Abstract
Chickpea is a highly nutritious protein-rich source and one of the major crops to alleviate global malnutrition, but poor seed quality affects its productivity. Seed quality is essential for better crop establishment and higher yields, particularly in the uncertain climate change. The present study investigated the impact of botanical priming versus hydropriming and bavistin seed treatment on chickpea seeds. A detailed physiological (germination percentage, root and shoot length, vigour index) and biochemical (amylase, protease, dehydrogenase, phytase, and lipid peroxidation) analysis was carried out in order to assess the effect of priming treatments. Turmeric-primed seeds showed better germination rate (94.5%), seedling length, enzyme activity, and lower malondialdehyde (MDA) content. Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis revealed the expression of minor polypeptides of albumin and globulin in the primed seeds. Moreover, field experiments indicated increased crop growth, vigour, days to 50% flowering, yield and its attributing traits in turmeric-primed seeds. Botanical priming can increase chickpea yield by up to 16% over the control group. This low-cost and eco-friendly technique enhances seed and crop performance, making it a powerful tool for augmenting chickpea growth. Therefore, chickpea growers must adopt botanical priming techniques to enhance the quality of seed and crop performance. Moreover, this approach is environmentally sustainable and can help conserve natural resources in the long term. Therefore, this new approach must be widely adopted across the agricultural industry to ensure sustainable and profitable farming practices.
Similar content being viewed by others
Introduction
Global climate change and water scarcity generally affect crop performance from germination and eventually reduce grain yield and quality with losses of up to 40–50% in crop productivity. Chickpea, a cool-season legume, are an excellent source of protein (18–20%) and the minerals phosphorus, calcium, magnesium, iron, and zinc that play a significant role in achieving global food security1. Over 90% of the world's chickpeas are grown in arid and semi-arid regions, making them vulnerable to various stresses2,3. Even though it is considered the candidate crop for rainfed conditions, the uncertain climatic condition adversely affects the crop's performance and makes it vulnerable to several biotic and abiotic stresses4.
Successful seedling and crop establishment are critical to higher crop production5. Poor seedling establishment is one of the most significant barriers primarily caused by subpar seed quality6. The improper storage is the primary factor for quick loss of seed vigour, which ultimately affects germination and crop performance. Moreover, crop germination due to adverse climatic conditions and delayed sowing affects production and productivity. Seed quality enhancement techniques provide a comprehensive solution to unlock the full genetic potential of seeds. Seed quality enhancement, a pre-sowing treatment, enhances germination and crop vigour during early planting and contributes to a higher crop yield7.
Seed priming, a technique in seed enhancement, involves the initial absorption of water by seeds to initiate the early stages of germination. However, this absorption is insufficient for radical emergence, and the seeds return to their original moisture content8. During the priming process, biochemical changes occur, including activating enzymes, producing compounds that promote growth, metabolizing molecules that inhibit germination, and repairing damaged cells9. The imbibition of seeds triggers a cascade of metabolic processes, such as the activation of hydrolytic enzymes (such as amylase, protease, lipase, dehydrogenase, and phytase), which lead to the hydrolysis of stored starch, lipids, proteins, polyphosphates, and other storage materials, converting them into simpler forms that readily absorbed by the embryo and ultimately influencing seed vigour10. Alpha-amylase breaks down starch into sugars for the developing embryo11. Proteolytic enzymes use seed storage proteins12, while dehydrogenase catalyzes the stored products during the anaerobic phase of seed germination13.
Phytase is an enzyme that unequivocally converts phytate into inositol/phosphoric acid, making a remarkable contribution to seed germination and growth14. On the other hand, lipid peroxidation is an unequivocally detrimental process that destabilizes the membrane and degrades proteins, leading to inevitable cell death, which hinders the capacity for ionic transport15. Similarly, the hydrolysis of seed storage proteins such as globulin and albumin unequivocally generates free amino acids, essential for germination and seed vigour16.
Over the past few decades, there has been a significant increase in the utilization of non-hazardous chemicals and fertilizers as alternatives to enhance crop productivity in the agriculture system. Among these alternatives, priming has emerged as a highly effective method to tackle this issue. Numerous efficacious priming agents, such as salts, polyamines, hormones, compatible solutes, and aqueous plant extracts, have been documented by various researchers17. Botanical priming, a priming technique that employs plant extracts as its agents, has proven particularly advantageous. This botanical priming method can stimulate metabolic processes, is non-toxic and environmentally friendly, and has excellent potential to control pathogenic microorganisms.
There are few reports on using turmeric rhizome and neem seed extracts for aphid control, plant growth, and crop yield. However, the effect of leaf extracts on seed physiology and plant growth in pulse crops has yet to be studied. No studies have investigated globulin and albumin expression in chickpeas during priming and germination at different durations. In the present investigation, we hypothesized that, first, the effect of turmeric and neem leaf extracts as seed priming agents positively influences physiological parameters and enhances seed vigour in chickpeas. Second, the improved enzymatic activities during botanical priming lead to increased seed germination and growth. Third, priming also increases the expression of different seed storage proteins compared to non-primed conditions. Our objective was to investigate the effect of botanical priming on physiological, biochemical and yield in chickpeas, for which we performed comprehensive laboratory and field experiments.
Results
Effect of botanical priming on seed germination and seedling characteristics
The present study was carried out to analyze the impact of seed priming with botanicals (turmeric, neem) on the physiological parameters of chickpeas and compare it with conventional hydropriming and fungicide seed treatment. In the present investigation, physiological parameters, viz germination percentage and seedling characteristics, were significantly increased by priming the seeds with botanicals, as shown in Table 1. The botanical primed seed had substantially higher seed germination and seedling growth than the control. Seeds primed with turmeric and neem leaves aqueous extract had higher germination and seedling growth followed by hydro priming. The results demonstrate that turmeric had a higher germination percentage (94.5%) than control (82.5%), indicating that botanical priming successfully enhanced the germination rate by 15% over control. Seedling characteristics, including root length, shoot length, seedling length, seedling dry weight and vigour indices, were significantly similarly affected by botanical priming. Table 1 shows marked enhancement in seedling length by 29%, vigour index Ι by 42% and vigour index II by 63% were observed in turmeric primed seed over control. Seed physiological parameters, such as seedling length, vigour index I, and vigour index II, were significantly affected by turmeric priming compared to other treatments such as neem and hydropriming. As illustrated in Table 1 and supplementary information file 1, a marked enhancement in seedling length by 8.6%, vigour index I by 30.4%, and vigour index II by 21.8% was observed in turmeric-primed seeds compared to those treated with neem
Biochemical changes upon botanical priming
The dynamic changes in hydrolytic enzymes and MDA content are shown in Figs. 1, 2, 3, 4, 5 and supplementary information file 2. Figure 1 shows that botanical priming and hydropriming significantly affected amylase activity, which increased with the increase in priming duration. In contrast, the amylase activity was decreased as the germination proceeded. Conversely, the amylase activity was constant at different priming hours in non-primed and bavistin-treated seeds; however, they showed a similar decreasing trend during germination as botanicals primed seed. Compared to non-primed seeds, maximum amylase activity was observed in turmeric-primed seeds, and it was increased to 4.8-fold at 12 h and 5.2-fold at 18 h of priming. The amylase activity was also assessed during germination, and it showed a decreasing trend with the increasing days of germination, and it was highest in turmeric primed seed. The highest amylase activity, 0.71 mg maltose/min, was observed on the first day of germination on the first day, and its activity decreased to 1.7-fold on the third day and 2.0-fold on the fifth day of germination.
Effect of different priming agents on amylase activity during 6 h, 12 h and 18 h duration of priming and 1st, 3rd, and 5th days of germination in primed and non-primed seed of chickpea variety PG-186. Data presented are means of four replicates with standard deviation. Different letters indicate significant differences within each treatment by Post Hoc test at P = 0.05 levels.T1 = No priming (control), T2 = Conventional practice (Bavistin@2 g/kg), T3 = Turmeric leaf priming, T4 = Neem leaf priming, T5 = On-farm priming (Hydropriming).
Effect of different priming agents on protease activity during 6 h, 12 h and 18 h duration of priming and 1st, 3rd, and 5th days of germination in the primed and non-primed seed of chickpea variety PG-186. Error bars are the representative of the standard deviation of four replicates. Different letters indicate significant differences by Post Hoc test at P = 0.05 levels within the treatment. T1 = No priming (control), T2 = Conventional practice (Bavistin@2 g/kg), T3 = Turmeric leaf priming, T4 = Neem leaf priming, T5 = On-farm priming (Hydropriming).
Effect of botanical priming on dehydrogenase activity during 6 h, 12 h and 18 h duration of priming and 1st, 3rd, the 5th days of germination in primed and non-primed seed of chickpea variety PG-186.vertical bar presented are means of four replicates with standard deviation. Different letters indicate significant differences within each treatment by Post Hoc test at P = 0.05 levels. T1 = No priming (control), T2 = Conventional practice (Bavistin@2 g/kg), T3 = Turmeric leaf priming, T4 = Neem leaf priming, T5 = On-farm priming (Hydropriming).
Effect of different priming agents on phytase activity during 6 h, 12 h and 18 h duration of priming and 1st, 3rd, and 5th days of germination in primed and non-primed seed of chickpea variety PG-186. Error bars are the representative of the standard deviation of four replicates. Different letters indicate significant differences within each treatment by Post Hoc test at P = 0.05 levels. T1 = No priming (control), T2 = Conventional practice (Bavistin@2 g/kg), T3 = Turmeric leaf priming, T4 = Neem leaf priming, T5 = On-farm priming (Hydropriming).
Effect of different priming agents on MDA content during 6 h, 12 h and 18 h duration of priming and 1st, 3rd, and 5th days of germination in primed and non-primed seed of chickpea variety PG-186. Data presented are means of four replicates with the standard deviation. Different letters indicate significant differences within each treatment by Post Hoc test at P = 0.05 levels. T1 = No priming (control), T2 = Conventional practice (Bavistin@2 g/kg), T3 = Turmeric leaf priming, T4 = Neem leaf priming, T5 = On-farm priming (Hydropriming).
Botanical priming significantly affected the protease activity (P < 0.05; Fig. 2). The protease activity significantly increased with increasing priming duration and till the third day of germination; after that, on the fifth day of germination, irrespective of treatments, the protease activity decreased. The protease activity was maximum during priming durations and germination days in seeds primed with 1% aqueous extract of turmeric leaves. Its activity increased to 1.3-fold in 6 h, 1.8-fold in 12 h, two fold in 18 h, 1.2-fold on the first day and 3.2-fold on the third day compared to control in turmeric primed seed. On the fifth day, there was a drastic reduction in the protease activity, which decreased by 1.8-fold compared to the third day of the same treatment. A similar pattern was observed in 1% aqueous extract of neem leaves and hydro-priming, whereas activity was constant throughout the priming duration in control and bavistin-treated seed.
In the current study, botanical priming significantly affected dehydrogenase activity (P < 0.05; Fig. 3) and differed among treatments. The dehydrogenase activity was significantly increased with priming duration and days of germination. The maximum dehydrogenase activity of 0.98 mg formazan/min was observed on the fifth day of germination in seeds primed with 1% aqueous extract of turmeric leaves. Its activity increased to 1.84-fold in 18 h and 1.3-fold on the fifth day of germination compared to the control.
To confirm the activation of dormant phytase zymogens induced by botanical priming, we investigated the dynamic changes in phytase activity during priming durations and days of germination. As shown in Fig. 4, the phytase activity significantly increased with increasing priming duration and days of germination. The maximum activity was observed in turmeric primed seed during the fifth day of germination, and it was 0.74 µM trypsin/min (1.9-fold increased over control at a particular time). Compared to the control, its activity in turmeric primed seed increased to 1.7-fold in 6 h, 2.4-fold in 12 h, and 2.9-fold in 18 h of priming. The phytase remained active during germination and increased by 2-fold on the first day, 2.1-fold on the third day and 1.9-fold on the fifth day of germination.
Botanically primed seed showed a significant reduction in lipid peroxidation activity, measured in terms of MDA content (µmol MDA/gr F-W). In the present study, as shown in Fig. 5, on increasing the priming duration, the MDA content was not found to change up to 12 h in turmeric primed seed; after that, it decreased sharply at 18 h of priming and during days of germination. Compared to the control, the MDA content decreased to 2.49-fold in 18 h of priming, 3.4-fold on the first day, four fold on the third day, and 3.6-fold on the fifth day of germination and the maximum MDA content was observed in the case of the control seed (T1).
Effects of botanical priming on total protein and the expression of seed storage protein
The impact of botanical priming on total protein content was significant in our present work; as illustrated in Fig. 6, a considerable upsurge (p < 0.05) in protein content was evident during priming hours and days of germination with both turmeric and neem leaf aqueous extract. In this, the total protein content increased with the priming duration up to the first day of germination. However, upon increasing the days of germination, irrespective of the priming material, the total protein decreases significantly from the third to the fifth days. Meanwhile, in the control and bavistin-treated seeds, protein content did not vary much throughout the priming duration and during germination. The total protein content increased 1.4-fold on the first day after that, decreased to 1.1-fold on the third day and one fold on the fifth day of germination compared to the control. Seed storage proteins (SSP) of chickpeas, i.e., globulin and albumin, were fractionalized by the Osborne method18 and the molecular weight of the protein was determined using SDS PAGE. Subunits separated from chickpea proteins are shown in Table 2. Supplementary information file 4 and Figure 7 shows that the expression of proteins was higher in primed seeds than in the control. In the present work, the estimated molecular weights of subunits of globulin, i.e., legumin, were (40 kDa, 39 kDa, 26 kDa, 23 kDa), vicillin Mw (50 kDa, 39 kDa, 19 kDa, 15 kDa), and glutelin (58 kDa, 55 kDa, 54 kDa). Three minor bands of 36 kDa, 42 kDa, and 56 kDa, the globulin subunits, were detected in the electrophoregram. Table 2 also reveals that the significant globulin subunits, i.e., 42 kDa and 56 kDa, were present in all primed seeds, whereas control and bavistin polypeptides of 42 kDa proteins were not observed. Our work in SDS-PAGE showed a progressive accumulation of the 11-S globulin during priming. In contrast, a slower accumulation of 11S occurred in control seeds, which resulted in an intense polypeptide of 56 kDa and 42 kDa in primed seeds.
Impact of different priming treatments on total protein content in chickpea during 6 h, 12 h and 18 h duration of priming and 1st, 3rd, and 5th days of germination in primed and non-primed seed of chickpea variety PG-186. Data presented in each column are means of four replicates with standard deviation within treatments. Different letters indicate significant differences by post-hoc test at the P = 0.05% level. T1 = No priming (control), T2 = Conventional practice (Bavistin@2 g/kg), T3 = Turmeric leaf priming, T4 = Neem leaf priming, T5 = On-farm priming (Hydropriming).
SDS- PAGE (12%) of chickpea seed storage protein extracted and characterized by Osbornes methods using the borate buffer. Shows the effect of different priming treatments on the expression of globulin and albumin subunits during 6,12,18 h of priming.12 kDa and 14 kDa are the subunits of albumin and 36 kDa, 42 kDa and 56 kDa are the globulin subunits. Seed treatments include T1 = No priming (control), T2 = Conventional practice (Bavistin@2 g/kg), T3 = Turmeric leaf priming, T4 = Neem leaf priming, T5 = On-farm priming (Hydropriming).
Effects of botanical priming on plant establishment, growth and yield of chickpea
An experiment with turmeric leaf extract priming in chickpea was compared with conventional seed treatment (+ control) and non-priming (control) under field conditions and data provided in supplementary information file 3. Table 3 shows that turmeric priming significantly improved field emergence, plant height at different growth stages, days to 50% flowering, and yield attributing characters (Tables 3 and 4). Turmeric priming significantly increased the field emergence by 27%, plant heights at 15 DAS, 45 DAS, and maturity by 5%, 11%, and 4.7%, respectively, compared to the non-priming. About the days to 50% flowering, it was significantly (p < 0.05) affected by priming and obtained three days earlier than in the control plant (Table 3). The effect of turmeric leaf priming on yield-attributing characteristics in chickpeas is shown in Table 4, which presents the data on-field performance, where turmeric priming considerably increased the number of pods per plant, biological yield, economic yield, and harvest index compared to the control. In comparison to the control, turmeric priming significantly raised, the number of pods per plant by 31%, the biological yield by 22% and the harvest index by 22.1%.
Discussion
Seed germination is an intricate process involving several physiological and biochemical changes modulated by several biochemical enzymes, and seed priming has been proven to alleviate the adverse effects of any stress during early seedling establishment. The present study suggested that seeds primed with botanicals accelerated seed germination rate and significantly enhanced seed vigour, as indicated by longer root lengths, shoot lengths, seedling length and seedling dry weight compared with the control (Table 1). Our findings are similar to previous results of botanical priming on black gram using neem and prosopus, demonstrating a significant increase in standard germination, shoot length, root length, seedling length and vigour19. Various physiological, biochemical, and molecular changes occurred during seed priming, contributing to improved germination rate and seedling vigour under various environmental conditions20. The increase in seedling characteristics was due to enhanced enzyme activity from bioactive substances like curcumin and phenols in the turmeric leaf extract. Similar results were reported in greengram21, clusterbean22, Vigna sinensis23, maize23 and wheat24. The increase in dry weight with botanical treatment may be due to the faster growth and development of seedlings and the hike in vigour index25. The high vigour index in botanically primed seeds may be due to growth-promoting compounds and secondary metabolites translocated during seedling growth. Priming improves chickpea starch metabolism by increasing amylase content during germination (Fig. 1). Similar results were obtained by Mukasa and coworkers in sugar beets26, where the level of amylase activity in primed sugar beets was 1.9 to 11.5 times higher than in the control group. In similar work on rice, seed priming increases the α-amylase activity and total soluble sugar content, resulting in a higher starch degradation process under chilling stress27.
Seed priming induced primary memory, which activates pre-germinative metabolism in seed that triggers gibberellin biosynthesis, antioxidants28,29, protein synthesis28, amylase and protease activation (Fig. 2), which helps in radical protrusion and enhances the antioxidant defense system against DNA damage30. Proteins stored in the seed are utilized during germination to provide amino acids and amides for the embryo's development. Proteases play a crucial role in protein degradation during the maturation process. Protease activity in chickpea declines after the 5th day of germination due to accelerated enzyme degradation. During Initial germination phase, facilitates the mobilization of stored proteins into amino acids and peptides for seedling growth. As the seedling develops and transitions to active growth, the demand for stored proteins decreases; henceforth, the decline in activity in proteases is observed. Protease activity was found highest in primed seeds mainly due to botanical priming (Fig. 2), which showed improved nitrogen metabolism in primed seeds, as reported in the pearl millet31. In our investigation, increasing priming duration led to enhanced protease activity (Fig. 2); similar results were obtained in beans where the proteolytic enzyme activity increased during the first seven days of seed germination32. Dehydrogenase enzymes are essential components of the electron transport chain, facilitating the transport of electrons and ATP production33, and their activity is considered a positive biomarker for testing seed viability and vigour34. The present study observed a relatively high amount of dehydrogenase after priming. Similar trends were reported in cucumber35 and cowpea36, suggesting the role of priming in accelerating dehydrogenase activity.
Seeds store phytic acid (phytate), the phosphorous storage form in the plants37, which can bind with essential cations like calcium, magnesium, and zinc, reducing their availability for digestion38. However, phytic acid is broken down by the enzyme phytase during germination, releasing cations, phosphates, and inositol utilized by the seedlings39. Priming significantly increased the activity of the phytase enzyme (Fig. 4); this may result from the de novo synthesis of phytase during germination40. A similar trend was reported for germinating rice41, lupin42, barley40 and soybean43. A maximum of seven fold increase in phytase activity was observed on the 10th day of germination in rice41. Significant differences in the phytase activity of wheat, rye, barley, and oats grains were observed, with rye grains showing the highest activity and oats being the lowest. After four days, wheat, barley, and oat activities increased approximately 4.5, 6, and nine fold, respectively, and rye activities increased approximately 2.5-fold after three days of germination44.
Seed priming significantly decreased the rate of lipid peroxidation in terms of MDA content (Fig. 5). MDA content was reduced after priming due to increased antioxidant enzyme activity45. In similar work, malondialdehyde content was 9% lower in primed pea seeds at 42 h of germination against unprimed seeds46. This decreased level of MDA indicated reduced lipid peroxidation, which helps maintain the integrity of the membrane in primed seeds47. In our finding, the protein content was significantly affected upon botanical priming, where the protein content was increased during priming and subsequently decreased during germination (Fig. 6). This was in accordance with the reports documented from black chickpea primed with MgO nanoparticles28. After priming, two minor bands of 12 kDa and 14 kDa subunits of albumin were identified (Fig. 7); our findings are similar to previous research48, where it was reported that chickpea 2S albumin (∼20 kDa) is composed of two polypeptides of 10 and 12 kDa. In similar findings, it was hypothesized that the extra peptide could be a peptide of 2S albumin with Mw 4–10 kDa49. Similar trends were reported in cucumber35 and black beans50. The probable reason for the extra one band in all treated seeds is priming, resulting in the synthesis of lost proteins and some new ones.
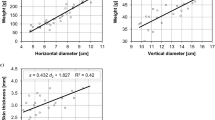
Chickpea seeds primed with aqueous leaf extract of turmeric had higher field emergence and plant heights during early establishment and at maturity than the control (Table 3). In the present study, turmeric leaf extract priming improved the seedling growth attributes by triggering the biosynthesis of nucleic acid, proteins, and hydrolytic enzymes and consequentially enhanced the cell division, cell enlargement, and metabolic activity and increased the photosynthetic process of the plant, resulting in increased uptake of more nutrients by efficient and more robust roots (Fig. 8). In similar works on nanoparticle priming, AgNPs accumulated in the seeds might activate the metabolic events vital for seed germination and seedling growth51. Significant differences were observed in the number of pods, seed yield, and harvest index in turmeric primed seed over control (Table 4). The results are in accordance with the studies on maize, where seeds primed with prosopis and moringa leaf extracts led to higher seed yield and yield-related parameters52. Physiologically active substances in the turmeric leaf may have activated the embryo growth, resulting in early seedling emergence from the soil. The early growth of roots is vital for establishing a robust and efficient root system, which contributes to the development of higher seedling vigour. The elasticity of the cell walls plays a significant role in ensuring effective water absorption, which is essential for the healthy growth of plants. As a result, the seedlings are better equipped to cope with the challenges of their environment, leading to more robust and healthier plant growth53. A similar observation was made in blackgram25, greengram54 and okra55.
Conclusions
This study conclusively demonstrates the effectiveness of botanical priming as a low-cost and eco-friendly technique to improve seedling attributes, hydrolytic enzymes, and yield significantly. Multiple lab and field studies suggest that botanicals can internalize seed coats and support water uptake inside seeds, accelerating various enzymatic activities and promoting seed germination. The study also indicated that priming with botanicals increases the root length due to the presence of phenol, which indirectly influences the germination % and increases the vigour index Ι and ΙΙ. Furthermore, as evidenced by biochemical activities, it can be hypothesized that the active ingredients in the botanicals, such as phenol and curcumin, accelerate the enzymes' activities, total proteins, and seed storage proteins. The low concentrations of botanicals help support early seedling establishment and prevent the attack of fusarium wilt disease during plant establishment, which leads to a healthy plant population and growth, ultimately resulting in higher crop yield. Therefore, botanical priming could be a cost-effective means of increasing the production and productivity of rainfed chickpea crops, which can further support the sustainable development of agriculture and improve the farmers' socio-economic condition. The study can be a boon for botanical priming applications for sustainable agricultural practices and the Agri-seed industry in the future.
Material and methods
Experimental materials
The present research used a medium-vigour seed lot of the desi type chickpea cv. PG-186. The seed lot that exhibited less than 85% germination was considered medium-vigour, and the standard germination test was conducted as per the International Seed Testing Association (ISTA) standard. Further, all the experiments and field study complies with local and national guidelines and regulations.
Priming materials and techniques
Fresh turmeric and neem leaves were dry shaded for 5–6 days and then dried in a hot air oven for 2–3 h at 60 °C. Dried leaves were ground into fine powder using mixer grinder, and further dissolved in 100 mL distilled water, and left overnight at room temperature. Further, the solution was filtrated using Whatman filter paper no. 1. Hydropriming was carried out by soaking the seeds in distilled water. The seeds without priming and farmers' standard practice of 2 g/kg bavistin treatment were taken as control. Seeds were placed in petri dishes between moist filter paper for 18 h at (20 ± 1 °C) and (80–85%) relative humidity. They were then air-dried for 48 h to their original moisture content.
Physiological analysis of chickpea upon priming
Physiological analysis was carried out of all the treatments, namely T1 (without priming), T2 (bavistin treatment), T3 (1% aqueous turmeric leaf extract), T4 (1% aqueous neem leaf extract), and T5 (hydropriming) by following completely randomized design. All the experiments were conducted in four replicates. Seeds of different treatments were placed in rolled paper towels and kept in a germination chamber at 20 ± 1 °C and 85% RH for a standard period of 8 days by following International Seed Testing Association (ISTA) protocols. All the physiological evaluations were carried out on the 8th day of germination.
Seedling characteristic determination
The seeds were assessed for different morphological indexes of seedlings, such as germination percentage, root length (RL), shoot length (SL), seedling length (RL + SL), seedling dry weight, and seedling vigour indices I and II56,57. Once the dry weight of the seedlings was determined, ten of them were carefully wrapped in wax paper and kept in a hot air oven maintained at a precise temperature of 80 ± 2 °C for 17 h. After this, the seedlings were allowed to cool for 45 min in desiccators before being weighed using an electronic scale. The dry mass of each seedling was calculated and expressed in grams. Additionally, the germination rate (the percentage of average germinated seed out of all tested seeds at the end of the entire test) was calculated, and seedling vigour indices (seedling vigour index I and seedling vigour index II) were estimated using the standard formulae57. Seedling vigour index I determine the seeding vigour on the basis of seedling length which is calculated using germination (%) x seedling length (cm), while, seedling vigour index II determines the seeding vigour which is calculated on the basis of dry matter of seedling by using formula germination (%) x seedling dry weight (g).
Assays of biochemical activities
Amylase activity was measured using Bernfeld's method58 with some modifications. 1 g of seed sample was ground in 10 ml of 10 mM CaCl2, and the supernatant was used for enzymatic activities. Starch and enzyme solutions were incubated at 27 °C for 30 min, and DNS reagent was added. After heating and adjusting the solution, optical density was measured at 560 nm, and a maltose standard curve was used. The enzymes were extracted from a 1 g seed sample in acetone and centrifuged to assess protease activity59. Add casein solution to each tube to assay enzyme activity and incubate at 35 °C. The enzyme solution was added and incubated again. TCA and sodium carbonate with FCR were added and incubated. The solution was filtered, and optical density was measured using a UV–Vis spectrophotometer at 600 nm. The dehydrogenase activity of primed and non-primed seed was quantified60, where imbibed seed samples of 200 mg were soaked in a freshly made, pH-7.0, 0.2% TTC solution in 10 ml. The solution was then incubated in the dark for 3 h at 30 °C. After draining the TTC solution, acetone was added to each tube for crushing, and the sample was incubated overnight before being centrifuged for 10 min at 10,000 rpm. Using a UV–Vis spectrophotometer, collect the supernatant and measure the absorbance at 480 nm.
The phytase activity was measured using a modified method61. One gram of seed sample was homogenized in 0.1 M sodium acetate buffer (pH 5.0) and centrifuged. Phytase activity was determined by adding buffer and sodium phytate solution, incubating, adding a crude enzyme, and incubating and measuring phosphate liberation with the ammonium molybdate method. MDA content was determined using 20% TCA and 0.5% TBA solution to quantify lipid peroxidation62. The seed sample was ground in 4 ml of 1% TCA solution. After centrifuging, the supernatant was collected. 1 ml of (20% TCA and 0.5% TBA) was added to each sample. The absorbance was accurately measured at 532 nm and 600 nm for specific and non-specific samples, following a rigorous incubation and centrifugation process. MDA content was measured in moles/ml.
Calculation
The extinction coefficient of this MDA-TBA abduct at 532 nm is 155 mM−1 cm−1.
Characterization of seed storage proteins
The total protein was estimated from chickpea seed, and a standard curve was produced using Bradford's standard solution63. A sample of dried chickpea seeds was ground using a hammer mill; fine powder was obtained, passed through a 0.185 mm mesh grid, and kept in air-tight plastic containers at room temperature to prevent spoilage. Chickpea flours were defatted overnight using a horizontal shaker with hexane in a ratio of 1:10 and then washed twice with ethyl ether, followed by drying for 1 h at − 20 °C. Protein fractions were obtained using the Osborne (1907) fractionation method64. Seed flours were extracted by stirring in borate buffer at pH-7.6 with NaCl and sodium azide for 2 h, followed by centrifugation at 30,000 rpm for 30 min.
Polyacrylamide Gel Electrophoresis analysis
SDS-PAGE was carried out using the 5% stacking gel and 12% resolution gel65. Sample solutions were prepared from 10 mg of freeze-dried protein extract or precipitates dissolved in 1 ml sample buffer (distilled water, 0.5 M Tri-HCl pH 6.8, glycerol, 10% SDS, 1% bromophenol blue and β-mercaptoethanol heated at 98 °C for 10 min, then applied to the sample wells. Electrophoretic migration was monitored at a constant current (12 mA/gel) for 1.5 to 2 h. SDS gels were stored for two hours, and distaining was done for 12 h (Supplementary Information file 4).
Field experiment
The field research trial used a randomized complete block design with eight replications and three priming treatments: T1 (dry seed as control), T2 (bavistin treated as positive control), and T3 (turmeric leaf extract primed). The seed was sown in 5 rows per plot, with a plot size of 2.8 × 1.8 m2. Field emergence percentage (no. of seedlings emerged/total no. of seed sown) was calculated 15 days and 30 days after sowing. Five randomly selected plants from each replicated plot of all treatments were tagged to take all the observations, such as plant height (at 15 DAS, 30 DAS, 45 DAS and maturity) and yield parameters. The biological yield was calculated before threshing by taking the total weight of harvested crop plants from the net plot area. The biological yield was given in kg ha−1. After sun drying for a few days, the harvested crop from the respective net plot was threshed with a thresher. Seed yield was recorded and expressed as kg/ha. The Harvest Index (HI) was calculated using formulae-
Statistical analysis
The collected data were analyzed using the statistical software SPSS and the analysis of variance technique. The treatment means were compared using a post-hoc test at a 5% significance level to determine whether there were any significant differences between them. Furthermore, a graphical representation of the data was created using Microsoft Excel to provide a clear and visual presentation of the results.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Agrawal, T. et al. Correlation and path coefficient analysis for grain yield and yield components in chickpea (Cicer arietinum L.) under normal and late sown conditions of Bihar. Int. J. Curr. Microbiol. Appl. Sci. 7(2), 1633–1642. https://doi.org/10.20546/ijcmas.2018.702.197 (2018).
Krishnamurthy, L., Johansen, C. & Sethi, S. C. Investigation of factors determining genotypic differences in seed yield of non-irrigated and irrigated chickpeas using a physiological model of yield determination. J. Agron. Crop Sci. 183(1), 9–17. https://doi.org/10.1046/j.1439-037x.1999.00306.x (1999).
Srinivasan, A., Johansen, C. & Saxena, N. P. Cold International Journal of Current Microbiology and Applied Science tolerance during early reproductive growth of chickpea (Cicer arietinum L.) characterization of stress and genotypic variation in pod set. Field Crops Res. 57, 181–193. https://doi.org/10.1016/S0378-4290(97)00118-4 (1998).
Jame, Y. W. & Cutforth, H. W. Simulating the effects of temperature and seeding depth on germination and emergence of spring wheat. Agric. Meteorol. 124(3–4), 207–218. https://doi.org/10.1016/j.agrformet.2004.01.012 (2004).
Farooq, M., Basra, S. M. A., Ahmad, N. & Hafeez, K. Thermal hardening: A new seed vigour enhancement tool in rice. J. Integr. Plant Biol. 47(2), 187–193. https://doi.org/10.1111/j.1744-7909.2005.00031.x (2005).
Farooq, M., Aziz, T., Wahid, A., Lee, D. J. & Siddique, K. H. Chilling tolerance in maize: Agronomic and physiological applications. Crop Pasture Sci. 60(6), 501–516. https://doi.org/10.1071/CP08427 (2009).
Taylor, A.G., Thomas, B.D.J. & Murphy, B.G. Seed treatments, in: Encyclopedia of Applied Plant Science pp.1291–1298, https://doi.org/10.1016/B0-12-227050-9/00049-1 (2003).
Heydecker, W. & Gibbins, B. M. The “priming” of seeds. Sympos. Seed Problems Horticult. 83, 213–224. https://doi.org/10.17660/ActaHortic.1978.83.29 (1977).
Farooq, M., Basra, S. M., Wahid, A. & Ahmad, N. Changes in nutrient-homeostasis and reserves metabolism during rice seed priming: consequences for seedling emergence and growth. Agril. Sci. China 9(2), 191–198. https://doi.org/10.1016/S1671-2927(09)60083-3 (2010).
Kaur, S., Gupta, A. K. & Kaur, N. Seed priming increases crop yield possibly by modulating enzymes of sucrose metabolism in chickpea. J. Agron. Crop Sci. 191(2), 81–87. https://doi.org/10.1111/j.1439-037X.2004.00140.x (2005).
Karunagaran, D. & Rao, P. R. Mode and control of starch mobilization during germination of seeds of horse gram. Plant Sci. 73(2), 155–159. https://doi.org/10.1016/0168-9452(91)90023-2 (1991).
Tan-Wilson, A. L. & Wilson, K. A. Mobilization of seed protein reserves. Physiol. Plant 145(1), 140–53. https://doi.org/10.1111/j.1399-3054.2011.01535.x (2012).
Oaikhena, E. E., Ajibade, G. A., Appah, J. & Bello, M. Dehydrogenase enzyme activities in germinating cowpea (Vigna unguiculata (L)Walp). J. Biol. Agric. Healthc. 3(20), 32–36 (2013).
Oberleas, D. The determination of phytate and inositol phosphates. Methods Biochem. Anal. https://doi.org/10.1002/9780470110393 (1971).
Yamamoto, Y., Kobayashi, Y. & Matsumoto, H. Lipid peroxidation is an early symptom triggered by aluminium but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 125(1), 199–208. https://doi.org/10.1104/pp.125.1.199 (2001).
Jambunathan, R. Distribution of seed protein fractions and amino acids in different anatomical parts of chickpea (Cicer arietinum L.) and pigeon pea (Cajanus cajan L.). Plant Foods Hum. Nutr. 32, 347–54. https://doi.org/10.1007/BF01094046 (1982).
Hatice, S., Duygu, S., Tuba, E., Zeybek, A. & Toker, C. Effect of seed priming on germination of relict beautiful (Vavilovia Formosa). Al. Fed. Mediterranean Agril. Sci. 34(1), 101–108. https://doi.org/10.29136/mediterranean.785458 (2021).
Osborne, T.B. The proteins of the wheat kernel. Carnegie Institution of Washington, Publication no. 84, Judd & Detweiler. Inc., Washington, DC (1907).
Gunasekar, J., Kamaraj, A. & Padmavathi, S. Effect of botanical seed priming on seed quality characters in black gram (vigna mungo L.) Hepper cv. CO6. Plant Arch. 17(2), 1383–1387 (2017).
Chen, K. & Arora, R. Priming memory invokes seed stress- tolerance. Environ. Exp. Bot. 94, 33–45. https://doi.org/10.1016/j.envexpbot.2012.03.005 (2013).
Tamilmani, U. Studies on effect of various seed management practices on quality seed production in greengram (Vigna radiata L.) cv. ADT 3 under abiotic stress condition. M.Sc. (Ag.) Thesis, Annamalai University, Annamalainagar (2012).
Prakash, M., Pallavamallan, S., Sathiyanarayanan, G. & Rameshkumar, S. Effect of seed pelleting with botanicals on germination and seedling growth of cluster bean under induced saline condition. Legume Res. Int. J. 44(1), 88–93 (2021).
Hussein, M. H., Eltanahy, E., Al Bakry, A. F., Elsafty, N. & Elshamy, M. M. Seaweed extracts as prospective plant growth bio-stimulant and salinity stress alleviator for Vigna sinensis and Zea mays. J. Appl. Phycol. 33(2), 1273–91 (2021).
Hamouda, M. M., Saad-Allah, K. M. & Gad, D. Potential of seaweed extract on growth, physiological, cytological and biochemical parameters of wheat (Triticum aestivum L.) seedlings. J. Soil Sci. Plant Nutr. 22(2), 1818–1831. https://doi.org/10.1007/s42729-022-00774-3 (2022).
Narayanan, G. S., Prakash, M. & Reka, M. Influence of seed hardening treatments on growth, gas exchange and yield parameters in black gram under drought condition. Legume Res. Int. J. 39(2), 248–255. https://doi.org/10.18805/lr.v0iOF.7480 (2016).
Mukasa, Y. et al. Accumulation of soluble sugar in true seeds by priming of sugar beet seeds and the effects of priming on growth and yield of drilled plants. Plant Prod. Sci. 6(1), 74–82. https://doi.org/10.1626/pps.6.74 (2003).
Hussain, S., Khan, F., Hussain, H. A. & Nie, L. Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front Plant Sci. 7, 116. https://doi.org/10.3389/fpls.2016.00116 (2016).
Sharma, P., Gautam, A., Kumar, V. & Guleria, P. MgO nanoparticles priming promoted the growth of black chickpea. J. Agric. Food Res. 10, 2666–1543. https://doi.org/10.1016/j.jafr.2022.100435 (2022).
Saadat, H., Sedghi, M., Seyed Sharifi, R. & Farzaneh, S. Evaluation of gibberellin synthesis genes (ga3ox) expression and antioxidant capacity in common bean (Phaseolus vulgaris L. cv. Sadri) seeds induced by chitosan under salinity. Iran. J. Plant Physiol. 13(4), 4715–4728. https://doi.org/10.30495/ijpp.2023.1978837.1460 (2023).
Bailly, C., Bogatek-Leszczynska, R., Côme, D. & Corbineau, F. Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci. Res. 12(01), 47–55. https://doi.org/10.1079/SSR200197 (2002).
Ramana, T. & Radhakrishnan, T. M. De novo synthesis of protease during germination of pearl millet seeds. Curr. Sci. 59, 347–400 (1987).
Gepstin, S. & Han, I. Evidence for the involvement of cytokinin in the regulation of proteolytic activity in cotyledons of germinating beans. Plant Cell Physiol. 21(1), 57–63. https://doi.org/10.1093/oxfordjournals.pcp.a075990 (1980).
Robert, K. M. et al. Harper’s illustrated biochemistry. Biologic Oxidation 12, 99–100 (2009).
França-Neto, J. D. B. & Krzyzanowski, F. C. Tetrazolium: An important test for physiological seed quality evaluation. J. Seed Sci. 41(3), 359–366. https://doi.org/10.1590/2317-1545v41n3223104 (2019).
Pandey, P., Bhanuprakash, K. & Umesha,. Effect of seed priming on biochemical changes in fresh and aged seeds of cucumber. J. Agril. Studies 5(3), 62. https://doi.org/10.5296/jas.v5i3.11637 (2017).
Arun, M. N., Bhanuprakash, K., Hebbar, S. S. & Senthivel, T. Effects of seed priming on biochemical parameters and seed germination in cowpea [Vigna unguiculata (L.) Walp]. Legume Res. Int. J. 40(3), 562–570. https://doi.org/10.18805/lr.v0i0.7857 (2017).
Karmakar, A. et al. RNAi-mediated silencing of ITPK gene reduces phytic acid content, alters 27 transcripts of phytic acid biosynthetic genes, and modulates mineral distribution in rice seeds. Rice Sci. 27, 315–328 (2020).
Tiwari, B. K. & Singh, N. Pulse Chemistry and Technology (RSC Publishing, 2012).
Shi, H., Bressan, R., Hasegawa, P. M. & Zhu, J. K. In Sodium in Plant Nutritional Genomics (eds Broadlay, M. & White, P.) 127–149 (Blackwell Publishing, 2005).
Sung, H. G. et al. Effect of germination temperature on characteristics of phytase production from barley. Bioresour. Technol. 96(11), 1297–1303. https://doi.org/10.1016/j.biortech.2004.10.010 (2005).
Kikunaga, S., Katoh, Y. & Takahashi, M. Biochemical changes in phosphorus compounds and in the activity of phytase and a-amylase in the rice (Oryza sativa) grain during germination. J. Sci. Food Agric. 56, 335–343. https://doi.org/10.1002/jsfa.2740560309 (1991).
Greiner, R. Purification and characterization of three phytases from germinated lupine seeds (Lupinus albus Var. Amiga). J. Agric. Food Chem. 50, 6858–6864. https://doi.org/10.1021/jf025619u (2002).
Prazeres, J. N., Ferreira, C. V. & Aoyama, H. Acid phosphatase activities during the germination of Glycine max seeds. Plant Physiol. Biochem. 42, 15–20. https://doi.org/10.1016/j.plaphy.2003.10.009 (2004).
Bartnik, M. & Szafrańska, I. Changes in phytate content and phytase activity during the germination of some cereals. J. Cereal Sci. 5(1), 23–28. https://doi.org/10.1016/S0733-5210(87)80005-X (1987).
Mconald, M. B. Orthodox seed deterioration and its repair. In Handbook of Seed Physiology: Applications to Agriculture (eds Sanchez, R. A. & Benech-Arnold, R. L.) 273–304 (Food Products Press, 2004).
Bhardwaj, J., Anand, A., Pandita, V. K. & Nagarajan, S. Pulsed magnetic field improves seed quality of aged green pea seeds by homeostasis of free radical content. J. Food Sci. Tech. 53, 3969–3977 (2016).
Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 14, 93–107. https://doi.org/10.1079/SSR2004159 (2004).
Vioque, J. et al. Purification and partial characterization of chickpea 2S albumin. J. Agric. Food Chem. 47(4), 1405–1409. https://doi.org/10.1021/jf980819k (1999).
Shewry, P. R. & Halford, N. G. Cereals seed storage proteins, structures, properties and role in grain utilization. J. Expert. Bot. 53, 947–958. https://doi.org/10.1093/jexbot/53.370.947 (2002).
Rocha, T. S. et al. Germination of Phaseolus vulgaris and alcalase hydrolysis of its proteins produced bioactive peptides capable of improving markers related to type-2 diabetes in vitro. Food Res. Int. 76(1), 150–159. https://doi.org/10.1016/j.foodres.2015.04.041 (2015).
Acharya, P., Jayaprakasha, G. K., Crosby, K. M., Jifon, J. L. & Patil, B. S. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 10, 5037. https://doi.org/10.1038/s41598-020-61696-7 (2020).
Srimathi, S., Gokulakrishnan, J. & Prakash, M. Effect of seed priming with botanical leaf extracts on seed quality and yield of maize hybrid COH (M) 4. J. Res ANGRAU 49, 37–44 (2021).
Basra, S. M., Farooq, M., Wahid, A. & Khan, M. B. Rice seed invigoration by hormonal and vitamin priming. Seed Sci. Tech. 34(3), 753–7. https://doi.org/10.15258/sst.2006.34.3.23 (2006).
Devi, K., Barua, P. & Meghali, B. Integrated effect of pre-sowing seed treatment, sowing windows and seasons on seed yield and quality of greengram. Legume Res. Int. J. https://doi.org/10.18805/LR-4174 (2019).
Muhammad, U. et al. Effects of neem (Azadirachta indica) seed and turmeric (Curcuma longa) rhizome extracts on aphids control, plant growth and yield in okra. J. Appl. Bot. Food Qual. 91, 194–201. https://doi.org/10.5073/JABFQ.2018.091.026 (2018).
Rajani, K. et al. Physiological and biochemical assesement of chickpea and lentil grown in different agroclimatic zones of Bihar. Curr. J. Appl. Sci. Technol. 39(10), 68–78. https://doi.org/10.9734/cjast/2020/v39i1030629 (2020).
Abdul-Baki, A. A. & Anderson, J. D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 13(6), 630–633. https://doi.org/10.2135/cropsci1973.0011183X001300060013x (1973).
Bernfeld, P. Amylases α and β. In Methods in enzymology (eds Colowick, S. P. & Kaplan, N. O.) (Academic, 1955).
Anson, M. L. The estimation of pepsin, trypsin, papain, and cathepsin with haemoglobin. J. Gen. Physiol. 22(1), 79–89. https://doi.org/10.1085/jgp.22.1.79 (1938).
Kittock, D. L. & Law, A. G. Relationship of seedling vigour to respiration and tetrazolium reduction in germinating wheat seeds. Agronomy J. 60(3), 268–288. https://doi.org/10.2134/agronj1968.00021962006000030012x (1968).
Azeke, M. A., Egielewa, S. J., Eigbogbo, M. U. & Ihimire, I. G. Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum). J. Food Sci. Tech. 48(6), 724–9. https://doi.org/10.1007/s13197-010-0186-y (2011).
Hodges, D. M., DeLong, J. M., Forney, C. F. & Prange, R. K. Improving the thiobarbituric acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611. https://doi.org/10.1007/s004250050524 (1999).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193(1), 265–75. https://doi.org/10.1016/S0021-9258(19)52451-6 (1951).
Osborne, T. B. The Vegetable Proteins 2nd edn. (Longmans, Green and Co, 1924).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. https://doi.org/10.1038/227680a0 (1970).
Acknowledgements
The authors acknowledge Bihar Agricultural University, Sabour, for providing the infrastructural facilities to carry out the research work and the BAU communication number of the manuscript. The author also acknowledges the fund support by the Science and Engineering Research Board, Government of India.
Author information
Authors and Affiliations
Contributions
K.K.: Conducted the experiments, collected the data, helped in data interpretation and compilation of the results. K.R.: Conceptualized the research idea and designed the experiments, significantly contributed in drafting and finalization of the manuscript. R.R.K.: Designed the molecular related experiments and refined the manuscript draft. T.R.: Contributed in preparation of protein samples, execution of molecular experiments, and proofreading of the manuscript. A.K.: Designed field experiments and analyzed field data. M.F.A.: Proofreading of the manuscript and technical inputs. V.K.: Contributed to the standardization of biochemical assays. V.K.: Participated in collection of the research materials and A.K.: Conducted field experiments and recorded high-quality data accurately.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaushal, K., Rajani, K., Kumar, R.R. et al. Physio-biochemical responses and crop performance analysis in chickpea upon botanical priming. Sci Rep 14, 9342 (2024). https://doi.org/10.1038/s41598-024-59878-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59878-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.