Abstract
The Plasmodium is responsible for malaria which poses a major health threat, globally. This study is based on the estimation of the relative abundance of mosquitoes, and finding out the correlations of meteorological parameters (temperature, humidity and rainfall) with the abundance of mosquitoes. In addition, this study also focused on the use of nested PCR (species-specific nucleotide sequences of 18S rRNA genes) to explore the Plasmodium spp. in female Anopheles. In the current study, the percentage relative abundance of Culex mosquitoes was 57.65% and Anopheles 42.34% among the study areas. In addition, the highest number of mosquitoes was found in March in district Mandi Bahauddin at 21 °C (Tmax = 27, Tmin = 15) average temperature, 69% average relative humidity and 131 mm rainfall, and these climatic factors were found to affect the abundance of the mosquitoes, directly or indirectly. Molecular analysis showed that overall, 41.3% of the female Anopheles pools were positive for genus Plasmodium. Among species, the prevalence of Plasmodium (P.) vivax (78.1%) was significantly higher than P. falciparum (21.9%). This study will be helpful in the estimation of future risk of mosquito-borne diseases along with population dynamic of mosquitoes to enhance the effectiveness of vector surveillance and control programs.
Similar content being viewed by others
Introduction
Mosquitoes are small dipteran insects, which belong to the Culicidae family, and they are cosmopolitan; their larva and pupa grow in different water bodies under favourable conditions1. The environmental factors such as temperature, humidity, rainfall, and species habitats are strongly correlated with distribution, survivability and abundance of mosquitoes2. The breeding sites/habitats of mosquitoes may vary with seasons and climatic conditions resulting in fluctuation in the population density of mosquitoes3,4. They play an important role in the transmission of protozoa, helminths, bacteria and viruses in livestock, pets, wildlife animals and human populations, globally5,6,7,8,9,10,11.
A total of 3594 species of mosquitoes have been reported worldwide and only 134 species were found in Pakistan12. Anopheles (An.) spp. such as An. nigerrimus Giles, An. peditaeniatus Leicester, An. subpictus Grassi sensu lato, An. pulcherrimus Theobald, An. stephensi Liston s.l., and An. culicifacies Giles s.l. were found in the irrigated areas of South Punjab, Pakistan13. Four species of Anopheles and nine species of Culex mosquitoes were reported in Murree Hills, Punjab, Pakistan1.
Plasmodium (Haemosporida: Plasmodiidae), a genus of mosquito-borne protozoan parasites, is responsible for malaria in humans. There are five different species of mosquito-borne Plasmodium (P.) which infect humans, including P. falciparum, P. malariae P. vivax, P. ovale and P. knowlesi14. Among these species, P. vivax causes morbidity, and P. falciparum is responsible for mortality. The vivax malaria transmission may be found sustained in areas with low transmission intensity due to presence of its dormant forms in hepatic cells15,16. Due to complications like algid malaria, kidney malfunction and cerebral malaria, falciparum malaria is considered as more fatal17,18. The two malarial parasite species, P. vivax and P. falciparum, are present in Pakistan19.
Approximately, 247 million human lives were at risk of Plasmodium infection with 619,000 fatalities in 2021 around the globe according to the latest world malaria report20. Among the mosquito-borne diseases, malaria, dengue, and chikungunya are the most prevalent in Pakistan. Malaria among the communicable infections is still considered the leading source of morbidity and mortality in Pakistan19. About 0.47 million cases of malaria were reported across Pakistan during 2020. From 1960’s to early 80’s, P. vivax was considered the most prevalent species of malarial parasite in Pakistan. However, an increasing trend in the prevalence of P. falciparum was observed over the last few decades. Recently, P. vivax (79.13%) has been the major cause of malaria in humans in Pakistan followed by falciparum malaria (16.29%) and mixed infections (3.98%) of these two species21. The P. falciparum species is rising in Balochistan and Sindh with 108,262 confirmed cases out of 1,484,821 suspected cases across the Sindh province in 2018 which is alarming and 50,000 mortalities occurs every year in Pakistan19,20.
Pakistan is among the WHO Eastern Mediterranean regions which is reported as moderate malaria-endemic with 63 million people at high risk and 217 million at moderate risk of malaria. The disease is still present in an endemic state in Pakistan and the 98% proportion of total malaria burden is covered by Pakistan among the seven countries in the region19,21. There is dire need of the estimation of future risk of mosquito borne diseases based on mosquito fauna. Therefore, this study is based to estimate the relative abundance of mosquitoes on the conventional microscopy, and finding out the correlation of the meteorological parameters (temperature, humidity and rainfall) with the abundance of the mosquitoes. In addition, this study is also focused on the use of nested PCR to explore the Plasmodium spp. in the female Anopheles mosquitoes from three climatic zones of Punjab province, Pakistan.
Results
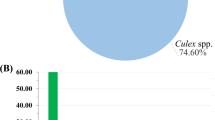
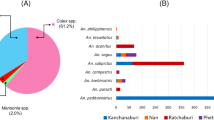
Out of total 27,428 collected mosquitoes, the percentage abundance of Anopheles and Culex mosquitoes in Mandi Bahauddin, Jhang, and Multan observed from the three climatic zones is given in Fig. 1. In addition, 4435 Anopheles and 6800 Culex were identified out of total 11,235 mosquitoes collected from three tehsils of district Mandi Bahauddin. About 3833 Anopheles and 5042 Culex were found out of 8875 mosquitoes from four tehsils of district Jhang. Similarly, 3346 Anopheles and 3972 Culex were segregated out of total 7318 collected from four tehsils of district Multan. The results have shown that the Culex spp. were the most abundant among the mosquitoes in the study areas (Table 1).
Among all the study districts, the highest average relative humidity (%) was reported in Mandi Bahauddin in January (79.5%), highest temperature (36.45 °C) in Multan in June and maximum rainfall (291 mm) was recorded in August in Mandi Bahauddin. The collective abundance of adult mosquitoes belonging to two genera, Anopheles and Culex was found to be higher during March, April and October in the study areas (Figs. 2, 3, and 4). The Table 2 shows the output of the Pearson’s correlation between abundance of mosquitoes and monthly average temperature (°C), average relative humidity (%) and rainfall (mm) for three study areas. It is evident that in Mandi Bahauddin, average temperature showed weak positive correlation (0.015) with Anopheles, and weak negative correlation with Culex (− 0.021). Similarly, average relative humidity had weak negative correlation (− 0.045) with Anopheles and Culex (− 0.065). However, rainfall was found to be negatively correlated to Anopheles (− 0.201) and Culex (− 0.106). In the district Jhang, the average temperature was weakly and negatively correlated to the Anopheles (− 0.084) and Culex (− 0.032), while the average relative humidity was positively and weakly correlated to the Anopheles. In addition, rainfall was recorded weakly and positively correlated to the Anopheles (0.073) and Culex (0.293). Similarly, In the district Multan, average temperature showed negative and weak correlation with the Anopheles (− 0.150) and Culex (− 0.184). However, average relative humidity was weakly and negatively correlated to the Anopheles (− 0.026) while weakly and positively correlated to Culex (0.018). Lastly, rainfall was found to be weakly and positively correlated to Anopheles (0.040) and Culex (0.174). However, the relationship of environmental parameters (relative humidity, average temperature and rainfall) with the abundance of mosquitoes in districts Mandi Bahauddin, Jhang and Multan was observed as non-significant (p > 0.05). The highest number of mosquitoes was found in March in district Mandi Bahauddin at 21 °C (Tmax = 27, Tmin = 15) average temperature, average relative humidity 69% and rainfall 131 mm (Fig. 2).
The first round PCR targeted 18S rRNA gene of Plasmodium genus revealed a band of 160 bp. The band revealed at 160 bp as shown in Fig. 5a was considered as positive result for the presence of genus Plasmodium. The overall prevalence of Plasmodium in the female Anopheles mosquitoes of the study areas was found to be 41.3% (64/155 pools) with a higher Plasmodium distribution in district Mandi Bahauddin followed by Multan and Jhang (Table 3). The administrative division-wise (tehsil-wise) prevalence of Plasmodium among vectors of the study districts was highest in the Mandi Bahauddin (66.6%), followed by Malikwal (44.4%), Ahmedpur Sial (44.4%), Multan (42.8%), Multan Saddar (40%), Jalalpur (40%), Phalia (38.1%), Jhang (37.5%), Athara Hazari (25%), Shujabad (25%), and Shorkot (22.7%). A non-significant (p > 0.05) difference among prevalence of Plasmodium spp. in Anopheles mosquitoes was observed in various study administrative divisions.
Molecular identification of Plasmodium spp.: (a) Gel representation of 160 bp cDNA fragments of first round amplification; (b) 110 bp cDNA fragments of second round amplification. [Lane L: 100 base pair (bp) plus DNA ladder, Lane C + : positive control, Lane A1–A5: female Anopheles mosquito PCR products of first reaction of nest-PCR, Lane N1–N4: PCR products of Plasmodium species specific primers (for vivax [N1], falciparum [N2], ovale [N3] and malariae [N4]), Lane C-: negative control, Lane B: blank].
For the 2nd round PCR (species specific), the desired band revealed at 110 bp indicating the species of Plasmodium. Among Plasmodium species, P. vivax and P. falciparum produced the desired band as shown in Fig. 5b. A total of 50 out of 64 positive pools were found positive for P. vivax (78.1%), and 14 pools for P. falciparum (21.9%). No P. ovale and P. malariae were found in the present study.
Discussion
Mosquitoes have medical importance as they act as vectors of various disease agents affecting millions of humans, annually. Aedes, Anopheles, Culex, Mansonia and Culiseta are the mosquito genera which may act as vectors27,28,29. The abundance of Anopheles mosquitoes was low as comparison to Culex spp. which could be due to various factors including sampling approach and better vector control strategies in urban areas with proper sanitation. The Culex mosquitoes attracts more towards livestock and human population than any other mosquitoes, therefore this might be the one reason of higher abundance30,31. In different studies, Culex were also found in abundance than other mosquitoes in Kenya32, in North Kent, UK33, and in Iran31. The watery habitat of rice field is more suitable for the development of Culex larvae and can be a factor for higher Culex abundance34,35.
These results showed that mosquitoes in Mandi Bahauddin were present abundantly as compared to Jhang and Multan at average relative humidity 50–80%, average temperature 15–30 °C and rainfall 130 mm. This might be due to the change in the climatic conditions of these three districts as Multan is situated in North zone of Punjab and Jhang in Central zone where climatic conditions are slightly different as compared to Mandi Bahauddin where climatic conditions are favorable for survival of mosquitoes. Numerous studies36,37,38,39,40 support the findings here that also show association between climatic factors and mosquito seasonal activity. Moreover, these results are also in agreement with other studies which showed that on the increase in temperature, the abundance of mosquitoes reduced due to higher metabolic rate and lower respirations rate leading to increase in mortality, and 15–28 °C temperature range found to be more favorable for the survivability and proper growth of mosquito fauna41,42. It has been presented in studies that ovipositing, larval growth and mosquito activity increased on high humidity levels43,44. In some studies, 44–69% RH range has been reported as more favourable for mosquito abundance42,44. The average rainfall has positive impact on mosquito abundance due to increase in suitable breeding sites44. The precipitation range 80–120 mm was reported suitable for mosquito population in a study37. The population density, diversity and distribution of mosquito fauna are strongly affected by the fluctuations in the climatic (including average relative humidity, average temperature and rainfall) and ecological conditions4,45.
In the present study, through nested PCR, P. vivax and P. falciparum among Plasmodium species have been identified in the anopheline vectors, which is in agreement with the study of Soomro et al.46. The WHO reports on malaria in Pakistan state that P. vivax and P. falciparum are the only dominant malaria-causing species in Pakistan47, and similarly, in our study, only two species, P. vivax and P. falciparum were identified in the study regions.
A similar study conducted in Faisalabad (Pakistan) reported the prevalence of the genus Plasmodium about 46% (14/30 pools) in Anopheles mosquitoes, along with P. falciparum and P. vivax25. The infection rate of 0.46% (566/123,286) for Plasmodium species was reported in the anopheline vectors in a systematic review and meta-analysis conducted in Thailand48. A study in Ubon Ratchathani Province, Thailand, detected P. vivax-210 and P. vivax-247 in the pooled Anopheles (1/29 pools; 3.45%) through ELISA and nested PCR49. Through the nested PCR technique, P. vivax was identified in DNA extracts (3/109; 2.75%) of Anopheles mosquitoes from the two provinces in Thailand50.
All the following studies on malaria prevalence in Pakistan have been conducted on blood samples, either through conventional microscopy or molecular techniques. A slide positivity rate of 35% was reported for the Plasmodium genus along with two species of P. vivax (66.8%) and P. falciparum (30.7%) in Quetta, Pakistan51. Similarly, a study conducted in Punjab province (including five cities) presented P. vivax (73%), P. falciparum (18%), and 5% of mixed infections of these two species52. Moreover, a higher prevalence of P. vivax in comparison to P. falciparum (99.4% vs. 0.53%) was reported in Lal Qilla Lower Dir, Khyber Pakhtunkhwa (KPK), Pakistan53. In Multan, 60.5% P. vivax and 37.2% P. falciparum were reported54. In Northern and Southern Punjab (10 cities), P. vivax (53.4%), P. falciparum (18.7%), and mixed infections of these two species (12.7%) were reported based on molecular analysis of blood samples. In another study of 1,127 malaria-susceptible patients in Pakistan's KPK province, molecular analysis (nPCR) revealed an overall prevalence of 38% of the genus Plasmodium, with 87% P. vivax and 13% P. falciparum55. Furthermore, based on nPCR, P. vivax (81.1%), P. falciparum (13.8%), and mixed infections of these two species (4.9%) were reported56. The study conducted in tribal areas revealed a higher prevalence of P. vivax than P. falciparum in March, while a contrast was observed in October57.
In contrast, the study conducted in Malakand district found a Plasmodium prevalence of 26.7%58. Likewise, in Karachi, Pakistan, in 2002, a higher prevalence of P. falciparum (65%) as compared to P. vivax (35%) was reported among the malaria-suspected children59. A high percentage of P. falciparum to P. vivax (88.5% vs. 9%) was reported in another study conducted in Karachi, Pakistan60. Based on 175 suspected malarias patients at the Khyber Teaching Hospital, Peshawar, Pakistan, from 2011 to 2014, P. falciparum was found in 71.43% of samples25. A similar pattern of prevalence of P. falciparum was observed in the southwestern region of Pakistan. For example, it was 58.9% (P. falciparum) and 41% (P. vivax) in the Larkana district61. The infection rates of P. falciparum and P. vivax were reported at 52.8% and 47.1% in another study in the Larkana district, respectively46. Furthermore, in Hyderabad, it was 52.5% and 46.5% in one study62, while in another research study, it was 47% and 53%63. Similarly, in Quetta, Dera Ismail Khan, and Karachi, a higher prevalence of P. falciparum than P. vivax (65%, 10.7%, and 58.1%) was reported, respectively59,64,65.
Malaria transmission is not always stable but rather found to be unstable, with P. falciparum transmission peaking between August and December while P. vivax transmission gains its peak level in two phases (first, from June to September, and second, from April to June) in Pakistan13,66,67. Therefore, the non-probability sampling and/or collection of mosquitoes might be related to a higher vivax prevalence as compared to falciparum, and these findings are also in agreement with those of Shahwani et al.68. In addition, the higher prevalence of malaria might be due to the development of resistance to chloroquine69. and ineffective vector control strategies in the area70,71. The development of resistance against most antimalarial drugs has been reported in Plasmodium spp.72 Moreover, the socio-demographic factors affecting the disease dynamics include exponential population growth, housing condition, occupation, awareness about health care services, urbanization, age, gender, excessive monsoon rains, and strategies used to avoid mosquito bites27,66,73.
The molecular tools have been proven more sensitive and accurate for the diagnosis of malarial infections than the conventional (classic) methods. The infection of P. vivax and P. falciparum through this technique has also been identified in a similar study in the Caribbean74. However, the nested PCR protocol used in this study was optimized using the same primers and conditions reported by Han et al.24 and Hayat et al.25, both of which detected Plasmodium infection in blood samples and in female Anopheles, respectively. In our study, there were no findings for P. ovale or P. malariae, which is aligned with a study conducted in Multan75 and Faisalabad, Pakistan25 and with the WHO World Malaria Report-2019. Moreover, no clinical cases in humans caused by P. ovale or P. malariae have been reported in Pakistan so far19,21.
Along with microscopy, immunochromatographic tests, and a rapid diagnostic test (RDT) have been used for the confirmation of antigens using monoclonal antibodies. On the other hand, these procedures are not sensitive or specific for the diagnosis of certain species of Plasmodium76. These RDTs and conventional techniques are quick, uncomplicated, and simple in interpretation, but they have the potential only for the identification of P. falciparum and P. vivax. Molecular assays that are more sensitive and specific than RDTs or conventional microscopy include nested multiplex-PCR, semi-nested-PCR, and nested-PCR77.
In conclusion, nested-PCR assay resulted in the identification of P. vivax and P. falciparum in this study. The molecular techniques, being costly, are regarded as less useful in routine clinical diagnosis, but their potential for specific Plasmodium spp. identification in blood and mosquito vectors can help in early surveillance, ultimately reducing the disease threat. Further parallel studies are recommended to confirm the different species of Plasmodium in mosquitoes as well as malaria-susceptible humans to ensure the presence of different Plasmodium species in a specific area in the future. In addition, climate change has resulted in variations in the pattern of precipitation (rainfall) and temperature, which affect the mosquito population directly or indirectly. However, climatic factors (relative humidity, temperature, and rainfall) have shown correlation with the numbers of mosquitoes, but their effect is complex, therefore, the influence of weather variables on mosquito vector behavior should be monitored through tools like the Global Positioning System (GPS) to enhance the understanding of these correlations. Moreover, the findings of this study provide a way forward to implement strict vector control measures to reduce the chances of vector-borne diseases in humans and animals.
Methods
Study areas
Three districts Mandi Bahauddin (73° 36′ to 73° 37′ E and 30° 8′ to 32° 40′ N), Jhang (71.37-to-73.13° E and 30.37-to-31.59° N) and Multan (71° 28′ 11′′ E and 30° 11′ 52′′ N) situated in South, Central and North climatic zones of Punjab were selected for sampling of mosquitoes (Fig. 6). The district Mandi Bahauddin is consisting of three tehsils (administrative divisions) including Mandi Bahauddin, Malikwal, and Phalia; district Jhang consisting of four tehsils including Ahmedpur Sial, Athara Hazari, Jhang and Shorkot, and district Multan consisting of four tehsils like Multan, Multan Saddar, Jalalpur, and Shujabad.
Mandi Bahauddin district has cold winter and hot summer which depict moderate climate of this district with average temperature range 3–48 °C, average rainfall 9.19–166.73 mm and relative humidity 22–51%. District Jhang climate scenarios include hot and dry summer season while winter is dry and cold with annual average temperature range 19.7–41.1 °C, average rainfall 4–76 mm and relative humidity 36–66%. While Multan district topography includes loose, flat, unconsolidated soil or sediments which are very suitable for agriculture. Climate of Multan is arid representing clement winters and scorching summers with max temperature recorded 52 °C and minimum temperature − 1 °C, average rainfall 7–55 mm and relative humidity 35–60%.
Entomological sampling, data collection and processing
Mosquitoes were collected from areas of human population, hospitals, livestock farms, poor households, lavatories, standing water ponds and sewage contaminated fields using two methods at each location: insect collecting nets and light baited traps (DP. LED Light, Guangzhou, China) during year 2022. Monthly mosquito collection was performed in three districts with a total of 45 locations (15 locations per district). Collection was done for 3 consecutive nights per month at each location between 17:00 and 7:00 h and preserved in 70% ethanol. Mosquitoes were brought to the Molecular Parasitology Laboratory, University of Agriculture, Faisalabad (UAF), Pakistan for morphological identification. Mosquitoes were identified under stereoscopic microscope using standard taxonomic keys provided by Soulsby22 and Becker et al.23. The data related to meteorological factors (temperature, humidity and rainfall) of the selected districts for the study duration was obtained from Pakistan Meteorological Department (Regional Meteorological Center, Lahore) (https://rmcpunjab.pmd.gov.pk/www/index.php). Then, the correlation of abundance of the mosquitoes with the meteorological factors (temperature, humidity and rainfall) was analyzed.
The female Anopheles were procured, identified and separated from the rest of mosquitoes for molecular analysis. Female Anopheles were pooled into 1.5 mL Eppendorf tubes after microscopic identification (15 females per pool) and total 155 pools of mosquitoes were made from the February-June collection and from the August-December collection. Then, gDNA was extracted using EZ-10 Spin Column Genomic DNA Miniprep Kit, Animal Samples (CAT. #: BS427; Bio Basic Canada Inc.) following the given protocols. Quantification and quality assessment of the extracted DNA were performed by Nanodrop spectrophotometer 2000 @A260/A280 purity ratio (ThermoFisher Scientific, USA).
Nested PCR
The extracted DNA of Anopheles were subjected to amplification of 18S rRNA genes (species-specific nucleotide sequences) using nested PCR capable of detecting individual species including P. falciparum, P. vivax, P. ovale and P. malariae and mix infection as described by Han et al.24 and Hayat et al.25. The nested PCR consisted of two reactions and during the first-round amplification, 25 μL reaction was prepared containing 12.5 μL of Red Dye PCR (2x) Master Mix (GeNeiTM consisting of 400 μM of each DNTP, 0.6 units of Taq DNA Polymerase, Tris buffer pH 8.5 and 3 mM of MgCl2), 1 μL (0.5 μM) each of Plasmodium universal forward (P1: 5′-ACGATCAGATACCGTCGTAATCTT-3′) and reverse (P2: 5′-GAACCCAAAGACTTTGATTTCTCAT-3′) primers, 3 μL of sample DNA and 7.5 μL nuclease free water. The DNA amplification protocol used for the first round of the nested PCR was initial denaturation at 94 °C for 10 min followed in order by 35 cycles of 92 °C for 30 s, 60 °C for 1.5 min, 72 °C for 1 min and final extension at 72 °C for 5 min. The 2nd round PCR was targeted on species specific gene fragments of various Plasmodium genus members. The species-specific primers were utilized in 2nd round for each Plasmodium spp. The second round of amplification was same as that of first round except the following; 1 μL of each species-specific reverse primer in a separate PCR tube (P. malariae: 5′-GGAAGCTATCTAAAAGAAACACTCATAT-3′, P. ovale: 5′-ACTGAAGGAAGCAATCTAAGAAATTT-3′, P. falciparum: 5′-CAATCTAAAAGTCACCTCGAAAGATG-3′, P. vivax: 5′-CAATCTAAGAATAAACTCCGAAGAGAAA-3′), 1 μL of 20 folds diluted first reaction DNA with 20 cycles of amplification (using same thermocycling temperatures as first round). The amplified products were visualized on ethidium bromide stained 1.5% agarose gel under Gel Doc™ XR + Gel Documentation System (Bio-Rad, USA). Moreover, the extraction of DNA, preparation of PCR reactions and replication of DNA were performed by different sets of pipettes and in clean and specific work areas to avoid cross-contamination. The positive bands were observed against a 100 bp plus molecular weight markers. The positive controls (DNA templates of Plasmodium) were provided by PMRC Research Center, Punjab Medical College [PMC], Faisalabad-38000, Pakistan, and the negative controls lacking template DNA were run on the gel as reference.
Statistical analyses
The relationship of abundance of mosquitoes with climatic parameters (temperature, relative humidity and rainfall) was statistically analyzed on monthly basis through Pearson’s correlation coefficient (r) to find out the direction and strength of the linear correlation4,26. However, the prevalence of Plasmodium spp. in the vector population of the study areas was statistically investigated through chi-square test of independence in contingency tables using R software (https://www.r-project.org/). Variables were considered statistically significant at p < 0.05.
Data availability
The data used to support the results of this study are included within the article.
References
Qasim, M., Naeem, M. & Bodlah, I. Mosquito (Diptera: Culicidae) of Murree Hills, Punjab, Pakistan. Pak. J. Zool. 46, 523–529 (2014).
Sinka, M. E. et al. A global map of dominant malaria vectors. Parasites Vectors 5, 69 (2012).
Emidi, B., Kisinza, W. N. & Mmbando, B. P. Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasites Vectors 10, 304 (2017).
Drakou, K. et al. The effect of weather variables on mosquito activity: A snapshot of the main point of entry of Cyprus. Int. J. Environ. Res. Public Health 17, 1403 (2020).
Armistead, J. S. et al. Infection of mosquitoes from in vitro cultivated Plasmodium knowlesi H strain. Int. J. Parasitol. 48, 601–610 (2018).
Pathak, A. K., Shiau, J. C., Thomas, M. B. & Murdock, C. C. Cryogenically preserved RBCs support gametocytogenesis of Plasmodium falciparum in vitro and gametogenesis in mosquitoes. Malar. J. 17, 1–15 (2018).
Pradeep, R. K. et al. Whether Dirofilaria repens parasites from South India belong to zoonotic Candidatus Dirofilaria hongkongensis (Dirofilaria sp. hongkongensis)?. Infect. Genet. Evol. 67, 121–125 (2019).
Souza-Neto, J. A., Powell, J. R. & Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 67, 191–209 (2019).
Yaseen, K. et al. Metagenomics of mosquito-borne flaviviruses in various geoclimatic districts of Punjab, Pakistan. Pak. Vet. J. 40, 407–412 (2020).
Baz, M. M., Hegazy, M. M., Khater, H. F. & El-Sayed, Y. A. Comparative evaluation of five oil-resin plant extracts against the mosquito larvae, Culex pipiens say (Diptera: Culicidae). Pak. Vet. J. 41, 191–196 (2021).
Jabeen, A., Ansari, J. A. & Ikram, A. First report of Aedes albopictus (Diptera: Culicidae) in District Mirpur, Azad Jammu and Kashmir, Pakistan. J. Med. Entomol. 58, 943–946 (2021).
Manzoor, F., Shabbir, R., Sana, M., Nazir, S. & Khan, M. A. Determination of species composition of mosquitoes in Lahore, Pakistan. J. Arthropod. Borne Dis. 14, 106 (2020).
Herrel, N. et al. Adult anopheline ecology and malaria transmission in irrigated areas of southern Punjab, Pakistan. Med. Vet. Entomol. 18, 141–152 (2004).
Woldearegai, T. G. et al. Characterization of Plasmodium infections among inhabitants of rural areas in Gabon. Sci. Rep. 9, 9784 (2019).
Cheng, Q., Cunningham, J. & Gatton, M. L. Systematic review of sub-microscopic P. vivax infections: Prevalence and determining factors. PLoS Negl. Trop. Dis. 9, e3413 (2015).
Oduma, C. O. & Koepfli, C. Plasmodium falciparum and Plasmodium vivax adjust investment in transmission in response to change in transmission intensity: A review of the current state of research. Front. Cell Infect. Microbiol. 11, 786317 (2021).
Josling, G. A. & Llinás, M. Sexual development in plasmodium parasites: Knowing when it’s time to commit. Nat. Rev. Microbiol. 13, 573–587 (2015).
Cibulskis, R. E. et al. Malaria: Global progress 2000–2015 and future challenges. Infect. Dis. Poverty 5, 61 (2016).
World Malaria Report, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO (accessed 10 September 2023); https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2019
World Malaria Report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO (accessed 22 October 2023); https://www.who.int/publications/i/item/9789240064898
Khan, M. I. et al. Malaria prevalence in Pakistan: A systematic review and meta-analysis (2006–2021). Heliyon 9, e15373 (2023).
Soulsby, E. J. L. Helminths, Arthropods and Protozoa of Domesticated Animals 7th edn. (Bailliere Tindal and Cassel Ltd, 1982).
Becker, N. et al. Mosquitoes and Their Control 2nd edn, 9–24 (Springer, 2010).
Han, E. T. et al. Detection of four Plasmodium species by genus-and species-specific loop-mediated isothermal amplification for clinical diagnosis. J. Clin. Microbiol. 45, 2521–2528 (2007).
Hayat, K. et al. PCR-based screening of Plasmodium species in mosquito vectors of Faisalabad district, Punjab, Pakistan. J. Anim. Plant Sci. 29, 1568–1574 (2019).
Taylor, R. Interpretation of the correlation coeffient: A basic review. J. Diagn. Med. Sonogr. 6, 35–39 (1990).
Robert, M. A., Okamoto, K. W., Gould, F. & Lloyd, A. L. Antipathogen genes and the replacement of disease-vectoring mosquito populations: A model-based evaluation. Evol. Appl. 7, 1238–1251 (2014).
Lagare, A. et al. First occurrence of Rift Valley fever outbreak in Niger, 2016. Vet. Med. Sci. 5, 70–78 (2019).
Alkenani, N. A. et al. Molecular identification and bio-control of mosquitoes using black seeds extract in Jeddah. Pak. Vet. J. 41, 359–364 (2021).
Kirby, M. J., West, P., Green, C., Jasseh, M. & Lindsay, S. W. Risk factors for house-entry by culicine mosquitoes in a rural town and satellite villages in the Gambia. Parasites Vectors 1, 41 (2008).
Faraji-Fard, P., Ahmadi-Angali, K. & Behbahani, A. Species variety of the calf and human-attracted mosquitoes in southwest Iran. J. Arthropod Borne Dis. 15, 162–170 (2021).
LaBeaud, A. D. et al. Arbovirus prevalence in mosquitoes, Kenya. Emerg. Infect. Dis. 17, 233 (2011).
Vaux, A. G. et al. Enhanced West Nile virus surveillance in the North Kent marshes, UK. Parasites Vectors 8, 1–8 (2015).
Ohba, S. Y., Van Soai, N., Van Anh, D. T., Nguyen, Y. T. & Takagi, M. Study of mosquito fauna in rice ecosystems around Hanoi, Northern Vietnam. Acta Trop. 142, 89–95 (2015).
Ha, T. V. et al. Spatial distribution of Culex mosquito abundance and associated risk factors in Hanoi, Vietnam. PLoS Negl. Trop. Dis. 15, e0009497 (2021).
Taylor, R. Interpretation of the correlation coefficient: A basic review. J. Diagn. Med. Sonogr. 6, 35–39 (1990).
Wang, L. et al. The role of environmental factors in the spatial distribution of Japanese encephalitis in mainland China. Environ. Int. 73, 1–9 (2014).
Couret, J. & Benedict, M. Q. A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: Culicidae). BMC Ecol. 14, 3 (2014).
Hii, Y. L., Zaki, R. A., Aghamohammadi, N. & Rocklöv, J. Research on climate and dengue in Malaysia: A systematic review. Curr. Environ. Health. Rep. 3, 81–90 (2016).
Asgarian, T. S., Moosa-Kazemi, S. H. & Sedaghat, M. M. Impact of meteorological parameters on mosquito population abundance and distribution in a former malaria endemic area, central Iran. Heliyon 7, e08477 (2021).
Asigau, S. & Parker, P. G. The influence of ecological factors on mosquito abundance and occurrence in Galápagos. J. Vector Ecol. 43, 125–137 (2018).
Jemal, Y. & Al-Thukair, A. A. Combining GIS application and climatic factors for mosquito control in Eastern Province, Saudi Arabia. Saudi J. Biol. Sci. 25, 1593–1602 (2018).
Costa, E. A. P. A., De Mendonça Santos, E. M., Correia, J. C. & De Albuquerque, C. M. R. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae). Rev. Bras. Entomol. 54, 488–493 (2010).
Khan, M. A. et al. The impact of climatic variables with GIS application on the abundance of medically important mosquitoes (Diptera: Culicidae) in Jeddah, Saudi Arabia. Int. J. Mosq. Res. 5, 12–18 (2018).
de Mello, C. F. et al. Spatial distribution and interactions between mosquitoes (Diptera: Culicidae) and climatic factors in the Amazon, with emphasis on the tribe Mansoniini. Sci. Rep. 12, 16214 (2022).
Soomro, F. R., Pathan, G. M., Gurbakhshani, A. L. & Kakar, J. K. Prevalence of malarial parasites in Larkano district, Sindh, Pakistan. Gomal J. Med. Sci. 8, 146–148 (2010).
WHO, 2017. Vector-Borne Diseases [online]. Geneva: WHO (accessed on 20 October 2023); Available online: https://www.who.int/en/news-room/fact-sheets/detail/vector-borne-diseases
Sukkanon, C. et al. Prevalence of Plasmodium spp. in anopheles mosquitoes in Thailand: A systematic review and meta-analysis. Parasites Vectors 15, 285 (2022).
Poolphol, P. et al. Natural Plasmodium vivax infections in Anopheles mosquitoes in a malaria endemic area of northeastern Thailand. Parasitol. Res. 116, 3349–3359 (2017).
Rattaprasert, P. et al. Detection of putative antimalarial-resistant Plasmodium vivax in Anopheles vectors at Thailand-Cambodia and Thailand-myAnmar borders. J. Trop. Med. Public Health 47, 182–193 (2016).
Sheikh, A. S., Sheikh, A. A., Sheikh, N. S. & Paracha, S. M. Endemicity of malaria in Quetta. Pak. J. Med. Res. 44, 41–50 (2005).
Khattak, A. A. et al. Prevalence and distribution of human Plasmodium infection in Pakistan. Malar. J. 12, 297 (2013).
Ahmed, S. et al. Malaria hotspots drive hypoendemic transmission in the Chittagong Hill districts of Bangladesh. PLoS One 8, e69713 (2013).
Tasawar, Z., Mannan, F. & Bhutta, A. Prevalence of human malaria at Multan, Pakistan. J. Med. Sci. 3, 1236 (2001).
Karim, A. M. et al. Prevalence of clinical malaria and household characteristics of patients in tribal districts of Pakistan. PLoS Negl. Trop. Dis. 15, e0009371 (2021).
Hussain, I. et al. Prevalence and distribution of human Plasmodium infection in federally administrative tribal areas of Pakistan. Acta Parasitol. 61, 537–543 (2016).
Karim, A. M. et al. Epidemiology and clinical burden of malaria in the war-torn area, Orakzai Agency in Pakistan. PLoS Neg. Trop. Dis. 10, e0004399 (2016).
Khan, W., Rahman, A. U., Shafiq, S., Ihsan, H. & Khan, K. Malaria prevalence in Malakand district, the north western region of Pakistan. J. Pak. Med. Assoc. 69, 946–949 (2019).
Akbar, J. U. Malaria in children at a children hospital. Pak. J. Surg. 7, 20–22 (2002).
Mahmood, K. H. Malaria in Karachi and other areas in Sindh. Pak. Armed Forces Med. J. 55, 345–348 (2005).
Junejo, A. A., Abbasi, K. A., Chand, H. & Abbasi, S. Malaria in children at Children Hospital Chandka Medical College Larkana. Med. Channel 18, 55–57 (2012).
Shaikh, M. A. et al. Platelet count in Malaria patients. J. Ayub Med. Coll. Abbottabad. 23, 143–145 (2011).
Devrajani, B. R., Jaffery, M. H. & Shah, S. Z. A. Spectrum of Malaria (Six months hospital based cross sectional descriptive study). Med. Channel 15, 30–33 (2009).
Yasinzai, M. I. & Kakarsulemankhel, J. K. Incidence of malaria infection in rural areas of Quetta, Pakistan. J. Biol. Sci. 3, 766–772 (2003).
Khan, H. U., Khattak, A. M., Khan, M. H., Mahsud, I. U. & Humayun, S. A study of prevalence of malaria in adult population of D. I. Khan, Pakistan. Biomedica 22, 99–104 (2006).
Erdman, L. K. & Kain, K. C. Molecular diagnostic and surveillance tools for global malaria control. Travel Med. Infect. Dis. 6, 82–99 (2008).
Malaria Report, 2018. Directorate of malaria control program, 2018 (accessed on 20 February 2022); Available online: http://dmc.gov.pk/
Shahwani, M. N. et al. Amplification of mitochondrial DNA for detection of Plasmodium vivax in Balochistan. J. Pak. Med. Assoc. 67, 677–681 (2017).
Shah, I. et al. Chloroquine resistance in Pakistan and the upsurge of falciparum malaria in Pakistani and afghan refugee populations. Ann. Trop. Med. Parasitol. 91, 591–602 (1997).
Yasinzai, M. I. & Kakarsulemankhel, J. K. Incidence of human malaria infection in northern hilly region of Baluchistan, adjoining with NWFP, Pakistan: District Zhob. Pak. J. Biol. Sci. 11, 1620–1624 (2008).
Zakeri, S. et al. Genetic structure of Plasmodium vivax isolates from two malaria endemic areas in Afghanistan. Acta Trop. 113, 12–19 (2010).
Rizwan, H. M., Abbas, H., Maqbool, M., Sajid, M. S. & Jones, M. K. Drug resistance in protozoal infections. In Biochemistry of Drug Resistance 1st edn (eds Sarfraz, A. et al.) 95–142 (Springer, 2021).
Hasyim, H., Dale, P., Groneberg, D. A., Kuch, U. & Müller, R. Social determinants of malaria in an endemic area of Indonesia. Malar. J. 18, 134 (2019).
Fontecha, G. A. et al. Comparison of molecular tests for the diagnosis of malaria in Honduras. Malar. J. 11, 119 (2012).
Yar, H. M., Masood, K., Maqbool, A. & Malik, G. Q. Prevalence of malarial parasite species in Multan district. Prof. 5, 183–187 (1998).
Orish, V. N., De-Gaulle, V. F. & Sanyaolu, A. O. Interpreting rapid diagnostic test (RDT) for Plasmodium falciparum. BMC Res. Notes 11, 850 (2018).
Fitri, L. E. et al. Malaria diagnostic update: From conventional to advanced method. J. Clin. Lab. Anal. 36, e24314 (2022).
Funding
The current study is funded by National Institute of Health Islamabad, Pakistan (Project Number: NHCG-21-41). In addition, the authors extend their appreciation to Researchers Supporting Project number (RSPD2024R965), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
The following statements should be used “Conceptualization, H.A., M.S.S., H.M.R., S.H.F., U.B.T., M.A.M., M.M. and K.Y.; methodology, H.A., M.A.M., H.M.R., M.M. and K.Y.; formal analysis, H.A., M.S.S., U.B.T., D.F and C.; investigation, H.A., M.S.S., M.M., K.Y. and M.A.M.; resources, H.A., M.S.S., Z.I., F.A.R, M.R. and D.F; data curation, H.A., S.H.F., M.M., D.F., M.R., F.A.R., and U.B.T.; analysis and inter-pretation of data, M.S.S., H.M.R., Z.I., and M.R; writing—original draft preparation, H.A., S.H.F., U.B.T., M.R., H.M.R. and M.A.M. ; writing—review and editing, M.R., Z.I., S.H.F., H.M.R., U.B.T., D.F., and F.A.R.; English editing, H.A., and M.S.S; supervision, M.S.S.; project administration, H.A., F.A.R., Z.I., M.A.M., and K.Y.; funding acquisition, M.S.S. and D.F. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbas, H., Sajid, M.S., Rizwan, H.M. et al. Exploring mosquito abundance and Plasmodium infection through nested-PCR: implications for disease surveillance and control. Sci Rep 14, 9871 (2024). https://doi.org/10.1038/s41598-024-60662-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60662-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.