Abstract
The sex ratio shift was observed in peoples who underwent ART treatment. Moreover, there is limited evidence on differences in sex ratio between single frozen-thawed blastocyst morphology, insemination type and transfer days. So further research is needed in this area with regard to factors possibly affecting the sex ratio. Retrospective study based on multicenter including two large assisted reproduction centers in Shanghai and Wuhan in China. A total of 6361 singleton delivery offspring after frozen-thawed blastocyst transfer. Propensity score weighting and logistic regression models were used to estimate the associations between blastocyst morphology grading and child sex ratio. The main outcome measures is singleton sex ratio. In our study, the primary outcome measure was sex ratio which was calculated as the proportion of male newborns among all live births. Higher quality blastocysts resulted in a higher sex ratio than single poor-quality frozen-thawed blastocyst transfer. Among the three blastocyst morphological parameters of trophectoderm (TE), Grade A and B were significantly associated with a higher sex ratio than Grade C. The similar trend was observed in both IVF and ICSI treated subgroups. As compared with expansion (4 + 3), expansion degree 6 achieved a higher sex ratio in overall populations and IVF treated subgroup. Transferring blastocysts of day 6 had the highest sex ratio both in IVF group and ICSI group. A 6.95% higher sex ratio in transferring blastocysts of day 5 in IVF group than those in ICSI group. No significant association between inner cell mass degree and sex ratio was observed. However, as compared with IVF treatment, all morphology parameters achieved the similar or the biased sex ratio favoring female in ICSI treated subgroup. Quality of blastocysts was positively associated with sex ratio. TE score and expansion degree rather than ICM were significantly associated with sex ratio at birth. ICSI treatment promotes the biased sex ratio favoring female.
Similar content being viewed by others
Introduction
The sex ratio at birth is often defined as the proportion of males in all live births1. Evolutionary theory suggests that some animal species, including human beings, may adjust their offspring sex ratio in response to maternal health or environmental conditions2. Variation in sex ration was commonly observed within human populations, deviating from the equality ratio of males to females (50%)3,4,5,6. This sex ratio shift was also observed in peoples who underwent assisted reproductive technology (ART) treatment for infertility7.
Accumulating evidence suggest that single blastocyst transfer induces a male-biased imbalance in the sex ratio. Sex ratios favoring males have been reported after transfer at the blastocyst stage in in vitro fertilization (IVF) cycles8,9,10. On the other hand, it appears that significantly more females were born than expected after blastocyst transfer using ICSI treatment for male infertility11,12. As well, a subgroup analysis comparing IVF, intracytoplasmic sperm injection (ICSI) and frozen embryo replacement revealed more females born after ICSI and frozen embryo replacement9. However, no difference in sex ration was observed after fresh blastocyst transfer in IVF cycles in another study by Gareth W. et al.13. In humans, the sex is determined at the moment of fecundation, while it is actually unclear how single blastocyst transfer in ART induced an imbalance of sex ratio in birth after fecundation. And these conflicting studies suggest that further research is needed in this area with regard to factors possibly affecting the sex ratio.
The selection of human blastocyst for transfer is most often based on morphological criteria and development rate. Blastocyst grading for embryo transfers usually occurs on day 5 of culture, and is composed of three parameters: the cellularity of both the inner cell mass (ICM) and trophectoderm (TE) and the degree of blastocoel expansion14. Several studies have attempted to identify the relationship between morphology parameters and sex ratio15. Ebner et al. reported that male blastocysts had a 2.53 higher chance of obtaining TE of quality A than female ones16. The association of Grade B trophectoderm with a higher sex ratio was also observed in a cohort study of transferring 1210 frozen blastocysts via IVF and ICSI treatments10. In a large scale study including 7246 women, who underwent fresh or frozen thawed blastocysts transfer, showed that developmental stage and TE score affected the probability of being a boy17. However, the limited samples or mixed groups of IVF and ICSI cycles, or fresh and frozen blastocyst transfer, compromised the power of these conclusions in above studies.
To address the relationship of morphology parameters of blastocyst with sex ratio of birth, this study retrospectively analyzed 6361 singleton frozen-thawed blastocyst transfer cycles from two of the large reproductive centers in Shanghai and Wuhan of China. This study will advanced the understanding of the blastocyst morphology-driven sex allocation in ART.
Methods
Patients
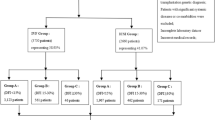
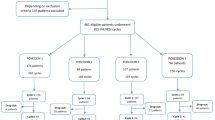
The present retrospective study was a multicenter cohort study with retrospectively conducted at the Department of Assisted Reproduction of the Ninth People’s Hospital affiliated with Shanghai Jiao Tong University School of medicine involving 3304 frozen-thaw blastocysts during the period from January 2007 to May 2020 and at the Reproductive Medicine Center of Tongji Hospital affiliated with Huazhong University of Science and Technology involving 3057 frozen-thaw blastocysts during the period from January 2019 to December 2020. The routine ovarian stimulation protocols were applied in 6161 patients in this study. The gonadotropin-releasing hormone (GnRH) agonist long and short protocols, progestin-primed ovarian stimulation (PPOS), gonadotropin-releasing hormone-antagonist (GnRH-A) protocol and micro-stimulus accounted for 36.16%, 30.92%, 24.37%, and 8.55%. The cycles with fertilization of IVF or ICSI followed by frozen-thawed embryo transfer (FET) cycles with single embryo transfer resulting in a single pregnancy and singleton live birth were included. In the case of identical twins, we analyzed only one newborn to ensure independence of observations. Patients lost to follow-up were excluded. Reproductive history includes infertility type –primary and secondary type and cause of infertility-male factor, female factor, both factor and unexplained infertility factor18. Female factors mainly include PCOS, tubal factors, uterine disorders, and endometriosis. Male factors mainly include oligospermia and asthenospermia and azoospermia.
Ethics statement
All methods were performed in accordance with the relevant guidelines and regulations. Approval for human retrospective analysis was obtained from the institutional Ethics Committee of Shanghai Ninth People’s Hospital and Wuhan Tongji Hospital. All participants provided informed consent after counseling for infertility treatments and routine IVF and ICSI procedures.
Patient and public involvement
This study involved the existing data and not included patients as study participants. There were no patients involved in setting the research question, the study design or the overall conduct of this study. No plans were involved patients in the dissemination of study findings.
Laboratory protocols
Oocytes were retrieved approximately 36 h after HCG administration. All the aspirated oocytes were fertilized with conventional insemination (IVF) or intracytoplasmic sperm injection (ICSI). Fertilization was assessed according to the presence of the pronuclear after 16–18 h insemination. One or two best-quality cleavage embryos for fresh cycle were choosed to transfer or cryopreservation 5 or 6 best-quality cleavage embryos according to the first cycle or not. The surplus embryos will be cultured continued and then the blastocyst were vitrified cryopreservation and thawed in FET cycles. In our study, we only analyzed the frozen-thawed blastocyst transfer. The cryoprotectant solution (KITAZATO@ Vitrofaction KIT, Japan) consisted of 15% dimethyl sulfoxide, 15% ethylene glycol and 0.5 M sucrose. We usually used laser objective and drill the zona pellucida through 2–3 laser pulses, then vitrified collapsed blastocysts within 30 min to prevent their re-expansion. When thawing blastocysts, the cryoprotectant dilutions (KITAZATO@ 129 Vitrofaction KIT, Japan) were sequential 1, 0.5, 0 M sucrose solutions. All the steps were carried out at room temperature except the first warming step (37 °C).
On day 5, 6, 7 of in vitro cultivation, blastocysts were evaluated morphologically according to Gardner and Schoolcraft´s classification19. The time of embryos observation is usually between 8 and 10 am. Blastocysts were assigned 3, 4, 5, 6 numeric score based on the degree of expansion and hatching status and ICM (A, B, C) and TE (A, B, C) evaluation was based on cell growth20,21.
In this study, blastocysts were classified into three groups: “good”, 3-6AA, 3-6AB, 3-6BA; “fair”, 3-6BB, 3-6AC, 3-6CA; “poor”, 3-6BC, 3-6CB, 3-6CC21,22,23. All embryos were cultured at 37 ° with 5% O2 and 6% CO2 concentration.
Embryo transfer and clinical outcomes
FET was performed after via a natural cycle or an artificial cycle. Women who have regular menstrual cycles underwent a natural cycle FET and women with irregular cycles an artificial cycle was offered with sequential oral administration of estradiol valerate and micronized vaginal progesterone as previously described24. The cycles with fertilization of IVF or ICSI followed by frozen-thawed embryo transfer (FET) cycles with single embryo transfer resulting in a single pregnancy and singleton live birth were included. In our study, the primary outcome measure was sex ratio which was calculated as the proportion of male newborns among all live births.
Statistical analysis
We performed matching using propensity scores calculated using logistic regression model in SPSS version 25.0. The inclusion criteria for good-fair-poor blastocyst groups was matched one-to-one on maternal age, maternal BMI, paternal age, duration of infertility, basal FSH, basal LH, type of infertility, main infertility cause (female factor, male factor, mixed factor and unexplained infertility), type of fertilization, day of embryo transfer which was found to potentially affect the sex ratio or significant different between these three groups. 791 of 1354 FET cycles of good blastocyst, 791 of 3592 FET cycles of fair blastocyst, 791 of 1415 FET cycles of poor blastocyst were obtained using nearest neighbor matching, with a caliper width of 0.02 and without replacement. All continuous data were presented as the mean and standard deviation (SD). Categorical variables were expressed as percentages and were analyzed using the chi-square test or Fisher’s exact test. A Binary logistic regression analysis was used to assess the association between the sex ratio of live birth and various morphological parameters. The data were reported as odds ratios (OR) and 95% confidence intervals (95% CI). A value of P < 0.05 was referred to as a statistical significance.
Ethics approval and consent to participate
Approval for human retrospective analysis was obtained from the institutional Ethics Committee of Shanghai Ninth People’s Hospital and Wuhan Tongji Hospital. All participants provided informed consent after counseling for infertility treatments and routine IVF and ICSI procedures.
Results
Demographics and basic characteristics
A total of 6361 singleton delivery offspring after frozen-thawed blastocyst transfer from two of the large reproductive centers in Shanghai and Wuhan of China were included in the cohort. 1354 good blastocyst, 3592 fair blastocyst and 1415 poor blastocyst were included. No significance were found in type of infertility, main infertility cause between these three groups (Supplementary Table 1). Also no significance were found in the rates of maternal age, paternal age, duration of infertility between good and fair blastocyst. However we found that there were significant differences in maternal age a, maternal BMI and paternal age between Good and Poor or Fair and Poor blastocysts. And Good, Fair and Poor blastocyst were significantly higher in IVF group than ICSI group. Also Day of embryo transfer was significant differences between these three groups (Supplementary Table 1). These differences may have effects on sex ratio outcomes, so we performed the propensity score matching to control for confounding parameters. After matching, there were 791 Good, 791 Fair and 791 Poor blastocysts in our data and the basic characteristic of these three groups were similar (P > 0.05, Table 1).
It was found that the sex ratio was significantly higher in IVF group than that of ICSI group before and after matching (56.6% versus 51.9%, P < 0.001; 57.4% versus 51.3%, P < 0.01, Supplementary Fig. 1A). Both Good and Poor quality blastocysts in IVF group resulted in a significant higher sex ratio than those in ICSI group before and after matching (P < 0.001, P < 0.05; P < 0.05, P < 0.05, respectively, Supplementary Fig. 1C). In addition, no difference was observed in birth weight between ICSI and IVF treated groups (Supplementary Fig. 1B). So we have reason to think that the matched data can be analyzed on behalf of all the data.
Association between blastocyst morphology parameters and sex ratio
We compared the possible influence of blastocyst quality on sex ratio. Transplantation of Good quality blastocyst resulted in a significantly higher sex ratio than those of poor-quality blastocyst (58.15% versus 51.71%, P = 0.011, odds ratio [OR], 1.295; 95% CI 1.062–1.581).
Since TE, ICM and degree of blastocyst expansion are kinetic parameters in morphological grades, we next explored their reference values for sex ratio. There was no significant difference among ICM grades with regard to sex ratio (Table 2). However, the group with A grade of TE achieved a much higher sex ratio of 61.68%, while the group with C grade of TE achieved a lower sex ratio of 49.35% (P < 0.001; odds ratio [OR], 1.653; 95% CI 1.281–2.134, Table 2). As well, the group with B grade of TE achieved higher sex ratio of 56.66% than that of C grade of TE (P = 0.003; odds ratio [OR], 1.322; 95% CI 1.098–1.593, Table 2). Moreover, blastocysts transferred with an expansion degree 6 had a 29.53% higher probability of sex ratio than those with an expansion degree 4 + 3 (P < 0.001; odds ratio [OR], 4.406; 95% CI 1.957–9.919, Table 2). Although, there was a decrease of sex ratio in the group of the expansion degree 5, no statistical differences were observed as compared with the group of expansion degree 4 + 3 (50.65% versus 54.91%, P = 0.1000.510, Table 2). We also found that there were no differences in sex ratio when transfer frozen-thawed blastocysts of day 5, day 6 or day 7 (55.07% versus 55.98% versus 45.45%, P = 0.935, P = 0.325, Table 2).
Effect of insemination type on sex ratio
To assess the effect of insemination type on sex ratio, blastocysts were allocated to IVF or ICSI derived group. It was found that the sex ratio was significantly higher in IVF group than that of ICSI group (Supplementary Fig. 1A). Both in IVF group and ICSI group, the blastocyst quality was significantly positive with the sex ratio (P < 0.05, Table 3). TE grades exhibited a strong effect on sex ratio likewise in both two groups (IVF group: 63.18% versus 58.62% versus 52.16%, P < 0.05; and ICSI group: 59.15% versus 52.52% versus 43.67%, P < 0.05, Table 3). Interestingly, blastocysts with expansion degree 6 in IVF group achieved a higher sex ratio (P = 0.001; odds ratio [OR], 4.957; 95% CI 1.919–12.805, Table 3), while in ICSI group, although blastocysts with expansion degree 6 has higher sex ratio than expansion degree 5 than expansion degree 4 + 3, there was no significantly differences between these three groups. In subgroup comparison, transferring blastocysts of day 6 had the highest sex ratio both in IVF and ICSI groups (57.48%, 52.63%, respectively, Table 3).
Interaction of insemination type and blastocyst morphology on sex ratio
To further explore the interaction of insemination type and blastocyst morphology on sex allocation, stratification analyses was performed and showed that good-quality and poor-quality blastocysts in IVF group resulted in a significant higher sex ratio than those in ICSI group (Supplementary Fig. 1C). Furthermore, there was a significant increase in sex ratio of IVF group both in A and B grade of ICM blastocysts as compared with ICSI group (ICM A: 60.13% versus 51.50%, P = 0.03; ICM B: 55.58% versus 50.48%, P = 0.031, Fig. 1A). In the perspective of TE grade, IVF-derived blastocysts with the B and C grade TE achieved higher sex ratio than those in ICSI group (58.62% versus 52.52%, P = 0.038; 52.16% versus 43.67%, P = 0.036, respectively, Fig. 1B). In addition, a higher sex ratio was observed in IVF-derived blastocysts with the expansion degree of 4 + 3 as compared with ICSI group (56.93% versus 51.04%, P = 0.008, Fig. 1C). Also, a 6.95% higher sex ratio in transferring blastocysts of day 5 in IVF group than those in ICSI group (57.46% versus 50.51%, P = 0.012, Fig. 1D).
Interaction of insemination type and blastocyst morphology on sex ratio. (A) Effects of ICM grade on sex ratio in IVF and ICIS treated groups. (B) Effects of TE grade on sex ratio in IVF and ICIS treated groups. (C) Effects of blastocysts expansion grade on sex ratio in IVF and ICIS treated groups. (D) Effects of blastocysts transfer day on sex ratio in IVF and ICIS treated groups. Data were presented with ratio with 95% confidence intervals (CIs). **P < 0.01, *P < 0.05, chi-square test.
Discussion
To our knowledge, this is the largest multicenter cohort study assessing the potential associations between morphology parameters of the frozen-thawed blastocysts and the sex ratio of singleton delivery offspring. We found that blastocyst quality, TE score, and expansion degree were associated with sex ratio both in IVF and ICSI groups, while no association was observed for ICM score and sex ratio. The major strength of this study is that we presented the sex allocation of a high number of competent blastocysts leading to a live birth from two big reproductive centers in China. The high number of outcome data led to relatively narrow CIs and therefore high precision of the results.
Although the studies about the association between blastocyst transfer and sex ratio published so far, some results are conflicting8,13,25,26,27,28. Here, our data showed that blastocyst transfer preferred male birth with 9.8% higher than female birth. It was reported that the female embryos with more active metabolism may be under oxidative stress29,30, which might make them more liable for growth retardation and poor quality than male embryos. Although high glucose metabolism was also observed in the male embryo from morula to blastocyst both in humans and cows31,32, these male embryos could develop to robust blastocysts probably due to being less susceptible to oxidative stress33. Therefore, more male blastocysts may be selected for fresh or frozen-thaw embryo transfer cycles, leading to a bias sex ratio.
Based on the morphology grading, competent blastocysts are selected for transfer at our IVF centers. To achieve satisfying pregnancies, those blastocysts showing complete expansion of the blastocoel cavity and hatching potential with good numbers of cells in both the TE and ICM were preferred for transfer. As a consequence, transfer of blastocysts with high quality contributed to more male birth in our study as well as reported previously8. Borgstrom et al. conducted a cohort study of 4842 deliver offspring from fresh or frozen-thawed embryo transfer and found that TE A blastocysts as compared with TE B blastocysts had a 31% increased probability of being a boy17. We also observed a significant increasing trend in the sex ratio in the context of TE A and B blastocysts transfer as compared with TE C blastocysts transfer both in ICSI and IVF groups, suggesting that high TE quality may increase the proportion of male infants. Similarly, blastocysts with expansion degree 6 owned a higher sex ratio, which is consistent in comparison of mixed group of IVF and ICSI derived blastocysts or IVF derived blastocysts alone. However, the ICSI derived blastocysts transfer with expansion degree 5 rather than 6 showed a higher sex ratio, which might be relative with the small number of samples in this subgroup. Interestingly, no statistically significant associations between ICM scores and sex ratio were found both in IVF and ICSI groups, although ICM was an important sign for high quality blastocysts.
It was reported that insemination type also affect sex ratio34,35,36,37. Here, we found that fewer male live-born infants are conceived via ICSI than IVF just as reported previously9,38,39. ICSI is the method of choice in cases with reduced sperm activity. In general, normal sperm with morphologically good are preferentially selected under high magnification for ICSI and thus induced female-biased births, since the percentage of sperm with good morphology carrying an X chromosome is higher39. Compared with IVF treated group, both the overall quality grading and the individual parameter score, including ICM, TE, and expansion degree, promoted the bias of sex ratio favoring female in ICSI treated group. These results indicated that the balance of sex ratio might be affected by insemination procedure and blastocyst morphology simultaneously or interactively.
As a large multicenter study, the limitation of the current study comes from the incomplete parent characteristic information for the different electrical data system. In addition, about half of the thawed blastocysts (from the center of Shanghai) were derived from the non-top quality cleavage embryos due to the freeze-all or fresh transfer strategy for all top quality embryos on day 3. It should be considered that the blastocysts with good quality would be higher if all embryos were cultured for blastocyst. As a consequence, the sex ratio favoring male would be higher possibly.
Perspective and significance
This large multicenter cohort study expands the current knowledge on associations between blastocyst morphology parameters and sex allocation of singleton delivery offspring. We demonstrated that the higher quality blastocysts resulted in a higher sex ratio after single frozen-thawed blastocyst transfer. TE score and expansion degree of blastocysts were significantly associated with sex ratio at birth, while ICM score showed no association with sex ratio whatever in ICSI or IVF treated groups. However, as compared with IVF treatment, all morphology parameters promoted the sex ratio bias favoring female in ICSI treated group, indicating the interaction of insemination type and blastocyst morphology on sex allocation. Embryologists should be aware of the effects of certain protocols on sex distribution, including blastocyst transfer and insemination method, in order to maintain the equilibrium of the sex ratio among offspring without affecting the pregnancy rate.
Data availability
The data used for this study comes from department of assisted reproduction of Shanghai Ninth People’s hospital and Tongji Hospital. Please contact corresponding author for data requests.
References
Jacobsen, R., Moller, H. & Mouritsen, A. Natural variation in the human sex ratio. Hum. Reprod. 14, 3120–3125. https://doi.org/10.1093/humrep/14.12.3120 (1999).
Trivers, R. L. & Willard, D. E. Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92. https://doi.org/10.1126/science.179.4068.90 (1973).
Catalano, R., Bruckner, T. & Smith, K. R. Ambient temperature predicts sex ratios and male longevity. Proc. Natl. Acad. Sci. U. S. A. 105, 2244–2247. https://doi.org/10.1073/pnas.0710711104 (2008).
Radin, R. G. et al. Sex ratio following preconception low-dose aspirin in women with prior pregnancy loss. J. Clin. Investig. 125, 3619–3626. https://doi.org/10.1172/JCI82357 (2015).
Fukuda, M., Fukuda, K., Shimizu, T., Andersen, C. Y. & Byskov, A. G. Parental periconceptional smoking and male: Female ratio of newborn infants. Lancet (London, England) 359, 1407–1408. https://doi.org/10.1016/S0140-6736(02)08362-9 (2002).
Moller, H. Change in male:female ratio among newborn infants in Denmark. Lancet (London, England) 348, 828–829. https://doi.org/10.1016/S0140-6736(05)65253-1 (1996).
Maalouf, W. E., Mincheva, M. N., Campbell, B. K. & Hardy, I. C. Effects of assisted reproductive technologies on human sex ratio at birth. Fertil. Steril. 101, 1321–1325. https://doi.org/10.1016/j.fertnstert.2014.01.041 (2014).
Luna, M. et al. Blastocyst embryo transfer is associated with a sex-ratio imbalance in favor of male offspring. Fertil. Steril. 87, 519–523. https://doi.org/10.1016/j.fertnstert.2006.06.058 (2007).
Hentemann, M. A., Briskemyr, S. & Bertheussen, K. Blastocyst transfer and gender: IVF versus ICSI. J. Assist. Reprod. Genet. 26, 433–436. https://doi.org/10.1007/s10815-009-9337-3 (2009).
Lou, H. et al. Does the sex ratio of singleton births after frozen single blastocyst transfer differ in relation to blastocyst development?. Reprod. Biol. Endocrinol. 18, 72. https://doi.org/10.1186/s12958-020-00623-x (2020).
Luke, B. et al. The sex ratio of singleton offspring in assisted-conception pregnancies. Fertil. Steril. 92, 1579–1585. https://doi.org/10.1016/j.fertnstert.2008.08.107 (2009).
Mohammad, N. S., Nazli, R., Zafar, H. & Fatima, S. Effects of lipid based multiple micronutrients supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 38, 219–226. https://doi.org/10.12669/pjms.38.1.4396 (2022).
Weston, G., Osianlis, T., Catt, J. & Vollenhoven, B. Blastocyst transfer does not cause a sex-ratio imbalance. Fertil. Steril. 92, 1302–1305. https://doi.org/10.1016/j.fertnstert.2008.07.1784 (2009).
Gardner, D. K., Lane, M., Stevens, J., Schlenker, T. & Schoolcraft, W. B. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil. Steril. 73, 1155–1158. https://doi.org/10.1016/s0015-0282(00)00518-5 (2000).
Baatarsuren, M. et al. The trophectoderm could be better predictable parameter than inner cellular mass (ICM) for live birth rate and gender imbalance. Reprod. Biol. 22, 100596. https://doi.org/10.1016/j.repbio.2021.100596 (2022).
Ebner, T. et al. Quantitative and qualitative trophectoderm grading allows for prediction of live birth and gender. J. Assist. Reprod. Genet. 33, 49–57. https://doi.org/10.1007/s10815-015-0609-9 (2016).
Borgstrom, M. B. et al. Developmental stage and morphology of the competent blastocyst are associated with sex of the child but not with other obstetric outcomes: A multicenter cohort study. Hum. Reprod. 37, 119–128. https://doi.org/10.1093/humrep/deab242 (2021).
Chen, M. et al. The sex ratio of singleton and twin delivery offspring in assisted reproductive technology in China. Sci. Rep. 7, 7754. https://doi.org/10.1038/s41598-017-06152-9 (2017).
Gardner, D. K. & Schoolcraft, W. B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 11, 307–311. https://doi.org/10.1097/00001703-199906000-00013 (1999).
Schoolcraft, W. B. et al. Blastocyst culture and transfer: Analysis of results and parameters affecting outcome in two in vitro fertilization programs. Fertil. Steril. 72, 604–609. https://doi.org/10.1016/s0015-0282(99)00311-8 (1999).
Zhao, Y. Y., Yu, Y. & Zhang, X. W. Overall blastocyst quality, trophectoderm grade, and inner cell mass grade predict pregnancy outcome in euploid blastocyst transfer cycles. Chin. Med. J. 131, 1261–1267. https://doi.org/10.4103/0366-6999.232808 (2018).
Irani, M. et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil. Steril. 107, 664–670. https://doi.org/10.1016/j.fertnstert.2016.11.012 (2017).
Capalbo, A. et al. Correlation between standard blastocyst morphology, euploidy and implantation: An observational study in two centers involving 956 screened blastocysts. Hum. Reprod. 29, 1173–1181. https://doi.org/10.1093/humrep/deu033 (2014).
Zhang, J. et al. Effect of endometrial thickness on birthweight in frozen embryo transfer cycles: An analysis including 6181 singleton newborns. Hum. Reprod. 34, 1707–1715. https://doi.org/10.1093/humrep/dez103 (2019).
Chang, H. J., Lee, J. R., Jee, B. C., Suh, C. S. & Kim, S. H. Impact of blastocyst transfer on offspring sex ratio and the monozygotic twinning rate: A systematic review and meta-analysis. Fertil. Steril. 91, 2381–2390. https://doi.org/10.1016/j.fertnstert.2008.03.066 (2009).
Menezo, Y. J., Chouteau, J., Torello, J., Girard, A. & Veiga, A. Birth weight and sex ratio after transfer at the blastocyst stage in humans. Fertil. Steril. 72, 221–224. https://doi.org/10.1016/s0015-0282(99)00256-3 (1999).
Csokmay, J. M. et al. Live birth sex ratios are not influenced by blastocyst-stage embryo transfer. Fertil. Steril. 92, 913–917. https://doi.org/10.1016/j.fertnstert.2008.07.1741 (2009).
Richter, K. S., Anderson, M. & Osborn, B. H. Selection for faster development does not bias sex ratios resulting from blastocyst embryo transfer. Reprod. Biomed. Online 12, 460–465. https://doi.org/10.1016/s1472-6483(10)61999-2 (2006).
Proctor, H. J. & Acai, S. A. Jr. Assets and liabilities of helicopter evacuation in support of emergency medical services. N. C. Med. J. 37, 25–28 (1976).
Perez-Crespo, M. et al. Differential sensitivity of male and female mouse embryos to oxidative induced heat-stress is mediated by glucose-6-phosphate dehydrogenase gene expression. Mol. Reprod. Dev. 72, 502–510. https://doi.org/10.1002/mrd.20366 (2005).
Tiffin, G. J., Rieger, D., Betteridge, K. J., Yadav, B. R. & King, W. A. Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. J. Reprod. Fertil. 93, 125–132. https://doi.org/10.1530/jrf.0.0930125 (1991).
Jimenez, A. et al. Experimental demonstration that pre- and post-conceptional mechanisms influence sex ratio in mouse embryos. Mol. Reprod. Dev. 66, 162–165. https://doi.org/10.1002/mrd.10345 (2003).
Nagata, C. et al. Sex ratio of infants born through in vitro fertilization and embryo transfer: Results of a single-institution study and literature review. JBRA Assist. Reprod. 25, 337–340. https://doi.org/10.5935/1518-0557.20200096 (2021).
Arikawa, M., Jwa, S. C., Kuwahara, A., Irahara, M. & Saito, H. Effect of semen quality on human sex ratio in in vitro fertilization and intracytoplasmic sperm injection: An analysis of 27,158 singleton infants born after fresh single-embryo transfer. Fertil. Steril. 105, 897–904. https://doi.org/10.1016/j.fertnstert.2015.12.009 (2016).
Bu, Z. et al. Live birth sex ratio after in vitro fertilization and embryo transfer in China–an analysis of 121,247 babies from 18 centers. PloS One 9, e113522. https://doi.org/10.1371/journal.pone.0113522 (2014).
Tarin, J. J., Garcia-Perez, M. A., Hermenegildo, C. & Cano, A. Changes in sex ratio from fertilization to birth in assisted-reproductive-treatment cycles. Reprod. Biol. Endocrinol. 12, 56. https://doi.org/10.1186/1477-7827-12-56 (2014).
Cai, H., Ren, W., Wang, H. & Shi, J. Sex ratio imbalance following blastocyst transfer is associated with ICSI but not with IVF: An analysis of 14,892 single embryo transfer cycles. J. Assist. Reprod. Genet. 39, 211–218. https://doi.org/10.1007/s10815-021-02387-8 (2022).
Maalouf, W., Maalouf, W., Campbell, B. & Jayaprakasan, K. Effect of ethnicity on live birth rates after in vitro fertilisation/intracytoplasmic sperm injection treatment: Analysis of UK national database. BJOG 124, 904–910. https://doi.org/10.1111/1471-0528.14241 (2017).
Setti, A. S., Figueira, R. C., Braga, D. P., Iaconelli, A. Jr. & Borges, E. Jr. Gender incidence of intracytoplasmic morphologically selected sperm injection-derived embryos: A prospective randomized study. Reprod. Biomed. Online 24, 420–423. https://doi.org/10.1016/j.rbmo.2012.01.007 (2012).
Acknowledgements
We gratefully acknowledge the staff of Department of Assisted Reproduction in Shanghai Ninth´s Hospital and Tongji Hospital in this study for their contribution.
Funding
This study was financially supported by the Natural Science Foundation of Shanghai (Grant number 19ZR1429300, awarded to HL).
Author information
Authors and Affiliations
Contributions
Tiantian Wang and Mingru Yin wrote the manuscript and analyzed the data. Lixia Zhu developed the experimental designs and analyzed the data. Jing Dong, Weina Yu and Wei Jin participated in data interpretation and critical discussion. Lei Jin and Qifeng Lyu revised the manuscript and analyzed the data. Hui Long developed the experimental designs, revised the manuscript and analyzed the data. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, T., Zhu, L., Yin, M. et al. Sex ratio shift after frozen single blastocyst transfer in relation to blastocyst morphology parameters. Sci Rep 14, 9539 (2024). https://doi.org/10.1038/s41598-024-59939-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59939-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.