Abstract
Cancer-directed surgeries (CDS) play a crucial role in prostate cancer (PCa) management along with possible survival and therapeutic benefits. However, barriers such as socioeconomic factors may affect patients’ decision of refusing recommended CDS. This study aimed to uncover risk factors and the impact on survival associated with CDS refusal. We retrospectively reviewed the Surveillance, Epidemiology, and End Results database for patients diagnosed with PCa between 2000 and 2019. Multiple sociodemographic and clinical characteristics were extracted to assess predictors for physicians’ surgical recommendations and patients’ surgical refusal, respectively. Propensity score matching was performed to balance the covariates. The impact of surgical refusal on mortality risk was also investigated. A total of 185,540 patients were included. The physician’s recommendation of CDS was significantly influenced by the patient’s age, race, income, home location, diagnosis year, Gleason score, prostate-specific antigen (PSA), and TNM stage. About 5.6% PCa patients refused CDS, most of whom were older, non-White race, lack of partners, living outside of metropolitan areas, with higher PSA or lower clinical TNM stage. Patients who refused CDS had an increased risk of cancer-specific mortality and overall mortality than those who performed CDS. Physicians may weigh a host of sociodemographic and clinical factors prior to making a CDS recommendation. Patients’ refusal of recommended CDS affected survival and was potentially modifiable by certain sociodemographic factors. Physicians should fully consider the hindrances behind patients’ CDS refusal to improve patient-doctor shared decision-making, guide patients toward the best alternative and achieve better outcomes.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer among men in the United States, with an estimated 3.5 million incident cases in 20221. It is still the second leading cause of cancer-related deaths in males, just behind lung cancer1. The vast majority (85%) of PCa survivors are 65 years or older, only and < 1% are younger than 502. Management algorithms vary based on the stage and grade of PCa as well as patients’ characteristics such as age, comorbidity, and personal preferences. Cancer-directed surgeries (CDS) play a crucial role in PCa management along with possible survival and therapeutic benefits3,4. For example, radical prostatectomy is regarded as a curative treatment for localized PCa or one part of multimodal therapy for advanced one. However, despite of well-defined treatment guidelines, a considerable number of patients are not recommended to perform CDS and the surgical outcomes remain inconsistent in those with CDS. Striking disparities in outcomes based on patient-level characteristics (e.g., demographic and socioeconomic variables) remain. For instance, PCa imposes a disproportionate burden on non-White patients as they experience more unfavorable tumor characteristics and a higher mortality (reaching up to three-fold risk) than their cohort counterparts 2,5,6. A significantly increased cancer-specific mortality (CSM) can also been noted in unmarried7, uninsured8 or rural9 PCa patients. Therefore, it is of great clinical significance to analyze the role and efficacy of CDS in treating PCa patients.
Respecting PCa patients’ choices is the highest priority, however, the choice of CDS might be also affected by the physician’s recommendation. Previous studies have demonstrated that primary care physician substantially influences the decision-making regarding PCa treatment and the type of treatment 10,11. Physician specialty type such as urologists or oncologists also affects the initiation of cancer-directed treatment11. Despite given clear benefits of recommended CDS, some PCa patients may still refuse these treatments due to various socioeconomic and demographic variables. As shown by prior analysis, age, race, marital status, insurance status, and income level are to be associated with surgery refusal7,12,13,14. Those without private insurance, or unmarried are less likely to undergo CDS. Surgery refusal could subsequently lead to an increased risk of overall mortality (OM) and CSM7. Particularly, given the less effect of sole CDS in late-stage PCa patients, the impact of refusing surgery may be underestimated in previous studies. Unfortunately, there is not enough evidence in the literature to suggest why these more vulnerable populations are more likely to refuse CDS as a cancer treatment.
The purpose of this study was to identify the demographic/socioeconomic variables associated with physician’s recommendations and patients’ refusal of CDS by using a large national cancer database. Additionally, we investigated the impact of CDS refusal on eventual survival. A better understanding of them will be of value to address the disparities in refusal and surgical outcomes as well.
Methods
Data source
This population-based cohort study was based on the national Surveillance, Epidemiology and End Results (SEER) database, which covers approximately 48.0% of the United States population (https://seer.cancer.gov/about/overview.html). Authorization was obtained from SEER to download PCa data for this study in June 2022. Within the SEER database (2000–2019), we identified and included all patients more than or equal to 18 years old with histologically confirmed PCa. Certain patients were excluded: cases with an unknown death certificate, autopsy only, or those who died before recommended surgery; a survival time of fewer than three months.
This study was exempt from local research ethics committee approval, considering that SEER data were de-identified and publicly available for research use.
Study population and variables
Sociodemographic and clinical characteristics of each patient were extracted for analysis as in our previous study15. A brief description of these variables is presented as follows: age at diagnosis, year of diagnosis, race (black (African American), white (Caucasian), others (American Indian/AK Native, Asian/Pacific Islander)), marital status (married, single, and unknown), annual household income (< $65 000, ≥ $65 000, and unknown), residential location (large city, small city and missing value), Gleason score (≤ 6, = 7, and 8–10), serum prostate-specific antigen (PSA) value (≤ 0.10 ng/ml, ≥ 98.00 ng/ml, others), systemic therapy (yes, no, unknown) and longitudinal follow-up of vital status. The PCa stage was identified by the American Joint Committee on Cancer Tumor-Node-Metastasis (AJCC-TNM) stage, seventh edition.
To identify the variables affecting physicians’ decisions, we set up a case–control cohort between patients with recommended CDS and not. According to SEER Program Coding and Staging Manual (https://seer.cancer.gov/tools/codingmanuals/), CDS-recommended (CDSR) was defined as the following items: surgery performed, surgery unknown if performed or recommended but not performed due to unknown reason, and surgery recommended but not performed due to patient’s, patient’s family member’s or the patient’s guardian’s refusal. CDS-not recommended (CDSnR) represented those patients not recommended to undergo CDS by medical service providers, regardless of whether the patients underwent the surgery or not.
To determine the variables contributing to the patient’s refusal of recommended surgery, another grouped comparison was conducted between those who underwent CDS (CDS accepted = CDSA) or not (CDS not accepted = CDSnA). CDSA was defined as patient accepted surgery treatment (surgery performed). Moreover, cancer-specific mortality (CSM) and overall mortality (OM) were collected to evaluate the benefit of CDSA for PCa patients. “SEER cause-specific death classification” and “vital status recode” in the database were used to calculate CSM and OM, respectively.
Statistics analysis
The statistical analyses consisted of three steps. Firstly, nonparametric independent-sample tests were used to compare two cohort groups (CDSR vs CDSnR, CDSA vs CDSnA) before and after propensity score matching (PSM). PSM was performed to adjust differences in potential covariates by a 1:1 matching ratio. A subset of variables was chosen for PSM matching: age, diagnosis year, race, marital status, income, and home location. PSA, GS, and AJCC stages were not adopted for matching due to > 50% missing records. Secondly, binary logistic regression in univariate and multivariable analyses were applied to determine the variables associated with CDS recommendation or CDS refusal, respectively. Thirdly, the Kaplan–Meier method and multivariable Cox proportional hazard models were used to analyze the impact of refusal of recommended CDS on CSM and OM. Adjusted model 1 adjusts for age, chemotherapy, radiotherapy, and systemic therapy. Adjusted model 2 adjusts for age, chemotherapy, radiotherapy, systemic therapy, race, partner, home, and income. Data analyses were performed by using SPSS version 27.0 (IBM, Armonk, NY, USA) and R software (R software for statistical computing, Vienna, Austria). A p-value < 0.05 was considered to be statistically significant.
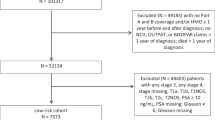
A flow diagram (Fig. 1) shows the details of inclusion and exclusion criteria and the case–control study design.
Study flowchart. Abbreviations: CDSR = cancer-directed surgery recommended; CDSnR = cancer-directed surgery not recommended; CDSA = cancer-directed surgery accepted; CDSnA = cancer-directed surgery not accepted; PSM = propensity score matching; SEER = the Surveillance, Epidemiology, and End Results.
Ethical approval
This study was exempt from local research ethics committee approval, considering that SEER data were de-identified and publicly available for research use.
Results
Demographic and clinical characteristics of patients with or without CDS recommendation
A total of 193,632 PCa cases diagnosed between 2000 and 2019 were extracted from the database. After considering inclusion and exclusion criteria, 185,540 cases were finally included for analysis. The median age at diagnosis for the total study population was 60 to 69 years old (40.2%). Of these patients, 94,964 cases (51.2%) were recommended CDS and 90,576 (48.8%) were determined not to be surgical candidates (Table 1). The comparison without PSM showed significant differences in multiple covariates (age, race, partner, income, home location, diagnosis year, Gleason score, PSA, clinical M stage, chemotherapy, radiotherapy, and system therapy) between CDSR and CDSnR groups (all p < 0.05). After PSM by 1:1 ratio, 59,833 cases were selected for each group. Significant differences could still be found in Gleason score, PSA, clinical TNM stage, chemotherapy, radiotherapy and system therapy between the two groups (all p < 0.05).
Particularly, patients in the CDSR group presented lower rates in CSM (8.3% vs. 14.8%) and OM (35.8% vs. 47.7%), and longer median survival time (143.0 vs. 122.0 months), all of which remained similar after PSM (9.3% vs 15.3%, 41.2% vs 47.7%, 138.0 vs. 129.0 months, respectively) (all p < 0.05).
Factors predicting physician’s CDS recommendation
Univariate analysis (Table 2) demonstrated that the physician’s recommendation of CDS was influenced by the patient’s age, race, income, home location, diagnosis year, Gleason score, PSA and TNM stage. Patients being younger, White in the race, living with a partner, living in outside of metropolitan area, with lower income, lower PSA, lower TNM stage but with Gleason score = 7 would be preferentially recommended CDS treatments by physicians. On multivariate analysis, only age, Gleason score, and clinical T and M stages were significantly associated with the increased recommendation of CDS treatment.
Demographic and clinical characteristics of patients with or without accepted CDS
Of the entire cohort, 74,074 cases were involved for comparison. The median age range was 60 to 69 years old (39.0%). 69,926 (94.4%) patients accepted CDS but 4148 (5.6%) refused CDS (Table 3). Before PSM, the comparison between CDSA and CDSnA showed significant differences in diverse covariates (age, race, partner, home location, diagnosis year, PSA, clinical M stage, radiotherapy and system therapy) (all p < 0.05). After PSM, 3869 cases were left in each group for comparison. Significant differences remained in PSA, chemotherapy, radiotherapy and system therapy when comparing the two groups (all p < 0.05).
Of note, patients who refused CDS treatment had higher rates in CSM (12.1% vs. 6.4%) and OM (49.5% vs. 28.4%), but a slightly longer median survival time (148.0 vs. 146.0 months), all of which were quite similar after PSM (11.9% vs 8.8%, 49.7% vs. 41.3%, 149.0 vs. 145.0 months, respectively) (all p < 0.05).
Factors predicting patient’s refusal of CDS treatment
As shown by univariate analysis (Table 4), patients refusing CDS treatment were more likely to be older, non-White race, lack partners, living outside of metropolitan area, higher PSA, lower clinical N and M stage and were diagnosed before the 2009 year (all p < 0.05). However, multivariate analysis indicated no factors significantly related to patients’ refusal of CDS treatment.
Refusal of CDS and Mortality
To further investigate the impact of the decision to refuse surgery on survival, Kaplan–Meier analysis and multivariable Cox proportional hazard models were adopted. As shown in Kaplan–Meier plots (Fig. 2A,B), significantly lower rates of CSM and OM (both p < 0.05) were determined in the CDSA group after 120 months. Cox proportional hazard models with or without PSM supported that CDS refusal could significantly increase the risk of CSM (hazard ratio, 0.54; 95% confidence interval, 0.49–0.59) and OM (hazard ratio, 0.59; 95% confidence interval, 0.56–0.61), respectively (Table 5). The Forest plot presented the subgroup analysis for CDSA vs CDSnA in CSM and OM, respectively (Fig. 3). The results demonstrated that patients refusing CDS obtained significantly poorer prognoses than those accepting CDS, particularly across age and diagnosis year subgroups. Younger patients diagnosed between 2010 and 2019 were more likely to have lower rates of CSM and OM.
Impact of surgical refusal on survival rate in unselected prostate cancer patients from SEER data base between 2010 and 2019. Shown are. (A) Kaplan–Meier curves of cancer-specific survival in patients with prostate cancers. (B) Kaplan–Meier curves of overall survival in patients with prostate cancers. (All p < 0.001) Abbreviations: CDSA = cancer-directed surgery accepted; CDSnA = cancer-directed surgery not accepted.
Discussion
Our study presented one of the largest pooled analyses of patients with PCa and highlighted the identifiable factors to predict the likelihood of a physician’s CDS recommendation and a patient’s CDS refusal. Particularly, we demonstrated that CDS refusal was associated with increased odds of CSM and OM. A better understanding of the effects of sociodemographic factors may enable to improve patients’ satisfaction, surgical utilization and treatment outcomes.
The decision to undergo CDS in PCa patients is personal and complex and undoubtedly, patients have full rights in their decision-making process. However, our results revealed that physicians’ recommendation of CDS could strongly affect patients’ final choices. Univariate analysis in this study demonstrated that physician’s recommendation of CDS was determined by the patient’s age, race, income, home location, diagnosis year, Gleason score, PSA and TNM stage. However, after multivariate analysis, only age, Gleason score, and clinical T and M stages were significantly associated with the increased recommendation of CDS treatment. In other words, physicians may only factor patients’ medical situation into their CDS recommendation and this decision process was not affected by patients’ socioeconomic factors. This finding was compatible with previous literature. Scherr et al. reported that PCa patients’ treatment decisions were chiefly decided by their urologists’ recommendations, which in turn were driven by medical factors (age, Gleason score, etc.) without patients’ preferences16. In addition, PCa patients diagnosed by urologists, rather than radiation oncologists, greatly preferred to receive up-front treatment such as CDS10. Different specialty types could lead to disparities in treatment outcomes in PCa patients11. Given the centrality of physicians’ recommendations in the decision-making process, physicians should strive for effective communication with the candidates and emphasize the important role of CDS in managing PCa.
CDS, such as radical prostatectomy and cytotherapeutic ablation, could serve as an established pillar of therapeutic options for PCa in particular localized ones3,4. When facing the selection of surgical interventions, patients undeniably weigh the potential tradeoffs between benefits and burdens. Despite the potential lifesaving or life-prolonging effect of CDS, a portion of PCa patients may still refuse to receive CDS treatments due to multiple reasons. Our study reported that about 5.6% PCa patients refused CDS, most of whom were older, non-White race, lack of partners, living outside of metropolitan areas. Particularly, PCa patients with higher PSA or lower clinical TNM stage proposed CDS refusal. These results were in parallel with prior studies. Islam et al. found that about 3.9% PCa patients refused the suggested surgery and those black, single, Medicaid/Medicare-covered, or early-stage ones had significantly increased odds of refusal rate12. Xu and colleagues reported a relatively lower refusal rate (2.47%) of CDS in PCa patients and pointed out that black and Asian/Pacific Islander patients were more likely to refuse CDS than White ones14. Quiet similarly, a recent study by Dee et al. indicated that older age, black/Asian, noninsurance or Medicaid, community facility type and later year of diagnosis were associated with increased odds of locoregional treatment (i.e., surgery ) refusal in PCa patients13. The influence of sociodemographic factors on CDS refusal can also be found in other cancer treatments such as lung cancer17, colon cancer18, breast cancer19 and so on. More attention should be paid to these factors that influence patients’ treatment decisions.
Sociodemographic factors especially age, race, marital status and cancer stage could act as vital predictors for patients’ CDS refusal due to nuanced and complex contributions. For instance, older patients may be more likely to refuse CDS due to a fear of a decrease in quality of life7, a perceived lack of social support20, an unaffordable surgical fee21, a group of comorbidities22, an existing communication gap between physicians23 and so on. Besides, the high rate of CDS refusal in non-White populations might be attributed to greater distrust toward the healthcare system24, late lacking medical insurance12 and different cultural competency25. Consequently, sociodemographic factors can play a crucial role in the decision of declining the CDS for PCa patients. Physicians, especially urologists, should fully consider the barriers behind patients’ refusal of CDS to improve patients’ satisfaction, surgical utilization, and treatment outcomes.
Of note, the most influential factors in PCa patients’ treatment decisions were the perceptions of therapeutic efficacy and side effects, mainly derived from physicians’ descriptions26. Our study revealed that PCa patients who refused CDS had an increased risk of death ( hazard ratio 0.54 in CSM and hazard ratio 0.59 in OM) than those who accepted. Consistently, Rapp et al.’s study identified an overall 1.60 higher mortality in PCa patients who refused CDS7. In other words, PCa patients could significantly benefit from CDS and achieve a longer survival time. Given surgery refusal increasing CSM, physicians should carefully and clearly inform PCa patients regarding their prognosis in case they are thinking of skipping surgical treatment. CDS may be a viable alternative option for those with locally advanced or even distant stages of PCa.
Admittedly, certain limitations in this study should be addressed. Above all, the retrospective nature may lead to inevitable selection bias even after PSM. Besides, the SEER database has the inherent limitation to provide all clinically significant variables for CDS analysis, including performance status, preoperative comorbidities, postoperative complications and subsequent treatments. It is difficult to parse out patients’ decision-making processes in “real-world” clinical practice. In addition, SEER cannot provide other related factors such as characteristics of the surgeons that probably influence receipt or refusal of CDS. Therefore, we should admit the difficulty to uncover the real truth of the past and accurately specify "recommended" and "accepted" CDS treatment. Moreover, our observational study provided insufficient information to clearly explain the causal relationship between sociodemographic factors and CDS refusal in PCa patients. Additionally, our study did not involve the medical insurance status and investigate how patients’ income and their ability to afford surgical fees, partially reduced the confidence power. On top of that, we conducted two adjusted models to explore the value of CDSA on CSM and OM. Unfortunately, due to the missing data in SEER database, we could not enroll several potentially related factors for analysis, such as PSA, Gleason score and TNM stage. Detailly, disease-related factors such as GS, PSA, and T stage was not adjusted with PSM in this study, which is a limitation to permit valid comparisons. Despite this, the main goal of this study is to uncover sociodemographic factors and their impacts on survival associated with cancer-directed surgeries refusal. The impacts of PSA, GS, and AJCC stages on survival time have been well discussed in studies27,28,29. Additionally, we did not perform subset analyses to identify whether CDS impacts on survival outcomes in GS 6 or ≤ T2 disease. GS 6 or ≤ T2 stage represents localised prostate cancer and CDS is one of the most effective treatments for this type of prostate cancer according to EAU guideline and other guidelines. These patients with CDS presented a longer survival time as supported by numerous studies30,31. In spite of these limitations, however, our present study was one of the largest SEER-based analysis to identify predictors for patients’ CDS refusal and subsequent effect on cancer survival. One strength of this study was the application of a series of statistical analyses such as PSM to mitigate limitations. Notably, our study shined a spotlight on physicians’ key role in patients’ decision-making process, providing valuable information for patient-doctor relationships and communication.
Conclusions
In conclusion, our study revealed that physicians may weigh a host of socio demographic and clinical factors prior to making a CDS recommendation to PCa patients. Patients’ acceptance of recommended CDS was potentially modifiable by certain sociodemographic factors. Physicians, especially urologists, should fully consider the hindrances behind patient’s refusal of recommended CDS, thus improving patient-doctor shared decision-making, guiding patients toward the best alternative and achieving better outcomes. Further studies are necessitated to confirm the generality of our results.
Data availability
The datasets generated for this study are available on request to the corresponding author.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 72(1), 7–33. https://doi.org/10.3322/caac.21708 (2022).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72(5), 409–436. https://doi.org/10.3322/caac.21731 (2022).
Mottet, N. et al. Eau-Eanm-Estro-Esur-Siog guidelines on prostate cancer-2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 79(2), 243–262. https://doi.org/10.1016/j.eururo.2020.09.042 (2021).
Cornford, P. et al. Eau-Eanm-Estro-Esur-Siog guidelines on prostate cancer. Part II-2020 update: Treatment of relapsing and metastatic prostate cancer. Eur. Urol. 79(2), 263–282. https://doi.org/10.1016/j.eururo.2020.09.046 (2021).
Powell, I. J., Vigneau, F. D., Bock, C. H., Ruterbusch, J. & Heilbrun, L. K. Reducing prostate cancer racial disparity: evidence for aggressive early prostate cancer Psa testing of African American men. Cancer Epidemiol. Biomark. Prev. 23(8), 1505–1511. https://doi.org/10.1158/1055-9965.Epi-13-1328 (2014).
Presley, C. J. et al. A new approach to understanding racial disparities in prostate cancer treatment. J. Geriatr. Oncol. 4(1), 1–8. https://doi.org/10.1016/j.jgo.2012.07.005 (2013).
Rapp, J., Tuminello, S., Alpert, N., Flores, R. M. & Taioli, E. Disparities in surgery for early-stage cancer: The impact of refusal. Cancer Causes Control. 30(12), 1389–1397. https://doi.org/10.1007/s10552-019-01240-9 (2019).
Abdelsattar, Z. M., Hendren, S. & Wong, S. L. The impact of health insurance on cancer care in disadvantaged communities. Cancer 123(7), 1219–1227. https://doi.org/10.1002/cncr.30431 (2017).
Stolzenbach, L. F. et al. Differences between rural and urban prostate cancer patients. World J. Urol. 39(7), 2507–2514. https://doi.org/10.1007/s00345-020-03483-7 (2021).
Hoffman, K. E. et al. Physician variation in management of low-risk prostate cancer: A population-based cohort study. JAMA Int. Med. 174(9), 1450–1459. https://doi.org/10.1001/jamainternmed.2014.3021 (2014).
Onukwugha, E. et al. Specialist visits and initiation of cancer-directed treatment among a large cohort of men diagnosed with prostate cancer. Urol. Oncol. 35(4), 150.e17. https://doi.org/10.1016/j.urolonc.2016.11.012 (2017).
Islam, K. M. & Wen, J. Prostate cancer patients’ refusal of cancer-directed surgery: A statewide analysis. Prostate Cancer 2015, 829439. https://doi.org/10.1155/2015/829439 (2015).
Dee, E. C. et al. Disparities in refusal of locoregional treatment for prostate adenocarcinoma. JCO Oncol. Pract. 17(10), e1489–e1501. https://doi.org/10.1200/op.20.00839 (2021).
Hu, X., Ye, H., Yan, W. & Sun, Y. Factors associated with patient’s refusal of recommended cancer surgery: Based on surveillance, epidemiology, and end results. Front. Public Health 9, 785602. https://doi.org/10.3389/fpubh.2021.785602 (2021).
Wei, Y. et al. Comparison of survival outcomes and risk factors between ductal carcinoma of the prostate and acinar adenocarcinoma of the prostate: A population-based propensity score-matching study. Eur. Urol. Open Sci. 46, 88–95. https://doi.org/10.1016/j.euros.2022.10.013 (2022).
Scherr, K. A. et al. Physician recommendations trump patient preferences in prostate cancer treatment decisions. Med. Dec. Mak. 37(1), 56–69. https://doi.org/10.1177/0272989x16662841 (2017).
Suh, W. N. et al. Risk factors associated with treatment refusal in lung cancer. Thoracic Cancer 8(5), 443–450. https://doi.org/10.1111/1759-7714.12461 (2017).
Delisle, M. et al. Refusal of colorectal cancer surgery in the United States: Predictors and associated cancer-specific mortality in a surveillance, epidemiology, and end results (Seer) cohort. Surg. Open Sci. 2(4), 12–18. https://doi.org/10.1016/j.sopen.2020.07.001 (2020).
Gaitanidis, A. et al. Refusal of cancer-directed surgery by breast cancer patients: Risk factors and survival outcomes. Clin. Breast Cancer 18(4), e469–e476. https://doi.org/10.1016/j.clbc.2017.07.010 (2018).
Aizer, A. A. et al. Marital status and survival in patients with cancer. J. Clin. Oncol. 31(31), 3869–3876. https://doi.org/10.1200/jco.2013.49.6489 (2013).
Groth, S. S. et al. Effect of insurance status on the surgical treatment of early-stage non-small cell lung cancer. Ann. Thorac. Surg. 95(4), 1221–1226. https://doi.org/10.1016/j.athoracsur.2012.10.079 (2013).
Puts, M. T. et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat. Rev. 41(2), 197–215. https://doi.org/10.1016/j.ctrv.2014.12.010 (2015).
Pham, A. K., Bauer, M. T. & Balan, S. Closing the patient-oncologist communication gap: A review of historic and current efforts. J. Cancer Educ. 29(1), 106–113. https://doi.org/10.1007/s13187-013-0555-0 (2014).
Jacobs, E. A. et al. An exploratory study of how trust in health care institutions varies across African American, Hispanic and white populations. Commun. Med. 8(1), 89–98. https://doi.org/10.1558/cam.v8i1.89 (2011).
Chao, G. F. et al. Asian Americans and prostate cancer: A nationwide population-based analysis. Urol. Oncol. 34(5), 233.e7. https://doi.org/10.1016/j.urolonc.2015.11.013 (2016).
Xu, J., Dailey, R. K., Eggly, S., Neale, A. V. & Schwartz, K. L. Men’s perspectives on selecting their prostate cancer treatment. J. Natl. Med. Assoc. 103(6), 468–478. https://doi.org/10.1016/s0027-9684(15)30359-x (2011).
Freedland, S.J., Hotaling, J.M., Fitzsimons, N.J., Presti Jr., J.C., Kane, C.J., Terris, M.K., et al. Psa in the New Millennium: A powerful predictor of prostate cancer prognosis and radical prostatectomy outcomes—Results from the search database. Eur. Urol. 53(4), 758–64 (2008); discussion 65–6. https://doi.org/10.1016/j.eururo.2007.08.047.
Epstein, J. I. et al. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur. Urol. 69(3), 428–435. https://doi.org/10.1016/j.eururo.2015.06.046 (2016).
Epstein, J. I. et al. The 2014 International Society of Urological Pathology (Isup) consensus conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 40(2), 244–252. https://doi.org/10.1097/pas.0000000000000530 (2016).
Bill-Axelson, A. et al. Radical prostatectomy or watchful waiting in prostate cancer—29-year follow-up. N. Engl. J. Med. 379(24), 2319–2329. https://doi.org/10.1056/NEJMoa1807801 (2018).
Lei, J. H. et al. Systematic review and meta-analysis of the survival outcomes of first-line treatment options in high-risk prostate cancer. Sci. Rep. 5, 7713. https://doi.org/10.1038/srep07713 (2015).
Acknowledgements
The authors thank the SEER Program for providing these valuable data.
Funding
This study was supported by the Natural Science Foundation of Fujian Province (2021J01359, YW; 2022J05211, RZ), by Natural Science Foundation of Hunan Province (2022JJ40708, YT) and by Xiaoxiang Cancer Clinical Research Public Welfare Project (P049-001, YT).
Author information
Authors and Affiliations
Contributions
Conceptualization: L.H., Y.W. Formal analysis and investigation: Y.G., R.Z., T.L., Y.Y. Writing - original draft preparation: Y.T. Writing - review and editing: L.H., Y.W.. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, Y., Gao, Y., Zhang, R. et al. A population-based propensity score matching analysis of risk factors and the impact on survival associated with refusal of cancer-directed surgery in patients with prostate cancer. Sci Rep 14, 9494 (2024). https://doi.org/10.1038/s41598-024-60180-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60180-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.