Abstract
Diabetes mellitus (DM) is a persistent, progressive, and multifaceted disease characterized by elevated blood glucose levels. Type 2 diabetes mellitus is associated with a relative deficit in insulin mainly due to beta cell dysfunction and peripheral insulin resistance. Metformin has been widely prescribed as a primary treatment option to address this condition. On the other hand, an emerging glucose-reducing agent known as imeglimin has garnered attention due to its similarity to metformin in terms of chemical structure. In this study, an innovative series of imeglimin derivatives, labeled 3(a–j), were synthesized through a one-step reaction involving an aldehyde and metformin. The chemical structures of these derivatives were thoroughly characterized using ESI–MS, 1H, and 13C NMR spectroscopy. In vivo tests on a zebrafish diabetic model were used to evaluate the efficacy of the synthesized compounds. All compounds 3(a–j) showed significant antidiabetic effects. It is worth mentioning that compounds 3b (FBS = 72.3 ± 7.2 mg/dL) and 3g (FBS = 72.7 ± 4.3 mg/dL) have antidiabetic effects comparable to those of the standard drugs metformin (FBS = 74.0 ± 5.1 mg/dL) and imeglimin (82.3 ± 5.2 mg/dL). In addition, a docking study was performed to predict the possible interactions between the synthesized compounds and both SIRT1 and GSK-3β targets. The docking results were in good agreement with the experimental assay results.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by a relative deficiency of insulin, primarily caused by beta cell dysfunction and external insulin resistance1. Genetic and environmental factors, such as obesity and sedentary behaviors, also play a role in its development2,3. The International Diabetes Federation (IDF) predicts that diabetes will affect around 700 million people worldwide in the next two decades, leading to a significant economic impact due to its rising incidence and prevalence4,5. The cornerstone of treating T2DM lies in lifestyle modifications, including maintaining a healthy weight, engaging in regular exercise, and adopting a balanced diet6. Subsequently, implementing effective medical treatments becomes essential in improving the quality of life and reducing mortality rates for individuals living with diabetes7. Diabetes substantially elevates the risk of developing cardiovascular disease, kidney failure, blindness, neuropathy, and premature mortality7,8.
Metformin, an antidiabetic agent, was introduced in the United Kingdom in 1958 and received approval from the United States Food and Drug Administration in 19949,10. Belonging to the class of drugs known as biguanides, metformin serves as the first-line treatment option for T2DM, effectively reducing liver glucose production and increasing body insulin sensitivity11. Notably, metformin exhibits favorable pharmacokinetic properties and demonstrates beneficial effects on cardiovascular health without causing hypoglycemia9. However, its derivatives, phenformin, and buformin, were once used as antidiabetic medications but were later withdrawn in many countries due to their association with an increased risk of lactic acidosis9.
1,3,5-triazine is well-known aromatic six-membered heterocyclic moiety and shows several biological and pharmacological properties. 1,3,5-triazines has a broad spectrum of biological activities including antibacterial12, antifungal10, antimalarial13, anticancer12,14, antiviral15, anti-inflammatory16, and antitubercular properties17.
Imeglimin is the first novel drug of the glimin class containing 1,3,5-triazine core which is synthesized from metformin18. The drug has been acting via various mechanisms, including targeting mitochondria bioenergetics, increasing insulin secretion, and protecting the endothelial or beta cells' death from oxidative stress19. Moreover, it demonstrated beneficial effects on the pancreas, liver, and skeletal muscles, which are adversely affected by diabetes5.
According to previous studies, several drugs that are used to treat the T2DM condition are often associated with various side effects, such as abdominal pain, hepatotoxicity, diarrhea, flatulence, and hypoglycemia20. Therefore, owing to their lack of safety, unsatisfactory efficacy, and cytotoxicity, further investigation for new antidiabetic agents is necessary.
Although imeglimin has not yet been approved by the United States Food and Drug Administration (FDA), imeglimin may provide a valuable new therapeutic option in the future. In fact, imeglimin has been shown to be more effective than metformin in various cases and has the potential to be used in various diseases17,19,21.
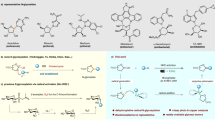
Therefore, imeglimin derivatives deserve further studies, especially in terms of anti-diabetic properties. In exploring the potential of this approach based on the distinct and specific pharmacological advantages of the imeglimin backbone (1,3,5-triazine scaffold), we prepared several novel and potent imeglimin derivatives by replacing methyl of imeglimin with different aromatic substituents and evaluated their antidiabetic activity on zebrafish diabetic model in vivo (Fig. 1).
Results and discussions
Chemistry
Despite the introduction of numerous novel antidiabetic compounds in the pharmaceutical market, metformin continues to be extensively prescribed as the first-line pharmacotherapy owing to its distinct and favorable attributes. Recently, imeglimin, akin to metformin as a antihyperglycemic agent, has emerged as one of these new compounds21. As part of this study, ten innovative compounds were synthesized to serve as potential antidiabetic agents, following the pathway illustrated in Scheme 1.
To create these novel imeglimin derivatives, we adopted a one-pot reaction involving diverse aromatic aldehyde derivatives and metformin, serving as the biguanide component. The reaction was carried out using acetic acid as the solvent and under reflux conditions for a duration of 24 h, resulting in the successful synthesis of the compounds 3(a–j).
To purify the reaction mixture the precipitate obtained after evaporation of acetic acid, was washed several times with chloroform to remove excess aldehyde until a pure product was obtained. The yields of final compounds ranged from 69 to 88%, indicating a satisfactory outcome.
To characterize the synthesized compounds, their structures were confirmed through the utilization of ESI–MS and NMR spectra. Mass spectra data from the positive ionization (ESI +) mode was used for confirmation molecular weight of syntheszied compounds and in ESI spectra, peaks corresponding the (M + H)+ were observed for all final compounds. All synthesized compounds displayed 1H NMR and 13C NMR spectra consistent with the assigned structure.
Antidiabetic activity
The anti-diabetic properties of the imeglimin derivatives 3(a–j) were investigated on the zebrafish diabetic model at a concentration of 10 μM for 48 h. Metformin and imeglimin were used as the positive control (reference drugs). The results presented in Table 1 show that the synthesized compounds (3b, 3e, and 3g) showed promising anti-diabetic effects, surpassing those of imeglimin and metformin.
Metformin and imiglimin significantly decreased fatsing blood sugar (FBS) in diabetic zebrafish (p < 0.0001). However, these two drugs did not change the FBS levels of non-diabetic zebrafish (p > 0.999). In addition, FBS reduction in the metformin and imiglimin groups was not statistically significant (p = 0.991).
All compounds synthesized in this study were able to reduce FBS in diabetic zebrafish (p < 0.0001). Compounds 3b (72.3 ± 7.2 mg/dL) and 3h (108.3 ± 11.0 mg/dL) had the strongest and weakest ability to reduce FBS in diabetic zebrafish, respectively. Compounds 3g and 3e were considered among the strongest compounds after compound 3b, respectively. With the exception of compound 3h, which could not reduce the FBS of zebrafish to that of non-diabetic zebrafish, other synthesized compounds were able to reduce FBS to similar level of non-diabetic zebrafish (p > 0.3–0.99). These three compounds (3b, 3e, and 3g) demonstrated FBS levels comparable to those of the reference compounds metformin (74.0 ± 5.1 mg/dL) and imeglimin (82.3 ± 5.2 mg/dL). On the other hand, compounds 3c and 3h showed less efficacy compared to the reference drugs and other synthesized compounds (Supplementary material Figs. S1& S2).
Structure–activity relationship (SAR) studies suggest that the presence of electron-donating groups at the benzyl rings is crucial for maximizing potency (Table 1). However, in the case of compound 3c, the hydroxyl substitution at the ortho-position of the phenyl ring likely attenuated the potency due to intramolecular hydrogen bonding with the triazine core. Surprisingly, compound 3g (2-pyridine analog) and compound 3b (with para-N,N-dimethylaminophenyl group) exhibited noteworthy antidiabetic properties and were considered as the most potent compounds in this series. These two compounds showed similar efficacy to metformin and were significantly more effective than imeglimin. The antidiabetic potential of compounds 3b and 3g may be attributed to the lone pair interaction of the respective nitrogen in the para-N,N-dimethylaminophenyl or pyridine moieties. In comparison to metformin, most of the imeglimin derivatives 3(a–j) demonstrated acceptable anti-diabetic activities, with compounds 3b and 3g standing out as the most effective derivatives. The triazine core exhibited remarkable antidiabetic activities, sparking interest in further structure modifications as potential lead compounds for future investigations.
Docking simulation method
There is no single protein, universally acknowledged as a valid target, responsible for biguanide’s bioactivities. In view of the wide variety of effects of these compounds, it is reasonable to assume that there is more than one pathway involved in their pharmacology. In addition, these imeglimin derivatives have a different effect compared to other anti-diabetic agents such as sulfonylureas and meglitinides, which work by modifying a single target.
Two proteins have been considered responsible for biguanide’s bioactivity in past computational studies22,23. Sirtuin 1 (SIRT1) is a novel target that has been introduced more recently than glycogen synthase kinase-3 beta (GSK-3β). SIRT1 regulates glucose/lipid metabolism through its deacetylase activity on many substrates. SIRT1 in pancreatic β cells positively regulates insulin secretion, protects cells from oxidative stress and inflammation, and modulates insulin signaling in the metabolic pathway. It has been proposed that biguanides such as metformin have a positive regulatory activity on SIRT122. GSK-3B has long been considered to be responsible for metformin bioactivity23. GSK-3β is a key enzyme in glycogen synthesis and plays a key role in the regulation of blood glucose. More importantly, GSK-3β is one of the key factors leading to insulin deficiency and insulin resistance. Inhibitors of GSK-3β have been shown to increase insulin sensitivity, glycogen synthesis, and glucose metabolism in skeletal muscles of diabetic patients23.
We performed molecular docking simulations to investigate the binding modes and possible interactions of the synthesized compounds 3(a–j) with the active sites of the SIRT1 and GSK-3β targets and compared them to the binding energies of metformin and imeglimin. Docking validation was done by re-docking the co-crystal ligands of the crystal structures of 1Q4L and 5BTR, and the RMSD values were lower than 2 Å, indicating the accuracy of the docking method.
Three compounds 3b, 3e, and 3g, which showed high antidiabetic activity against the zebrafish diabetic model, were selected for docking studies to determine the possible interaction with SIRT1 and GSK-3β. Table 2 lists the docking binding energies (kcal/mol) predicted by molecular docking.
The docking results of GSK-3β indicate that Leu188, Gln185, and Asp200, with the same bond type, have alkyl–pi interactions, with the free amine at the end of the biguanides, and Asp200 with an attractive charge, interact with all three compounds 3b, 3e, and 3g. The superior docking binding energy of 3b in the active site of GSK-3β may be related to the specific volume of the para-N,N-dimethylaminophenyl moiety, which is easily occupied and positioned in the active site by the two methyl groups (Fig. 2A–F).
The docking results of SIRT1 and Ala295, Pro213, and Pro291 with alkyl-pi interactions were the same as those of Asp298 and Asp292 with biguanides, and the electrostatic attractive charge bonds of Asp298 and Asp292 with biguanides were the same as those between SIRT1 and compounds 3b, 3e, and 3g. However, the highest binding energy between 3b and SIRT1 may be related to a more favorable van der Waals interaction (Fig. 3A–F).
The superimposed positions of the most potent compounds 3b, 3e, and 3g in the active sites of the SIRT1 and GSK-3β indicate that all compounds are well positioned inside the cavity with proper orientation (Fig. 4).
Despite the slight difference between the docking energies for compounds 3b, 3e, and 3g, there is a qualitative agreement between the FBS and docking binding energies for both SIRT1 and GSK-3β targets and all three ligands are good options for further consideration.
Conclusion
In summary, we designed and synthesized 10 new imeglimin analogs, labeled 3(a–j), and evaluated their antidiabetic effects on a zebrafish diabetic model. Some of these compounds demonstrated significant anti-diabetic activity and showed greater efficacy than standard metformin. The synthesized derivatives, 3(a–j), were characterized by 1H-NMR, 13C-NMR, and ESI–MS techniques. The two compounds 3b (72.3 ± 7.2 mg/dL) and 3g (72.7 ± 4.3 mg/dL), as the strongest compounds in this study, have the same potential as metformin in reducing FBS (74.0 ± 5.1 mg/dL).
The structure–activity relationship (SAR) study highlighted the significance of electron-donating groups on the aryl moiety of the target compounds in enhancing their antidiabetic activity. The remarkable anti-diabetic potential of compounds 3b and 3g warrants further investigation of their efficacy and safety, making them promising candidates for the development of more effective and safer antidiabetic drugs. These findings provide valuable insights for future research endeavors aimed at advancing diabetes treatment options.
Experimental
General methods
All the chemicals used in this study were procured from renowned sources, namely Merck and Sigma-Aldrich. These chemicals were used without any further purification. A Kofler hot stage apparatus was used to determine the melting points. The 1H and 13C NMR spectra were recorded using a Bruker FT-500 instrument with TMS as the internal standard. Chemical shifts (δ values) are expressed in ppm relative to internal solvent peaks, and coupling constants (J) are measured in Hertz. Signals are described as s (singlet), d (doublet), t (triplet), m (multiplet), and brs (broad singlet).
Reaction completion and purity of the synthesized compounds were verified using TLC on silica gel 250-μm F254 plastic sheets. The mass spectra were acquired using the SCIEX Triple Quad 5500 + LC–MS/MS instrument.
Docking method
Crystallographic structures of biguanide receptors with PDB ID: 1Q4L and 5BTR were obtained from a protein data bank (www.rcsb.org) for studying the bioactivity of compounds 3(a–j) on GSK-3β and SIRT1. These two structures were chosen because they already contain experimentally studied ligands; thus, choosing a grid box would be easier and more accurate. All water molecules, ligands, and ions were removed from the PDB file for the preparation of the input file. Polar hydrogens were then added, and the Kollman-united charge was used to calculate the partial atomic charge. The prepared file was saved in pdbqt format and used in the following steps. 3D structures of three target compounds and two controls were generated and energy minimized by the MM2 force field using Chem3D 12 and saved in PDB format. OpenBabel (version 2.3.1) was used to convert PDB to PDBQT. Data were prepared, and docking was performed by AutoDock Vina 1.2.324.
A 40 × 40 × 40 Å (x, y, z) grid box dimension with 0.375 nm spacing was centered in the reported pocket of each protein reported in previous studies for GSK-3β and SIT122,23. For GSK-3β, the grid box was centered in 40.118, 4.584, 34.328 (x, y, z), and for SIRT1, the grid box was centered in 21.647 52.885 12.831 (x, y, z), and the exhaustiveness was set at 8. Discovery Studio Visualizer version 17.2 and PyMol version 1.1 visualized the docking results. The docking results were visualized by Discovery Studio Visualizer version 17.2 and PyMol version 1.1.
Zebrafish assay
Ethical statement
All zebrafish experiments were conducted according to the standard animal guidelines approved by the Animal Care Committee of the Tehran University of Medical Sciences and Endocrinology and Metabolism Research Center (IR.TUMS.VCR.REC.1395.8, IR.TUMS.AEC.1401.060). In addition, all procedures for animal experiments described in this study were performed in accordance with the care and use of laboratory animals and ARRIVE guidelines.
Conditioning of adult Zebrafish
Three-month-old zebrafish (Danio rerio) with a body length of 2.0–2.5 cm was randomly chosen and divided into fourteen groups (a diabetic model group, two positive control groups, a negative group, and 10 experimental groups) and transferred into 6-well plates so that one fish placed in each well (20 ml). The fish plates were kept at constant filtration, continuous air conditioning, pH around 7, and a temperature of 28 ± 1 °C. They were then set aside in a photoperiod of 16 h of light, and 8 h of darkness daily and fed once a day with 7% of their body weight25. The average weight per fish was 3g. Each group experiment was repeated for three times.
Blood glucose measerment
On the last day of treatment, the fish were fasted for 24 h for blood sampling and blood glucose measurement. After blood sampling, a glucometer was used to measure the blood glucose levels. In biological studies, anesthesia in animals is usually carried out using substances approved and studied to ensure animal welfare. The clove powder extract (250 mg/L) was used for fish anesthesia in the present study. Several steps have been taken before blood sampling. In order to avoid potential side effects, it is necessary to prepare an appropriate concentration of clove extract and to know the correct dosage. Fish were placed in an incubator containing 250 mg clove powder extract in 1 L of water. Parameters, such as heart rate, respiration, and overall condition, were monitored in order to ensure the welfare of animals. After complete anesthesia by cutting the caudal fins, a glucometer was used to measure the blood glucose level in the fish.
Hyperglycemia induction
The Hyperglycemia modeling process induces a diabetic state in fish subjects for research. For acclimation (induction of glucose) gradually increasing glucose concentrations of 50, 100, and 200 mM were applied for 10, 7, and one day respectively25. To maintain accuracy, glucose solutions were exchanged every 12 h, ensuring sufficient oxygen supply, and preventing contamination. Fish were closely monitored for stress and movement issues, and fed daily. A control group without added glucose provided a comparison. This approach enhances diabetes research, providing valuable insights and potential therapeutic implications.
Hypoglycemic effect determination on Zebrafish diabetic model
In the zebrafish experimental groups, compounds 3(a–j) (10 µM) and metformin hydrochloride (10 µM) were administered for 48 h, and the culture medium was refreshed once. Subsequently, the culture medium was replaced with fresh water to eliminate glucose interference during the sampling process. Blood glucose levels were measured using a Match blood sugar test device. The fish's posterior tail was cut using tweezers, and the removed blood was immediately brought into contact with the test strip to obtain the fasting blood sugar (FBS) readings26.
General procedure for the synthesis of aryl 1,3,5-triazine derivatives 3(a–j)
Metformin (5 mmol) was dissolved in glacial acetic acid (35 ml) as a solvent to prepare the imeglimin derivatives. Subsequently, various aldehyde derivatives (5.5 mmol) were carefully added dropwise to the reaction medium. The reaction mixture was then refluxed overnight in an oil bath at 108 °C with continuous stirring. The progress of the reaction was periodically monitored using TLC. After completion of the reaction, the resulting mixture was evaporated under vacuum. Finally, the mixture was washed three times with chloroform to eliminate any excess aldehyde to obtain the desired and pure compounds 3(a–j). The successful synthesis of the imeglimin derivatives for further investigation is ensured by this process.
N 2 ,N 2-dimethyl-6-phenyl-1,6-dihydro-1,3,5-triazine-2,4-diamine (3a)
White powder; yield: 88%; m.p.: 257–259 °C; 1H NMR (500 MHz, D2O): δ (ppm) 7.60–7.31 (m, 5H, Ar), 5.83 (q, J = 5.2 Hz, 1H, N–CH–N), 3.08 (S, 3H, Me), 3.04 (S, 3H, Me); 13C NMR (125 MHz, D2O): δ (ppm) 157.77, 156.21, 138.97, 129.93, 129.41, 126.31, 63.14, 23.28; LRMS (ESI +): m/z: calcd for C11H15N5 + H+:218.13 [M + H]+, found: 218.13.
6-(4-(dimethylamino)phenyl)-N 2 ,N 2-dimethyl-1,6-dihydro-1,3,5-triazine-2,4-diamine (3b)
Brown powder; yield: 72%; m.p.: 287–289 °C; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 9.65 (1H, NH), 8.17 (d, J = 15.72 Hz, 4H, Ar), 2.52 (s, 6H, 2 × NCH3), 1.63 (s, 6H, 2 × NCH3); HRMS (ESI +): m/z: calcd for C13H20N6/2 + H+ + K+:150.07 [M/2 + H + K]2+, found: 150.00.
2-(4-amino-6-(dimethylamino)- 1,3,5-triazin-2-yl)phenol (3c)
White powder; yield: 69%; m.p.: 257–259 °C; 1H NMR (300 MHz, DMSO-d6) δ(ppm) 7.29–6.80 (m, 4H, Ar), 2.10 (s, 6H, 2 × NCH3); LRMS (ESI +): m/z: calcd for C11H13N5O + H+:232.11 [M + H]+, found: 232.08.
6-(2-methoxyphenyl)- N 2 ,N 2-dimethyl-1,6-dihydro-1,3,5-triazine-2,4-diamine (3d)
White powder; yield: 79%; m.p.: 210–213 °C; 1H NMR (500 MHz, D2O): δ (ppm) 7.37–7.25 (m, 1H, Ar), 7.11 (dd, J = 7.6, 1.7 Hz, 1H, Ar), 6.99 (d, J = 8.3 Hz, 1H, Ar), 6.91 (t, J = 7.5 Hz, 1H, Ar), 5.97 (s, 1H, N–CH–N), 3.78 (s, 3H, OCH3), 2.93 (s, 6H, 2 × NCH3); 13C NMR (125 MHz, D2O) δ(ppm) 157.43, 156.58, 155.98, 130.92, 126.46, 125.70, 120.59, 111.75, 59.07, 55.53, 36.89, 27.45; LRMS (ESI +): m/z: calcd for C12H17N5O + H+:248.14 [M + H]+, found: 248.07.
N 2 ,N 2-dimethyl-6-(3-nitrophenyl)-1,6-dihydro-1,3,5-triazine-2,4-diamine (3e)
White powder; yield: 79%; m.p.: 210–213 °C; 1H NMR (500 MHz, D2O): δ (ppm) 8.17–8.12 (m, 2H, Ar), 7.74 (d, J = 7.9 Hz, 1H, Ar), 7.58 (t, J = 7.9 Hz, 1H, Ar), 5.91 (s, 1H, N–CH–N), 3.02–2.94 (m, 6H, 2 × Me); 13C NMR (125 MHz, D2O) δ(ppm) 157.37, 155.87, 148.04, 140.82, 132.73, 130.44, 124.42, 121.09, 61.97, 37.17, 36.54; LRMS (ESI +): m/z: calcd for C11H14N6O2 + H+:263.12 [M + H]+, found: 263.08.
6-(furan-2-yl)- N 2 ,N 2-dimethyl-1,6-dihydro-1,3,5-triazine-2,4-diamine (3f)
White powder; yield: 86%; m.p.: 198–201 °C; 1H NMR (500 MHz, D2O): δ (ppm) 7.49 (s, 1H, Ar), 6.56–6.16 (m, 2H, Ar), 5.89 (d, J = 2.8 Hz, 1H, N–CH–N), 3.00 (d, J = 2.8 Hz, 6H, 2 × Me); 13C NMR (125 MHz, D2O) δ(ppm) 160.02, 156.12, 151.13, 144.39, 110.82, 108.29, 57.12, 37.74, 36.78; LRMS (ESI +): m/z: calcd for C9H13N5O + H+:208.11 [M + H]+, found: 208.07.
N 2 ,N 2-dimethyl-6-(pyridin-2-yl)-1,3,5-triazine-2,4-diamine (3g)
Brown powder; yield: 75%; m.p.: 292–296 °C; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 8.56 (s, 1H, Ar), 8.15 (d, J = 8.4 Hz, 1H, Ar), 7.99 (t, J = 7.3 Hz, 1H, Ar), 7.67 (s, 1H, Ar), 3.11 (S, 3H, Me), 2.99 (S, 3H, Me); 13C NMR (125 MHz, D2O): δ(ppm) 161.85, 159.42, 158.06, 148.19, 145.29, 140.54, 129.33, 124.18, 37.04; LRMS (ESI +): m/z: calcd for C10H12N6 + H+:217.11 [M + H]+, found: 217.08.
6-(4-fluorophenyl)- N 2 ,N 2-dimethyl-1,6-dihydro-1,3,5-triazine-2,4-diamine (3h)
White powder; yield: 67%; m.p.: 225–228 °C; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 7.60 (m, 2H, Ar), 7.41 (m, 2H, Ar), 2.51 (s, 3H, Me), 0.76 (s, 3H, Me). LRMS (ESI +): m/z: calcd for C11H14FN5 + H+:236.12 [M + H]+, found: 236.07.
2-(4-amino-6-(dimethylamino)-1,2-dihydro-1,3,5-triazin-2-yl)benzoic acid (3i)
White powder; yield: 78%; m.p.: 241–243 °C; 1H NMR (500 MHz, D2O): δ (ppm) 7.86 (S, 1H, Ar), 7.80 (d, J = 10.3 Hz, 2H, Ar), 7.66 (d, J = 14.7 Hz, 1H, Ar), 6.03–5.92 (m, 1H, N–CH–N), 3.22–3.16 (m, 3H, Me), 3.15 – 3.07 (m, 3H, Me); 13C NMR (125 MHz, D2O) δ(ppm) 166.36, 157.58, 152.93, 139.36, 135.85, 131.47, 128.94, 125.52, 124.78, 65.06, 38.42, 37.60; LRMS (ESI +): m/z: calcd for C12H15N5O2-OH-:244.12 [M-OH]+, found: 244.08.
4-(4-amino-6-(dimethylamino)-1,2-dihydro-1,3,5-triazine-2-yl)phenol (3j)
White powder; yield: 71%; m.p.: 246–247 °C; 1H NMR (500 MHz, D2O): δ (ppm) 7.24 (d, 2H, Ar), 6.86 (d, 2H, Ar), 5.90–5.56 (m, 1H, N–CH–N), 3.04 (s, 6H, 2 × Me); 13C NMR (125 MHz, D2O) δ(ppm) 157.76, 156.89, 156.25, 130.66, 128.23, 115.99, 63.02, 37.41, 36.68; LRMS (ESI +): m/z: calcd for C11H15N5O + H+:234.13 [M + H]+, found: 234.08.
Data availability
The all data necessary for confirming the conclusions presented in the article are represented fully within the article.
References
Abdelhaleem, I. A. et al. Efficacy and safety of imeglimin in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized clinical trials. Diabetes Metab. Syndr. 15(6), 102323 (2021).
Federation ID. IDF Diabetes Atlas, 10th edn. (2021).
The Lancet. Diabetes: A dynamic disease. The Lancet 389, 2163 (2017).
Shah, N., Abdalla, M. A., Deshmukh, H. & Sathyapalan, T. Therapeutics for type-2 diabetes mellitus: A glance at the recent inclusions and novel agents under development for use in clinical practice. Therapeut. Adv. Endocrinol. Metabol. 12, 20420188211042144 (2021).
Konkwo, C. & Perry, R. J. Imeglimin: Current development and future potential in type 2 diabetes. Drugs. 81(2), 185–190 (2021).
Vieira, R. et al. Sugar-lowering drugs for type 2 diabetes mellitus and metabolic syndrome—Review of classical and new compounds: Part-I. Pharmaceuticals. 12(4), 152 (2019).
Sudesna, C., Khunti, K. & Davies, M. J. Type 2 diabetes. The Lancet. 389(10085), 2239–2251 (2017).
Chevalier, C., Perrimond-Dauchy, S., Dubourg, J., Fouqueray, P. & Bolze, S. Lack of drug–drug interaction between cimetidine, a renal transporter inhibitor, and imeglimin, a novel oral antidiabetic drug, in healthy volunteers. Eur. J. Drug Metab. Pharmacokinet. 45, 725–733 (2020).
Raczyńska, E. D., Gal, J.-F., Maria, P.-C. & Fontaine-Vive, F. Biguanide antidiabetic drugs: Imeglimin exhibits higher proton basicity but smaller lithium-cation basicity than metformin in vacuo. ACS Omega 3(12), 17842–17852 (2018).
Singh, U., Bhat, H. & Gahtori, P. Antifungal activity, SAR and physicochemical correlation of some thiazole-1, 3, 5-triazine derivatives. J. Mycol. Méd. 22(2), 134–141 (2012).
Kato, E. T., Das, S. R. & McGuire, D. K. Antihyperglycemic therapies and cardiovascular outcomes in patients with type 2 diabetes mellitus: State of the art and future directions. Trends Cardiovasc. Med. 31(2), 101–108 (2021).
Guo, H. & Diao, Q.-P. 1, 3, 5-triazine-azole hybrids and their anticancer activity. Curr. Top. Med. Chem. 20(16), 1481–1492 (2020).
Gogoi, P. et al. In silico study, synthesis, and evaluation of the antimalarial activity of hybrid dimethoxy pyrazole 1, 3, 5-triazine derivatives. J. Biochem. Mol. Toxicol. 35(3), e22682 (2021).
Chalermnon, M., Cherdchom, S., Sereemaspun, A., Rojanathanes, R. & Khotavivattana, T. Biguanide-based synthesis of 1, 3, 5-triazine derivatives with anticancer activity and 1, 3, 5-triazine incorporated calcium citrate nanoparticles. Molecules 26(4), 1028 (2021).
Golankiewicz, B., Januszczyk, P., Ikeda, S., Balzarini, J. & De Clercq, E. Synthesis and antiviral activity of benzyl-substituted imidazo [1, 5-a]-1, 3, 5-triazine (5, 8-diaza-7, 9-dideazapurine) derivatives. J. Med. Chem. 38(18), 3558–3565 (1995).
Mogilski, S. et al. Aryl-1, 3, 5-triazine ligands of histamine H 4 receptor attenuate inflammatory and nociceptive response to carrageen, zymosan and lipopolysaccharide. Inflamma. Res. 66, 79–95 (2017).
Singh, S. et al. 1, 3, 5-Triazine: A versatile pharmacophore with diverse biological activities. Archiv der Pharmazie. 354(6), 2000363 (2021).
Huston, J. et al. Imeglimin in type 2 diabetes. Drugs Today 58(9), 437–449. https://doi.org/10.1358/dot.2022.58.9.3419555 (2022).
Giruzzi, M. Imeglimin. Clin. Diabetes 39(4), 439–440. https://doi.org/10.2337/cd21-0085 (2021).
Katzung, B. G. Basic and Clinical Pharmacology 14th edn. (McGraw Hill Professional, 2017).
Yendapally, R. et al. A review of phenformin, metformin, and imeglimin. Drug Dev. Res. 81(4), 390–401 (2020).
Cuyàs, E. et al. Metformin is a direct SIRT1-activating compound: Computational modeling and experimental validation. Front. Endocrinol. 9, 657 (2018).
Lakshmi Rajagopal, P. et al. Molecular docking analysis of metformin analogues with GSK-3β. Bioinformation. 18(3), 269 (2022).
Vina, A. improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading; O Trott, AJ Olson. J. Comput. Chem. 455–461.
Mohammadi, H., Manouchehri, H., Changizi, R., Bootorabi, F. & Khorramizadeh, M. R. Concurrent metformin and silibinin therapy in diabetes: Assessments in zebrafish (Danio rerio) animal model. J. Diabetes Metabol. Disord. 19, 1233–1244 (2020).
Zang, L., Shimada, Y., Nishimura, Y., Tanaka, T. & Nishimura, N. A novel, reliable method for repeated blood collection from aquarium fish. Zebrafish. 10(3), 425–432 (2013).
Acknowledgements
The authors wish to thank the support of the Vice-Chancellor for Research of Tehran University of Medical Sciences (Grant Nos. 95-01-158-31016 and 1400-2-411-53406).
Author information
Authors and Affiliations
Contributions
A.K.: Synthesis, data anylsis and Zebrafish assay; H.M.: Zebrafish assay; S.A.: Docking studies; S.P.: Synthesis; M.P.: Synthesis; Z.E.: Synthesis; M.T.: Data curation, Writing – original draft; M.B.: Zebrafish assay; M.R.K.: Zebrafish assay supervision. A.A.: Conceptualization, project administration, Supervision, Funding acquisition, Writing - review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khodakhah, A., Mohammadi, H., Abdoli, S. et al. Synthesis and molecular docking studies of new aryl imeglimin derivatives as a potent antidiabetic agent in a diabetic zebrafish model. Sci Rep 14, 9410 (2024). https://doi.org/10.1038/s41598-024-60206-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60206-3
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.