Abstract
Animal activity reflects behavioral decisions that depend upon environmental context. Prior studies typically estimated activity distributions within few areas, which has limited quantitative assessment of activity changes across environmental gradients. We examined relationships between two response variables, activity level (fraction of each day spent active) and pattern (distribution of activity across a diel cycle) of white-tailed deer (Odocoileus virginianus), with four predictors—deer density, anthropogenic development, and food availability from woody twigs and agriculture. We estimated activity levels and patterns with cameras in 48 different 10.36-km2 landscapes across three larger regions. Activity levels increased with greater building density, likely due to heightened anthropogenic disturbance, but did not vary with food availability. In contrast, activity patterns responded to an interaction between twigs and agriculture, consistent with a functional response in habitat use. When agricultural land was limited, greater woody twig density was associated with reduced activity during night and evening. When agricultural land was plentiful, greater woody twig density was associated with more pronounced activity during night and evening. The region with the highest activity level also experienced the most deer-vehicle collisions. We highlight how studies of spatial variation in activity expand ecological insights on context-dependent constraints that affect wildlife behavior.
Similar content being viewed by others
Introduction
Individual animals must decide when and for how long to remain active each day. Such decisions can depend on a complex array of potentially antagonistic forces including duration of daylight1, food availability2, reproduction or rearing of young3, and temporal pulses in predation risk or fear4. Theory predicts that animal decisions on activity strive to balance these factors to maximize energetic efficiency by remaining active as little as possible while simultaneously obtaining necessary nourishment and minimizing predation risk5.
Accurate estimation of activity level (i.e., how much of a day spent active) and pattern (i.e., when activity occurs) of animal populations requires sampling across the landscape that animals use throughout the day. If some used landcover types are not sampled, activity metrics may be biased and misleading. Camera traps have been used commonly to estimate activity metrics because they are able to sample continuously within a wide variety of landcover types6.
Unfortunately, the intensity of sampling needed to estimate several replicate activity distributions poses logistical difficulties in terms of purchasing and deploying camera traps, and classifying animals within captured images. Quantitative study of how activity changes across space is thus challenging and rare. Most comparisons of animal activity metrics have been restricted to qualitative comparisons of visual activity graphs and statistical tests between two activity levels or patterns at once6. More detailed examinations of how activity quantitatively changes across multiple explanatory variables, landscapes, and regions would improve our ecological understanding of behavioral ecology.
The white-tailed deer (Odocoileus virginianus; henceforth, deer) is a common New World ungulate that inhabits diverse landscapes7. Deer behavior changes along environmental gradients including, but not limited to, human development8, food availability or quality9, and density of conspecifics10. Like other previous activity research, field studies of deer activity have been predominantly descriptive in nature or only included statistical comparisons of two activity distributions11,12. Because deer can exhibit behavioral fluidity in response to environmental context, additional plasticity in activity might be expected. We sought quantitative understanding of how deer activity levels and patterns change across gradients of various environmental characteristics during the winter in the midwestern USA (henceforth, Midwest).
Frequent disturbances may generally cause higher activity levels due to avoidance of perceived danger. Disturbances from human activities can influence numerous deer behaviors13,14,15. Human development in the form of roads and buildings may specifically affect deer activity because development is consistently associated with mortality from deer-vehicle collisions16. Therefore, deer may change their activity along gradients of human activity or development.
Deer in the Midwest rely on several food resources during winter including low-quality woody twigs in forested sites and higher-quality crop residue from agricultural fields after harvest. But deer also must balance food acquisition with predation risk17. According to foraging theory, deer should bias feeding activity in favor of safer areas and either exhibit greater per capita vigilance or form larger groups in riskier areas18. Fear of predation in deer may be associated with time of day and vegetative concealment (i.e., how much vegetation is obstructing direct line of sight to deer19). Within home ranges, disproportionate changes in habitat use with availability (i.e., functional responses in habitat use20) may result from attempts by individuals to balance decisions about activity with spatially varying risks and rewards21. In Midwest landscapes, deer use of crop residue in open agricultural fields and woody twigs in forest patches may reflect attempts to balance tradeoffs of forage quality, quantity, or perceived predation risk. If so, interactions between the amount of agriculture and woody twigs may be important predictors of deer activity.
Density dependence in ungulates is of interest ecologically and because of its implications for management of game species10,22. Theory suggests that larger densities of ungulates can decrease the amount of available per capita food and thereby increase intraspecific competition for nutrients23. Density-dependent responses in activity of wild boar (Sus scrofa), red deer (Cervus elaphus), and roe deer (Capreolus capreolus) have been documented24. Moreover, elk (Cervus canadensis) exhibited density-dependent habitat selection25. But no quantitative assessment has examined how deer activity shifts as a function of population density.
Hypotheses and predictions
Our goals were to determine whether and to what extent deer winter activity levels and patterns vary spatially, and to discover if variables related to anthropogenic disturbances, food, and intraspecific density correspond to these activity changes. First, we hypothesized that deer would alter their activity levels in response to human development. We predicted that road length and density of buildings would exhibit positive relationships with deer activity levels.
Secondly, we hypothesized that deer activity levels would respond to intraspecific density. Greater density, by decreasing available per capita food, may increase the amount of time spent foraging23; accordingly, we predicted a positive relationship between activity levels and intraspecific density of deer.
Thirdly, we hypothesized that deer activity levels would respond to food availability. We predicted that deer activity levels would decrease as the density of woody twigs and the amount of agricultural land increased, because more available food should decrease the time required for foraging. Further, we predicted a significant interactive effect of woody twigs and agricultural land due to activity levels of deer requiring tradeoffs between resource acquisition and safety.
Fourthly, we hypothesized that deer activity patterns would respond to availability of different food types in a manner that incorporated time-dependent tradeoffs between forage acquisition and safety. Deer are crepuscular26, predominantly consuming agricultural products in open fields during nocturnal hours but consuming woody twigs during any time of day27. Moreover, deer may prefer diverse diets which can ensure proper nutrient intake and avoidance of overconsumption of any single mineral28,29. Therefore, in areas with small amounts of agricultural land, we predicted deer would alter their activity patterns to be more active at night in areas where woody twig density was low, because deer would rely more heavily on crops as a food source at night. In contrast, we predicted deer would be less active at night in areas where woody twig density was high, because deer would be able to consume adequate amounts of twigs during daytime in the relative safety of woodlands. However, in areas with plentiful amounts of agricultural land, we predicted deer would be more active during daytime in areas where woody twig density was low, because deer would need to search for and consume scarce woody twigs throughout daytime. In areas with plentiful agricultural land, we also predicted deer would be more nocturnal in areas where woody twig density was high, because woody twigs would be readily available for consumption during daytime and thus deer could concentrate their time spent active during nocturnal hours when crop consumption could more safely occur.
Results
We sampled 1,018 unique locations with camera traps (total trap nights = 13,816). Cameras did not detect deer at 187 locations. The average number of sampling locations and deer detections used for activity estimation was 21 and 536, respectively, per replicate 10.36-km2 landscape. We detected > 100 deer detections in 42 different landscapes.
Regional analysis
Activity distributions in RMUs 3 and 9 both exhibited bimodal peaks characteristic of crepuscular activity, whereas activity in RMU 4 peaked predominantly during the evening and to a much lesser extent in the morning (Fig. 1). Both activity levels and patterns varied significantly across regions. We estimated activity levels of 0.41 (standard error [SE] = 0.01), 0.39 (SE = 0.02), and 0.44 (SE = 0.01) in RMU 3, 4, and 9, respectively. Activity level in RMU 3 was similar to RMUs 4 (observed difference = 0.02, SE = 0.02, W = 1.27, P = 0.26) and 9 (observed difference = 0.03, SE = 0.02, W = 2.60, P = 0.21). However, differences arose in activity levels between RMUs 4 and 9 (observed difference = 0.05, SE = 0.02, W = 8.60, P = 0.010).
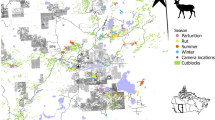
Landcover types and sampled landscapes within Regional Management Units (RMU) 3 (west central), 4 (southern), and 9 (northeastern, two separate areas) within Indiana, USA. Within each RMU, we estimated the activity distributions of white-tailed deer using camera traps deployed within landscapes during the winters from 2019 to 2021.
Deer spent more time active during morning and less at night in RMU 9, but the reverse was true for RMU 4 (Fig. 2). Deer allotted their activity more evenly throughout each of the four time-of-day categories in RMU 3 when compared to the other regions. Deer devoted similar fractions of total activity to evening hours in all three regions.
The fraction of deer activity levels (± 95% confidence intervals) observed during morning, daytime, evening, and night within Regional Management Units (RMU) 3, 4, and 9 in Indiana, USA (A). Fractions of deer activity levels observed between RMUs and within times of day (B) or within RMUs and between times of day (C) are similar (P > 0.05) if sharing an identically colored line. We collected activity data using camera traps during the winters of 2019 to 2021.
Replicate landscape analysis
Activity levels
Models containing predictors associated with anthropogenic development, twig density, and intraspecific density had evidence ratios < 3; neither area of agriculture nor models with interactions received support (Table 1, Table S1). The model containing number of buildings had the most support; in it, the number of buildings exhibited a strong positive relationship with deer activity levels (Table 2, Fig. 3).
Effects plot from a hierarchical Bayesian beta regression model predicting white-tailed deer activity levels (± standard error) as a function of the number of buildings within 3.2 × 3.2-km landscapes. Activity data were collected using camera traps during the winters of 2019 to 2021 in Indiana, USA.
Activity patterns
The only model with support for activity patterns contained a strong interaction between twig density and the amount of surrounding land devoted to agriculture (Tables 1, 3). Specifically, when the amount of agricultural land was small, increases in woody twig density were associated with reduced deer activity during night and evening periods, and greater relative activity during morning and (moderately) during daytime periods (Fig. 4). Conversely, when the amount of agricultural land was large, increases in woody twig density were associated with greater deer activity during night and evening periods, and reduced activity during morning and daytime periods (Fig. 4). We provide plots of posterior distributions, posterior predictive checks, and trace plots in Supplementary Figs. S1–S4.
Effects plot from a hierarchical Bayesian Dirichlet model regressing the fraction of white-tailed deer activity levels exerted during night (baseline), morning, daytime, and evening (± standard error, SE) across an interaction between the amount of land used for agriculture (0.13, 0.42, and 0.72 km2 = − 1, mean, and 1 standard deviation, respectively) within the 3.2 × 3.2-km landscape and the density of woody twigs within the same landscape (twigs/m2). Activity data were collected using camera traps during the winters of 2019 to 2021 in Indiana, USA.
Discussion
Activity patterns of deer responded to the interaction of natural and agricultural food sources, supporting our prediction. Other studies have demonstrated functional responses in habitat use by ungulates consistent with modulation of site-specific differences in risk and reward30,31. But our results are, to our knowledge, the first to document such modulation operating across landscapes in a manner that shifts the time of day during which activity occurs. In contrast to our prediction, we found no strong relationships between deer activity levels and these same sources of foods. Although we tested a large gradient of woody twig density and agricultural availability, food in our study areas may not have been limited enough to alter both activity levels and patterns simultaneously. Instead of changing activity levels, deer appeared to obtain sufficient amounts of both agriculturally and naturally sourced foods while adjusting their activity patterns based upon the availability of each food source.
Although previous research documented activity levels to be density dependent in other ungulates24, we found no strong relationship between deer density and deer activity levels. Limitation of food resources is a major contributor to intraspecific competition and ecological carrying capacity for deer32. But other research found minimal effects of deer use intensity (an index of population density) on the health of forest understories in the same areas and times we deployed camera traps33. Vast amounts of agricultural food are present in RMUs 3 and 9, and forests containing woody food are widespread in RMU 4. Therefore, plentiful food resources may have reduced or eliminated any potential intraspecific competition for food within our study system and thus negated our ability to detect any potential response in deer activity levels to food.
Deer activity may be more broadly related to other areas of interest in wildlife management. For instance, deer-vehicle collisions are a major concern of wildlife managers34. Population density has been a key focus of those attempting to reduce or model animal-vehicle collisions35,36,37, but animal activity may also play a role38,39. If animals either are active during a greater fraction of each day or shift their activity to coincide with periods of peak vehicular traffic volume, the chances of animals and vehicles colliding on the landscape likely will increase. In our study, we documented in RMU 9 the highest regional activity levels and a pattern characterized by a greater fraction of activity during the morning rush hours. Under such conditions, accidents involving collisions between motorists and deer might be expected. Indeed, deer-vehicle collisions occur at a rate 1.98 times higher in RMU 9 compared to RMU 3 and 440. Therefore, quantitative examinations of the relationships between characteristics of activity distributions and deer-vehicle collisions may help future management planning to reduce collisions. If positive relationships are found, incentivizing humans to hunt deer in close proximity to roadways may reduce occurrence of deer-vehicle collisions by causing deer to shift to nocturnal activity patterns, reduce movement rates, or select areas further from roads41,42.
Metrics from estimated animal activity distributions are fundamental parameters used for research purposes in energetics11, population ecology43, movement ecology44, species interactions11, and urban ecology45. In population estimation, activity levels are often used to estimate the sampling availability (i.e., fraction of the 24-h day that animals are moving and thus able to be detected by camera traps) of the target species for camera trapping38,46. In so doing, most studies have used a single estimate of animal activity to correct for the sampling availability of the target population. However, our results suggest that models of variation in activity level across study regions may yield more accurate estimates of sampling availability. Improvements may be especially dramatic for spatially explicit models of density that depict how the size of animal populations change across space while also relying on estimates of sampling availability. We encourage future research in population ecology to consider how sampling availability changes across space.
Quantitative modelling of spatial variation in animal activity metrics requires sampling intensity sufficient to estimate activity distributions within many spatial replicates. Such modeling has the capacity to enhance our understanding of animal behavior, and to improve knowledge useful for wildlife management and conservation. The sampling effort required for modeling of animal activity poses both logistical and financial challenges. Fortunately, large-scale camera trapping operations are becoming more common in wildlife research and management47,48,49 and should enable closer inspection of environmental and human factors affecting animal activity. Moreover, opportunities to integrate other data sources (e.g., bio logging50) should improve our ability to detect shifts and test for effects of risk:reward tradeoffs on activity levels and patterns across environmental gradients.
Methods
Study sites
We deployed camera traps within Regional Management Units (RMU) 3, 4, and 9 in Indiana, USA40. We used the same sampling design and camera deployment strategy outlined in previous research within the same study areas51. We placed cameras in random 10.36-km2 sub-areas (henceforth, landscapes). We sampled 20 landscapes across all three RMUs in each year. We repetitively sampled two landscapes in each RMU during each year to examine annual differences unrelated to spatial variation. Thus 48 unique landscapes were sampled. A thorough description of each RMU is presented in previous research52.
Data collection
We randomly deployed camera traps > 200 m apart within forests, wetlands, grasslands, and agricultural fields53. We affixed cameras at 1-m height to trees or metal poles that we hammered into the ground if trees were not present. We programmed cameras to capture a 3-photo burst when triggered, and specified a minimum delay between bursts of 1 s. We collected data from camera trap images during 2-week intervals from 12–25 February 2019, 9–22 March 2020, and 25 February–10 March 2021. After data collection, we classified photos containing deer and recorded the time of day of each encounter at each camera.
Estimation of activity levels and patterns
Times of day when animals are active and inactive are associated with greater and lesser numbers of captures by camera traps, respectively54. The distribution of detection times can thus be used to estimate animal activity level. To do this, we first double-anchored detection times of deer based on the average sunrise and sunset times at camera trap locations on days we collected data using methods of previous research1. Double anchoring detection times accounts for the differing sunset and sunrise times across the days and locations that we sampled, which is critical because deer are crepuscular26. We then fit circular kernel probability density functions to the double-anchored detection times of deer using the methods of previous research53. We used the area under each circular kernel probability density function as the estimate of deer activity level, and used nonparametric bootstrapping to estimate uncertainty associated with activity levels54. We used the “activity” package in R to double anchor detection times, fit probability density functions, and estimate activity levels55,56.
We estimated activity patterns by first defining four different time-of-day categories: night (defined as > 2 h after sunset to > 2 h before sunrise), morning (defined as < 2 h before sunrise to < 2 h after sunrise), daytime (defined as > 2 h after sunrise to > 2 h before sunset), and evening (defined as < 2 h before sunset to < 2 h after sunset). We then used numerical integration to estimate what fraction of the daily deer activity level that occurred within each time-of-day category by
where \({t}_{1}\) and \({t}_{2}\) are the two temporal bounds of a given time-of-day category, and \(f(x)\) is the circular kernel density used to model activity level.
Predictors of activity
Deer density
We estimated spatially explicit deer density using the density surface model from a previous project in the same study areas and times this study was conducted51. These density estimates utilized camera trap distance sampling and landscape covariates within a generalized additive model to predict deer density inside 30 × 30-m cells across each of the RMUs we sampled51. To estimate deer density within each landscape, we averaged the density estimate of each cell within the landscape of interest. Density estimates within landscapes ranged from 0.78 to 37.43 deer/km2 (mean = 9.06, SE = 1.08).
Anthropogenic development
We calculated the total road length and number of buildings within landscapes using the Indiana primary and secondary roads state-based shapefile57 and the US building footprints shapefile from Microsoft (https://github.com/Microsoft/USBuildingFootprints), respectively.
Food availability
We calculated the total amount of agricultural land within landscapes using the National Land Cover Database 2019 landcover raster file53. We also estimated the density of non-avoided twigs as an index of natural food using the methods and data from previous research in the same study areas58. Specifically, we sampled five 1-m2 quadrats placed every 10 m along randomly placed and oriented 50-m transects. The number of transects sampled per forest patch was determined by \({A}_{i}/2{NT}_{i}<{NT}_{i}\), where \({NT}_{i}\) = the number of transects in forest patch \(i\), and \({A}_{i}\) = the area (ha) of forest patch \(i\). We counted all living woody twigs in 3-dimensional space 20–180 cm above the quadrat59, and estimated twig density (\({D}_{i}\)) by \({D}_{i}={t}_{i}/{n}_{i}\), where \({t}_{i}\) = the total number of twigs counted in forest patch \(i\), and \({n}_{i}\) = the total number of quadrats surveyed within forest patch \(i\). We used a Pearson’s chi-square test for count data60 to classify twig species as non-avoided, which we defined as significantly (consumed at a higher rate than expected; α ≤ 0.05) or neutrally (consumed at a similar rate than expected; α > 0.05) selected for consumption.
Natural predators and human hunting
Deer will often alter their decision making when they co-occur with predators or perceived predators61,62. Although coyotes (Canis latrans) predominantly prey solely on juvenile deer63, past research has found behavioral responses in deer when exposed to coyotes64. However, during times of the year when juvenile deer are extremely young, past research found only groups containing juveniles to respond to coyote presence by altering their activity pattern11. Groups solely containing older deer did not alter their activity patterns when subjected to the presence of coyotes11. Our study was conducted later in the year when juvenile deer are much larger and thus less susceptible to predation by coyotes. For these reasons, we did not consider coyotes as a predictor of deer activity patterns in this work.
Human hunting is a major source of deer mortality16 and can affect several deer behaviors41,42. However, outside of times of year or areas where humans hunt deer, deer often become accustomed to nonhunting humans (e.g., human walking or dog walking) and often do not perceive humans directly as risky19,65. Our sampling did not occur during seasons when humans could harvest deer in Indiana. For these reasons, we did not test the effects of indices of human hunters on deer activity levels or patterns.
Regional analysis
We used a Wald χ2 statistic with 1 degree of freedom54 to test for differences between (1) activity levels within regions, (2) fraction of deer activity levels exerted within each time-of-day category between RMUs, and (3) fraction of deer activity levels exerted between each time-of-day category within RMUs. In each Wald test, \(W=\frac{{{(AL}_{1}-{AL}_{2})}^{2}}{SE({{AL}_{1})}^{2}+SE({{AL}_{2})}^{2}}\), where \({AL}_{1}\) and \({AL}_{2}\) are the two activity levels being compared and \(SE\) denotes the standard error. To minimize potential for Type 1 Error due to multiple pairwise comparisons, we implemented a Holm’s adjustment strategy66.
Replicate landscape analysis
We modelled activity levels of deer within 10.36-km2 landscapes using a mixed effects beta regression. We used a beta regression because activity level is a fraction bounded between 0 and 1. We modelled the fraction of deer activity levels exerted during each of the four time-of-day categories using a mixed effects Dirichlet regression. We used a Dirichlet regression because the four fractions of deer activity levels exerted during each of the time-of-day categories are naturally compositional and thus sum to 1. We did not include activity levels or patterns from landscapes that had < 100 deer detections, as other research suggested that coefficients of variation exceed 0.10 with sample sizes of < 100 and thus are imprecise54.
We fit all models within a hierarchical Bayesian framework in R using the “brms” package67. Because landscapes were naturally nested within larger regions, we included a random intercept for region. For all continuous predictors, we subtracted the mean and divided by the standard deviation to aid convergence. We ran 3 Markov chains for a total of 2500 iterations per chain. We discarded the first 1000 iterations per chain and specified Student-T priors for all regression coefficients and random effects in the beta and Dirichlet models (degrees of freedom = 3, mean = 0, standard deviation = 2.5; default in “brms” package).
To examine support for each of the models, we used weights based on approximate leave-one-out cross validation68 and evidence ratios of model weights (i.e., the weight of the top model divided by the weight of the model under consideration). We considered models with evidence ratios < 3 to have statistical support for being the “best” model in the candidate set, and only further reported statistics on regression coefficients from these models. Because 89% confidence intervals are more stable when posterior samples are < 10,000, we considered strong predictors those with associated 89% credible intervals that did not overlap zero69.
Data availability
The data used in this manuscript are included as Supplementary Information.
References
Vazquez, C., Rowcliffe, J. M., Spoelstra, K. & Jansen, P. A. Comparing diel activity patterns of wildlife across latitudes and seasons: Time transformations using day length. Methods Ecol. Evol. 10, 2057–2066 (2019).
Metcalfe, N. B., Fraser, N. H. & Burns, M. D. Food availability and the nocturnal vs. diurnal foraging trade-off in juvenile salmon. J. Anim. Ecol. 68, 371–381 (1999).
Schmidt, K. Variation in daily activity of the free-living Eurasian lynx (Lynx lynx) in Białowieża Primeval Forest. Poland. J. Zool. 249, 417–425 (1999).
Ross, J., Hearn, A. J., Johnson, P. J. & Macdonald, D. W. Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk. J. Zool. 290, 96–106 (2013).
Kronfeld-Schor, N. & Dayan, T. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 153–181 (2003).
Frey, S., Fisher, J. T., Burton, A. C. & Volpe, J. P. Investigating animal activity patterns and temporal niche partitioning using camera-trap data: Challenges and opportunities. Remote Sens. Ecol. Conserv. 3, 123–132 (2017).
Hewitt, D. G. Nutrition. In Biology and Management of White-Tailed Deer (ed. Hewitt, D. G.) 147–173 (CRC Press, 2011).
Storm, D. J., Nielsen, C. K., Schauber, E. M. & Woolf, A. Space use and survival of white-tailed deer in an exurban landscape. J. Wildl. Manage. 71, 1170–1176 (2007).
Hirth, D. H. Social behavior of white-tailed deer in relation to habitat. Wildl. Mon. 53, 3–55 (1977).
Kie, J. G. & Bowyer, R. T. Sexual segregation in white-tailed deer: Density-dependent changes in use of space, habitat selection, and dietary niche. J. Mammal. 80, 1004–1020 (1999).
Higdon, S. D., Diggins, C. A., Cherry, M. J. & Ford, W. M. Activity patterns and temporal predator avoidance of white-tailed deer (Odocoileus virginianus) during the fawning season. J. Ethol. 37, 283–290 (2019).
Crawford, D. A., Conner, L. M., Morris, G. & Cherry, M. J. Predation risk increases intraspecific heterogeneity in white-tailed deer diel activity patterns. Behav. Ecol. 32, 41–48 (2021).
Harveson, P. M., Lopez, R. R., Collier, B. A. & Silvy, N. J. Impacts of urbanization on Florida Key deer behavior and population dynamics. Biol. Conserv. 134, 321–331 (2007).
Nojoumi, M., Clevenger, A. P., Blumstein, D. T. & Abelson, E. S. Vehicular traffic effects on elk and white-tailed deer behavior near wildlife underpasses. PLoS ONE 17, e0269587 (2022).
Visscher, D. R. et al. Human impact on deer use is greater than predators and competitors in a multiuse recreation area. Anim. Behav. 197, 61–69 (2023).
VerCauteren, K. C. & Hygnstrom, S. E. Managing white-tailed deer: midwest north America. In Biology and Management of White-Tailed Deer (ed. Hewitt, D. G.) 514–549 (CRC Press, 2011).
Clare, J. D. et al. A phenology of fear: Investigating scale and seasonality in predator–prey games between wolves and white-tailed deer. Ecology 104, e4019 (2023).
Altendorf, K. B., Laundre, J. W., Lopez Gonzalez, C. A. & Brown, J. S. Assessing effects of predation risk on foraging behavior of mule deer. J. Mammal. 82, 430–439 (2001).
Schuttler, S. G. et al. Deer on the lookout: how hunting, hiking and coyotes affect white-tailed deer vigilance. J. Zool. 301, 320–327 (2017).
Mysterud, A. & Ims, R. A. Functional responses in habitat use: availability influences relative use in trade-off situations. Ecology 79, 1435–1441 (1998).
Godvik, I. M. R. et al. Temporal scales, trade-offs, and functional responses in red deer habitat selection. Ecology 90, 699–710 (2009).
Keyser, P. D., Guynn, D. C. Jr. & Hill, H. S. Jr. Density-dependent recruitment patterns in white-tailed deer. Wildl. Soc. Bull. 33, 222–232 (2005).
Becker, J. A. et al. Ecological and behavioral mechanisms of density-dependent habitat expansion in a recovering African ungulate population. Ecol. Monogr. 91, e01476 (2021).
Ramirez, J. I. et al. Density dependence of daily activity in three ungulate species. Ecol. Evol. 11, 7390–7398 (2021).
van Beest, F. M., McLoughlin, P. D., Mysterud, A. & Brook, R. K. Functional responses in habitat selection are density dependent in a large herbivore. Ecography 39, 515–523 (2016).
Beier, P. & McCullough, D. R. Factors influencing white-tailed deer activity patterns and habitat use. Wildl. Mon. 109, 3–51 (1990).
Larson, T. J., Rongstad, O. J. & Terbilcox, F. W. Movement and habitat use of white-tailed deer in southcentral Wisconsin. J. Wildl. Manage. 42, 113–117 (1978).
Forbes, J. M. & Provenza, F. D. Integration of learning and metabolic signals into a theory of dietary choice and food intake. In Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction (ed. Cronjé, P. B.) 3–20 (CABI Publishing, 2000).
Ceacero, F. et al. Avoiding toxic levels of essential minerals: A forgotten factor in deer diet preferences. PLoS ONE 10, e0115814 (2015).
Padié, S. et al. Roe deer at risk: teasing apart habitat selection and landscape constraints in risk exposure at multiple scales. Oikos 124, 1536–1546 (2015).
Huggler, K. S. et al. Risky business: How an herbivore navigates spatiotemporal aspects of risk from competitors and predators. Ecol. Appl. 32, e2648 (2022).
McCullough, D. R. The George Reserve Deer Herd: Population Ecology of a K-selected Species 271 (The University of Michigan Press, 1979).
Sample, R. D. et al. Selection rankings of woody species for white-tailed deer vary with browse intensity and landscape context within the Central Hardwood Forest Region. For. Ecol. Manage. 537, 120969 (2023).
Conover, M. R., Pitt, W. C., Kessler, K. K., DuBow, T. J. & Sanborn, W. A. Review of human injuries, illnesses, and economic losses caused by wildlife in the United States. Wildl. Soc. Bull. 23, 407–414 (1995).
Hedlund, J. H., Curtis, P. D., Curtis, G. & Williams, A. F. Methods to reduce traffic crashes involving deer: what works and what does not. Traffic. Inj. Prev. 5, 122–131 (2004).
DeNicola, A. J. & Williams, S. C. Sharpshooting suburban white-tailed deer reduces deer–vehicle collisions. Hum. Wildl. Conf. 2, 28–33 (2008).
Gkritza, K., Baird, M. & Hans, Z. N. Deer-vehicle collisions, deer density, and land use in Iowa’s urban deer herd management zones. Accid. Anal. Prev. 42, 1916–1925 (2010).
Hothorn, T., Müller, J., Held, L., Möst, L. & Mysterud, A. Temporal patterns of deer–vehicle collisions consistent with deer activity pattern and density increase but not general accident risk. Accid. Anal. Prev. 81, 143–152 (2015).
Stickles, J. H. et al. Using deer-vehicle collisions to map white-tailed deer breeding activity in Georgia. J. Southe. Ass. Fish Wildl. Agen. 2, 202–207 (2015).
Swihart, R. K., Caudell, J. N., Brooke, J. M. & Ma, Z. A flexible model-based approach to delineate wildlife management units. Wildl. Soc. Bull. 44, 77–85 (2020).
Kilgo, J. C., Labisky, R. F. & Fritzen, D. E. Influences of hunting on the behavior of white-tailed deer: implications for conservation of the Florida panther. Conserv. Biol. 12, 1359–1364 (1998).
Little, A. R. et al. Hunting intensity alters movement behaviour of white-tailed deer. Basic Appl. Ecol. 17, 360–369 (2016).
Howe, E. J., Buckland, S. T., Després-Einspenner, M. L. & Kühl, H. S. Distance sampling with camera traps. Methods Ecol. Evol. 8, 1558–1565 (2017).
Rowcliffe, J. M., Jansen, P. A., Kays, R., Kranstauber, B. & Carbone, C. Wildlife speed cameras: Measuring animal travel speed and day range using camera traps. Remote Sens. Ecol. Conserv. 2, 84–94 (2016).
Gaynor, K. M., Hojnowski, C. E., Carter, N. H. & Brashares, J. S. The influence of human disturbance on wildlife nocturnality. Science 360, 1232–1235 (2018).
Nakashima, Y., Fukasawa, K. & Samejima, H. Estimating animal density without individual recognition using information derivable exclusively from camera traps. J. Appl. Ecol. 55, 735–744 (2018).
Delisle, Z. J., Flaherty, E. A., Nobbe, M. R., Wzientek, C. M. & Swihart, R. K. Next-generation camera trapping: systematic review of historic trends suggests keys to expanded research applications in ecology and conservation. Front. Ecol. Evol. 9, 617996 (2021).
Swanson, A. et al. Snapshot Serengeti, high-frequency annotated camera trap images of 40 mammalian species in an African savanna. Sci. Data 2, 1–14 (2015).
Cove, M. V. et al. SNAPSHOT USA 2019: a coordinated national camera trap survey of the United States. Ecol. Soc. Am. 102, e03353 (2021).
McClintock, B. T., Russell, D. J., Matthiopoulos, J. & King, R. Combining individual animal movement and ancillary biotelemetry data to investigate population-level activity budgets. Ecology 94, 838–849 (2013).
Delisle, Z. J., Miller, D. L. & Swihart, R. K. Modelling density surfaces of intraspecific classes using camera trap distance sampling. Methods Ecol. Evol. 14, 1287–1298 (2023).
Delisle, Z. J. et al. Using cost-effectiveness analysis to compare density-estimation methods for large-scale wildlife management. Wildl. Soc. Bull. 47, e1430 (2023).
Dewitz, J. National Land Cover Database (NLCD) 2019 Products: U.S. Geological Survey data release. https://doi.org/10.5066/P9KZCM54 (2021).
Rowcliffe, J. M., Kays, R., Kranstauber, B., Carbone, C. & Jansen, P. A. Quantifying levels of animal activity using camera trap data. Methods Ecol. Evol. 5, 1170–1179 (2014).
Rowcliffe, J. M. R Package ‘activity’: Animal activity statistics. Version 1.3.3. https://cran.r-project.org/web/packages/activity/activity.pdf (2023).
R Core Team. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, (2022).
US Census Bureau, Department of Commerce. https://catalog.data.gov/dataset/tiger-line-shapefile-2015-state-indiana-primary-and-secondary-roads-state-based-shapefile (2018).
Sample, R. D. The Influence of Local and Landscape Characteristics on Deer Browsing, and Subsequently the Composition and Structure of Forest Understories, in Indiana. Purdue University. PhD Dissertation (2022).
Frerker, K., Sonnier, G. & Waller, D. M. Browsing rates and ratios provide reliable indices of ungulate impacts on forest plant communities. For. Ecol. Manage. 291, 55–64 (2013).
Ebbert, D. Package ‘Chisq.posthoc.test’: A post hoc analysis for Pearson’s chi-squared test for count data. Version 0.1.2. https://CRAN.R-project.org/package=chisq.posthoc.test (2019).
Lingle, S. Anti-predator strategies and grouping patterns in white-tailed deer and mule deer. Ethology 107, 295–314 (2001).
Cherry, M. J., Conner, L. M. & Warren, R. J. Effects of predation risk and group dynamics on white-tailed deer foraging behavior in a longleaf pine savanna. Behav. Ecol. 26, 1091–1099 (2015).
Watine, L. N. & Giuliano, W. M. Coyote predation effects on white-tailed deer fawns. Nat. Resour. 7, 628–643 (2016).
Gulsby, W. D., Cherry, M. J., Johnson, J. T., Conner, L. M. & Miller, K. V. Behavioral response of white-tailed deer to coyote predation risk. Ecosphere 9, e02141 (2018).
Price, M. V., Strombom, E. H. & Blumstein, D. T. Human activity affects the perception of risk by mule deer. Curr. Zool. 60, 693–699 (2014).
Holm, S. A simple sequentially rejective multiple test procedure. Scand. Stat. Theory Appl. 6, 65–70 (1979).
Bürkner, P. C. Brms: An R package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 80, 1–28 (2017).
Vehtari, A., Gelman, A. & Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27, 1413–1432 (2017).
McElreath, R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan (CRC, 2018).
Acknowledgements
We appreciate the thoughtful and constructive comments of two anonymous reviewers that improved the clarity of our manuscript. We thank P.G. McGovern for fieldwork coordination, L. Utley for GIS assistance, numerous technicians who helped collect field data, and hundreds of private-property owners who allowed us to conduct research on their land. We gratefully acknowledge the traditional homelands of the Indigenous Bodéwadmik (Potawatomi), Lenape (Delaware), Myaamia (Miami), and Shawnee People upon which our fieldwork was conducted; see native-land.ca for additional information. This paper is a contribution of the Integrated Deer Management Project, a collaborative research effort between Purdue University and the Indiana Department of Natural Resources Division of Fish and Wildlife (Grant W-48-R-02).
Author information
Authors and Affiliations
Contributions
Z.J.D, R.D.S, and R.K.S. coordinated the data collection. Z.J.D. led the data analysis and writing of the manuscript with contributions from all co-authors. All co-authors contributed to drafts of the manuscript and gave final approval for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Delisle, Z.J., Sample, R.D., Caudell, J.N. et al. Deer activity levels and patterns vary along gradients of food availability and anthropogenic development. Sci Rep 14, 10223 (2024). https://doi.org/10.1038/s41598-024-60079-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60079-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.