, uCys, uGSH, pCys, and pCysGly) (Table 5) some were also associated with fibrin clot properties (uHTL, uGSH, pCysGly) (Table 4). Other variables such as total cholesterol, LDL cholesterol, GFR, and age were also associated both with fibrin clot properties (Table 4) and stroke (Table 5). Taken together, these findings suggest that the sulfur-containing amino acid metabolites uHTL, uGSH, and pCysGly as well as the other variables can promote stroke by promoting unfavorable fibrin clot properties.
We also found factors that were not associated with fibrin clot properties but were nevertheless associated with stroke. Among those were the sulfur-containing amino acid metabolites uHcy and uCys. These findings suggest that uHcy and uCys can promote stroke without influencing fibrin clot properties.
In the present study we found that in logistic regression analyses, plasma and urinary sulfur-containing amino acid metabolites were associated with ischemic stroke, independently of established risk factors (Table 5). To our best knowledge, the independent associations of uHTL, uHcy, uCys, uGSH, pCys, and pCysGly with ischemic stroke have not been previously reported. The association of pHcy with stroke has been reported before27,28,29. Although in the present study pHcy was associated with stroke in unadjusted analyses, this association disappeared after adjustments for GFR, glucose, LDL cholesterol, HDL cholesterol, and earlier diseases (Table 5). Our findings suggest that sulfur-containing amino acid metabolites such as uHTL, uHcy, and pCysGly might be better predictors of stroke than pHcy. The role of sulfur-containing amino acids in stroke, and their diagnostic value remain to be investigated in future studies.
Strength and limitations
The present study is the first to evaluate sulfur-containing amino acid metabolites as determinants of fibrin clot properties in relation to stroke events. The case–control design of the present study allows the identification of associations whose mechanistic significance and causality need to be assessed in prospective studies. As our study was limited to Caucasian populations, our findings are still to be confirmed in other populations.
Conclusions
Sulfur-containing amino acid metabolites such as uHTL, uGSH, pCysGly were associated both with fibrin clot properties and stroke, suggesting that these metabolites can promote stroke by promoting unfavorable fibrin clot properties. Other sulfur-containing amino acid metabolites such as uHcy, uCys, pCys, and factors such as MTHFR C677T polymorphism were associated with stroke without influencing fibrin clot properties. Targeting sulfur-containing amino acid metabolites and their urinary excretion might be a useful therapeutic strategy to mitigate prothrombotic phenotypes that increase a risk of stroke.
Materials and methods
Patients
The present study included ischemic stroke patients (n = 200) and healthy control individuals (n = 291). Participant characteristics and blood/urine samples were collected at the admission to the study. Serum, citrated plasma, and EDTA plasma were prepared as previously described13. Stroke patients had earlier coronary stenosis (23.3%) (> 50% of cross-surface area obstructed in at least one of the major coronary arteries), myocardial infarction (8.7%), other heart disease (22.5%), diabetes (24.0%), and hypertension (77.5%). The frequency of these risk factors was up to 10 times less prevalent in control individuals: 2.3%, 0.7%, 4.3%, 3.7%, and 21.2%, respectively. Stroke patients were on medications at admission (78.3%), including antiplatelet drugs (11%), acetyl salicylic acid (30%), statins (30%), β-blockers (41%), and metformin (19%). Medication use in control individuals was 7.7%, including antihypertensives (3%), statins/anti-lipid (2%), β-blockers (1.4%), and metformin (0.3%). Samples were assayed by investigators blinded to the clinical data to avoid bias. Written informed consent was obtained from all the participants on the admission to the study. The study protocols were following the principles of the Declaration of Helsinki and was approved by the Bioethical Committee, Poznań University of Medical Sciences (#908/17, #909/17, approved Sep 2017; #1240/17, approved Dec 2017).
The clotting/lysis assay
The assay was changed from that described previously9. Briefly, 25 µL citrated plasma was added to 75 µL buffer (50 mM Tris–HCl, 150 mM NaCl, pH 7.6) containing 12.5 ng of tPA (Molecular Innovations), 83 ng/ml final concentration. The reaction was started with 50 µL of activation mix containing 0.09 U/mL of thrombin (Millipore-Sigma) and 22.5 mM CaCl2 in 50 mM Tris–HCl, 150 mM NaCl (pH 7.6) to each well of the 96-well plate using a multichannel pipette at 20 s intervals. The time of addition of the activation mix was recorded to enable the plate reader times to be adjusted to the start of clot initiation. Absorbance was read at 340 nm every 30 s for 1 h in a Tecan NanoQuant Infinite M200 Pro microplate reader. Complete lysis of fibrin clots occurred within 1 h at the tPA concentration used. Plasma samples were assayed in duplicates and the values were averaged.
Clotting/lysis data analysis
Kinetics of fibrin clot formation and lysis, illustrated in Supplemental Figure S1, were analyzed using a customized software kindly provided by Dr. Peter Grant9. Maximum absorbance at 340 nm (Absmax, a measure of fibrin network density) and fibrin CLT (i.e., a time at which A340 was reduced to 50% of the highest value, a measure clot’s susceptibility to lysis) were calculated from the kinetics. Inter-assay variabilities for Absmax and fibrin CLT were 1.5% and 4.7%, respectively. Correlations between the clotting and lysis variables for the stroke patients and the healthy individuals are shown in Supplementary Table S1.
Genotyping
Polymorphisms were established by using the PCR-RLFP (MTHFR C677T rs1801133, DHFR 19bpdel rs70991108, and CBS T833C rs5742905/CBS 844ins68) or TaqMan probes (ThermoFisher Scientific) (MTHFR A1298C rs1801131) according to previously published methods30,31.
Metabolite assays
Plasma and urinary sulfur-containing amino acids metabolites32,33 and uHTL34 were quantified as previously described. Creatinine and lipids were quantified by standard assays13.
Anti-N-Hcy-protein autoantibody assays
Anti-N-Hcy-protein autoantibodies were quantified as previously described14,35,36.
Statistics
Normality of distribution was tested with the Shapiro–Wilk’s statistic. Mean ± standard deviation (SD) or median was calculated for normally or non-normally distributed variables, respectively. An unpaired two-sided t-test was used for comparisons between two groups of variables with normal distribution. A Mann–Whitney rank sum test was used for comparisons between two groups of non-normally distributed variables. Associations between fibrin clot lysis time (CLT) or Absmax and other variables were studied by bivariate correlations, multiple regression, and logistic regression analyses. Statistical software packages Statistica, version 13 (TIBCO Software Inc., Palo Alto, CA, USA) and PSPP, version 1.0.1 (www.gnu.org) were used. Probability values were 2-sided and P value < 0.05 was considered significant.
Data availability
The data that support the findings of this study are available in the methods and/or supplementary material of this article.
References
Martin, S. S. et al. Heart disease and stroke statistics: A report of US and global data from the American Heart Association. Circulation https://doi.org/10.1161/CIR.0000000000001209 (2024).
Feigin, V. L., Norrving, B. & Mensah, G. A. Global burden of stroke. Circ. Res. 120, 439–448. https://doi.org/10.1161/CIRCRESAHA.116.308413 (2017).
Boehme, A. K., Esenwa, C. & Elkind, M. S. Stroke risk factors, genetics, and prevention. Circ. Res. 120, 472–495. https://doi.org/10.1161/CIRCRESAHA.116.308398 (2017).
Greco, A. et al. Antithrombotic therapy for primary and secondary prevention of ischemic stroke: JACC state-of-the-art review. J. Am. Coll. Cardiol. 82, 1538–1557. https://doi.org/10.1016/j.jacc.2023.07.025 (2023).
Zabczyk, M., Ariens, R. A. S. & Undas, A. Fibrin clot properties in cardiovascular disease: from basic mechanisms to clinical practice. Cardiovasc. Res. 119, 94–111. https://doi.org/10.1093/cvr/cvad017 (2023).
Undas, A. & Ariens, R. A. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 31, e88-99. https://doi.org/10.1161/ATVBAHA.111.230631 (2011).
Undas, A. et al. Altered fibrin clot structure/function in patients with cryptogenic ischemic stroke. Stroke 40, 1499–1501. https://doi.org/10.1161/STROKEAHA.108.532812 (2009).
Siegerink, B. et al. Clot lysis time and the risk of myocardial infarction and ischaemic stroke in young women; results from the RATIO case-control study. Br. J. Haematol. 156, 252–258. https://doi.org/10.1111/j.1365-2141.2011.08935.x (2012).
Carter, A. M., Cymbalista, C. M., Spector, T. D., Grant, P. J. & Euro, C. I. Heritability of clot formation, morphology, and lysis: The EuroCLOT study. Arterioscler. Thromb. Vasc. Biol. 27, 2783–2789. https://doi.org/10.1161/ATVBAHA.107.153221 (2007).
Pinzon, R. T., Wijaya, V. O. & Veronica, V. The role of homocysteine levels as a risk factor of ischemic stroke events: A systematic review and meta-analysis. Front. Neurol. 14, 1144584. https://doi.org/10.3389/fneur.2023.1144584 (2023).
Jakubowski, H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 99, 555–604. https://doi.org/10.1152/physrev.00003.2018 (2019).
Borowczyk, K. et al. Urinary excretion of homocysteine thiolactone and the risk of acute myocardial infarction in coronary artery disease patients: The WENBIT trial. J. Intern. Med. 285, 232–244. https://doi.org/10.1111/joim.12834 (2019).
Sikora, M., Skrzydlewski, P., Perla-Kajan, J. & Jakubowski, H. Homocysteine thiolactone contributes to the prognostic value of fibrin clot structure/function in coronary artery disease. PLoS One 17, e0275956. https://doi.org/10.1371/journal.pone.0275956 (2022).
Undas, A. et al. Autoantibodies against N-homocysteinylated proteins in humans: Implications for atherosclerosis. Stroke 35, 1299–1304 (2004).
Moll, S. & Varga, E. A. Homocysteine and MTHFR mutations. Circulation 132, e6-9. https://doi.org/10.1161/CIRCULATIONAHA.114.013311 (2015).
Chang, G. et al. The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: A meta-analysis of observational studies. BMC Geriatr. 19, 331. https://doi.org/10.1186/s12877-019-1304-y (2019).
Winther-Larsen, A. et al. The ABO locus is associated with increased fibrin network formation in patients with stable coronary artery disease. Thromb. Haemost. 120, 1248–1256. https://doi.org/10.1055/s-0040-1713753 (2020).
Sumaya, W. et al. Impaired fibrinolysis predicts adverse outcome in acute coronary syndrome patients with diabetes: A PLATO sub-study. Thromb. Haemost. 120, 412–422. https://doi.org/10.1055/s-0039-1701011 (2020).
Chwatko, G. & Jakubowski, H. Urinary excretion of homocysteine-thiolactone in humans. Clin. Chem. 51, 408–415 (2005).
Jakubowski, H., Boers, G. H. & Strauss, K. A. Mutations in cystathionine beta-synthase or methylenetetrahydrofolate reductase gene increase N-homocysteinylated protein levels in humans. FASEB J. 22, 4071–4076. https://doi.org/10.1096/fj.08-112086 (2008).
Jakubowski, H. Homocysteine is a protein amino acid in humans. Implications for homocysteine-linked disease. J. Biol. Chem. 277, 30425–30428 (2002).
Glowacki, R. & Jakubowski, H. Cross-talk between Cys34 and lysine residues in human serum albumin revealed by N-homocysteinylation. J. Biol. Chem. 279, 10864–10871 (2004).
Sauls, D. L. et al. Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: A potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry 45, 2480–2487 (2006).
Hortin, G. L., Seam, N. & Hoehn, G. T. Bound homocysteine, cysteine, and cysteinylglycine distribution between albumin and globulins. Clin. Chem. 52, 2258–2264. https://doi.org/10.1373/clinchem.2006.074302 (2006).
Klajmon, A. et al. Plasma thiol levels and methylenetetrahydrofolate reductase gene c.665C > T and c.1286A > C variants affect fibrin clot properties in Polish venous thromboembolic patients. Mol. Genet. Metab. 139, 107623. https://doi.org/10.1016/j.ymgme.2023.107623 (2023).
Dong, X. et al. MTHFR A1298C gene polymorphism on stroke risk: An updated meta-analysis. Genes Environ. 43, 40. https://doi.org/10.1186/s41021-021-00208-z (2021).
Spence, J. D. Cardioembolic stroke: Everything has changed. Stroke Vasc. Neurol. 3, 76–83. https://doi.org/10.1136/svn-2018-000143 (2018).
Lehotsky, J. et al. Role of homocysteine in the ischemic stroke and development of ischemic tolerance. Front. Neurosci. 10, 538. https://doi.org/10.3389/fnins.2016.00538 (2016).
Smith, A. D. & Refsum, H. Homocysteine—from disease biomarker to disease prevention. J. Intern. Med. 290, 826–854. https://doi.org/10.1111/joim.13279 (2021).
Skibola, C. F. et al. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc. Natl. Acad. Sci. USA 96, 12810–12815. https://doi.org/10.1073/pnas.96.22.12810 (1999).
Griffioen, P. H., de Jonge, R., van Zelst, B. D., Montserrate Brouns, R. & Lindemans, J. Detection and allele-frequencies of the 833T>C, 844ins68 and a novel mutation in the cystathionine beta-synthase gene. Clin. Chim. Acta 354, 191–194. https://doi.org/10.1016/j.cccn.2004.11.019 (2005).
Stachniuk, J., Kubalczyk, P., Furmaniak, P. & Glowacki, R. A versatile method for analysis of saliva, plasma and urine for total thiols using HPLC with UV detection. Talanta 155, 70–77. https://doi.org/10.1016/j.talanta.2016.04.031 (2016).
Kusmierek, K., Glowacki, R. & Bald, E. Analysis of urine for cysteine, cysteinylglycine, and homocysteine by high-performance liquid chromatography. Anal. Bioanal. Chem. 385, 855–860. https://doi.org/10.1007/s00216-006-0454-x (2006).
Glowacki, R., Bald, E. & Jakubowski, H. An on-column derivatization method for the determination of homocysteine-thiolactone and protein N-linked homocysteine. Amino Acids 41, 187–194. https://doi.org/10.1007/s00726-010-0521-7 (2011).
Sikora, M. et al. Serum proteome alterations in human cystathionine beta-synthase deficiency and ischemic stroke subtypes. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20123096 (2019).
Wloczkowska, O. et al. Anti-N-homocysteine-protein autoantibodies are associated with impaired cognition. Alzheimers Dement (N Y) 7, e12159. https://doi.org/10.1002/trc2.12159 (2021).
Acknowledgements
Supported in part by Grants from the National Science Center, Poland (2017/27/B/ST4/01476, 2018/29/B/NZ4/00771, 2019/33/B/NZ4/01760, 2021/43/B/NZ4/00339) and the American Heart Association Grant 17GRNT32910002.
Author information
Authors and Affiliations
Contributions
M.S., E.B., O.U., I.W.: Methodology, Investigation, Validation, Data curation. J.P.-K.: Data curation, Project Administration. J.P.-K., R.K.: Writing—review and editing. K.B., J.P., R.G.: Methodology, Investigation. H.J.: Conceptualization, Methodology, Investigation, Visualization, Validation, Writing—original draft preparation, Writing—review and editing, Supervision, Project administration, Resources, Data curation, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sikora, M., Bretes, E., Perła-Kaján, J. et al. Homocysteine thiolactone and other sulfur-containing amino acid metabolites are associated with fibrin clot properties and the risk of ischemic stroke. Sci Rep 14, 11222 (2024). https://doi.org/10.1038/s41598-024-60706-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60706-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.