Abstract
Urbanisation has contributed to a severe decline in biodiversity worldwide. However, urban ecosystems can also play an important role in the conservation of threatened species, including ground-nesting birds such as the Eurasian Oystercatcher (Haematopus ostralegus). While the coastal populations of this shorebird have declined sharply, there is growing evidence that pairs nesting on urban flat roofs have high reproductive success. However, the reasons for rooftop nesting and the species’ habitat use in urban areas remain poorly understood. In this study, we investigate the territory selection and foraging behaviour of the Eurasian Oystercatcher in the city of Münster (NW Germany). All nesting sites were located on flat roofs (N = 24), most of which were covered with gravel. Overall, reproductive success was high. This was mainly because the roofs provided protection from mammalian predators, leading to increased nest and chick survival. Moreover, breeding performance in the study area was favoured by the proximity of sports pitches. According to our observations, they provided a large amount of easily accessible prey throughout the breeding season. Overall, our study highlights that the reproductive success of the Eurasian Oystercatcher in urban environments is highly dependent on both safe nesting sites on flat roofs and the availability of suitable foraging habitats. Although our study suggests that breeding in urban areas can be beneficial for the model organism, the species’ strong territory fidelity makes it very sensitive to the rapid environmental changes occurring in cities. The value of urban ecosystems for bird conservation should therefore be better integrated into urban planning and management.
Similar content being viewed by others
Introduction
With increasing human population and rapid economic growth, urban areas have expanded during the last decades all over the globe1,2. Since urban sprawl has led to a severe loss of natural and semi-natural habitats, urbanisation is considered to be one of the most serious threats to biodiversity3,4. Although cities are usually considered hostile environments for most taxa and exhibit a high level of human disturbance, urbanisation can also contribute to the emergence of novel ecosystems that can play an important role in maintaining biodiversity, including the conservation of threatened species (e.g.5,6,7). This is especially true when cities are surrounded by intensively used landscapes where suitable habitats are limited for most species8,9. Moreover, recent studies found positive effects of reduced anthropogenic activity during the COVID-19 lockdown on urban wildlife (e.g.10,11). For instance, it has been shown that shorebirds benefitted from reduced human disturbance in urban beach ecosystems11,12. By contrast, the study of Seress et al.13 revealed that the breeding success of an urban adapter species did not change when human disturbance decreased during the lockdown.
Birds are an important component of urban biodiversity and are among the best-studied animal groups in urban ecosystems (e.g.14,15). Despite the great challenges of living in cities, many bird species have successfully adapted to urban habitats16,17,18. In particular, cities can provide suitable nesting conditions for habitat generalists and a high abundance of shrub and cavity-breeding birds (e.g.8,19,20,21). However, it has also been reported that some ground-nesting birds, which have suffered severe declines in European agricultural landscapes22, can breed successfully in urban areas (e.g.23,24).
This is also the case for the Eurasian Oystercatcher (Haematopus ostralegus, hereinafter referred to as Oystercatcher), which was originally restricted to coastal areas but has shifted inland along the floodplains of major rivers in Central and Western Europe during the last decades (e.g.25,26,27). While the coastal Oystercatcher population is declining at an alarming rate, the inland breeding population is increasing throughout Europe (e.g.28,29,30,31). According to Gedeon et al.26, inland breeding Oystercatchers account for at least 10% of the German breeding population. Several authors have observed that inland Oystercatcher populations often use urban flat roofs for breeding (e.g.32,33,34,35). Rooftop nesting is relatively common among seabirds such as gulls and terns (e.g.36,37,38). However, it rarely occurs among waders39. The Oystercatcher is an exception as it is one of the few European waders that feeds its chicks, which therefore do not need direct access to foraging habitats during the rearing period40. Although the Oystercatcher is among the best-studied shorebirds in Europe31,41, the breeding ecology of Oystercatchers nesting in urban environments is still poorly understood.
In this study, we investigated territory selection and foraging behaviour of the Oystercatcher in the city of Münster (NW Germany) in 2020. We compared the habitat use of both successful and unsuccessful breeding pairs to the habitat conditions in random sites. In particular, we aimed to identify the environmental factors that determine nesting success and improve our understanding of the species’ foraging preferences in urban areas. As we expected that the risk of predation is significantly reduced when Oystercatchers breed on rooftops (cf.37,38), we hypothesised that this strategy could result in higher reproductive success of the species. Furthermore, we assumed that a large proportion of urban green space is essential to provide sufficient food throughout the breeding season (cf.33,34). In line with these hypotheses, we assert that cities can provide good breeding conditions for the Oystercatcher but also address potential limitations of our study. The results of our study can be used to draw conclusions about measures that can be taken to mitigate the negative population trend of the species.
Background information
Study species
The Eurasian Oystercatcher (Haematopus ostralegus) is a migratory shorebird over most of its breeding range that includes much of the Palearctic. The European breeding population of the nominate subspecies Haematopus ostralegus ostralegus is currently estimated to be at least 300,000 breeding pairs, with a clear stronghold in the UK and Norway as well as the Wadden Sea in Germany and the Netherlands27,31, 42. The inland shift of the species has often been attributed to a population increase that took place until the 1980s, but is likely also due to reduced reproductive success following severe environmental changes in coastal habitats (e.g.25,26,31,43,44). Oystercatchers are long-lived birds that have a strong territory fidelity. Breeding mainly takes place in sparsely vegetated coastal habitats, such as salt marshes, beaches and dunes40,45. In addition to habitats associated with freshwater marshes, flat gravel rooftops are among the main breeding habitats in inland areas, especially in urban environments27,29,34,45. The availability of foraging habitats with high prey abundance plays an important role for successful breeding of the Oystercatcher31,46. On the coast, where the species’ diet consists mainly of marine molluscs and worms, foraging is highly dependent on tidal dynamics40,45. By contrast, inland breeders feed primarily on earthworms captured from short-turf grassland and urban green infrastructure such as lawns and sports pitches40,43,45,47. As a result of negative population trends throughout most of its range, the Oystercatcher is now listed as vulnerable in Europe and near threatened globally31,42.
Study area
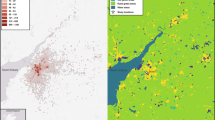
The study was conducted in the city of Münster (North Rhine-Westphalia, Germany, 51° 58ʹʹ N, 7° 38ʹʹ E; 39–99 m a.s.l, Fig. 1). Münster is a medium-sized, rapidly growing city with a human population of 320,000 inhabitants in an area of 303 km2. It is located in the North German Plain, 200 km south of the Wadden Sea coast. The study area covers the whole municipal area of the city of Münster (~ 300 km2). The climate in the study area is suboceanic with a mean annual temperature of 10.4 °C and an average annual precipitation of 763 mm (1991–2020, weather station Münster/Osnabrück48). Since the mid-twentieth century, the study area has been severely affected by agricultural intensification and urban sprawl28. Today, it is largely covered by built-up areas (34%) surrounded by intensive arable land (46%) and forests (18%), water bodies and wetlands together cover only 2%. Among the latter, the EU Bird Sanctuary’ Rieselfelder Münster’ is one of the most important inland stopover areas for shorebirds in north-western Germany. The first evidence of breeding of the Oystercatcher in the study area dates back to 196949. Since then, the local Oystercatcher population has steadily increased28. Some breeding pairs in the study areas nested in the same location for at least 20 years (own observation).
Location of Oystercatcher territories and random sites and the prevailing land-cover types in the city of Münster (NW Germany), for more details see Table 1.
Materials and methods
Bird census
Oystercatcher territories were located using standardised territory mapping. Bird census was done under favourable weather conditions between 6 and 10 a.m. The whole study area was searched for Oystercatchers over four surveys carried out between early April and mid-June 2020 with at least 7 days between two consecutive surveys50,51. Following Bibby et al.50, all observations of territorial behaviour (e.g. breeding or feeding adults, alarming birds and pairs at potential nesting sites) were recorded with their location during each survey. In order to separate the territories of different birds in close proximity, particular attention was paid to simultaneous observations of territorial behaviour52. If birds showing territorial behaviour were detected at potential nesting sites during at least two of the four surveys, breeding was assumed51.
To assess nesting success, territories were additionally checked weekly between mid-June and mid-July for the presence of juvenile birds30. Nesting success was confirmed if at least one fledgling was detected in a territory during this period53. If adults left the nesting site prior to the fledging of the chicks, breeding was considered unsuccessful. In the case of successful nesting, we also counted the number of fledglings in order to calculate the reproductive output of the Oystercatcher population in the study area.
In further analyses, we compared the habitat characteristics within territories of successful (N = 14) and unsuccessful breeding pairs (N = 10) and random sites (N = 12). Following the procedure of previous studies (e.g.37,54), the random sites were selected from all available flat roofs in the study area using the ‘create random points’ tool in ArcGIS 10.8.1. They thus represent a random selection of potential nesting sites in the city of Münster, which were not occupied by Oystercatchers, and reflect the general environmental conditions of the entire study area.
Foraging preferences
During the main rearing period (early June to mid-July40,45), we studied the foraging preferences of the Oystercatcher in a total of twelve territories. We examined foraging in the nearest (i) urban lawn, (ii) agricultural grassland and (iii) sports pitch (playing surface with natural grass turf, in the study area mainly used for playing football) in the vicinity of the nesting site for 15 min in each of the three habitat types. These habitats are among the most important foraging habitats for the Oystercatcher in urban areas (e.g.33,34,41,47). During the observation period, we measured the duration of foraging activity (time in minutes) and counted the foraging success (catches/attempt) of the Oystercatcher.
Environmental parameters
The environmental conditions within Oystercatcher territories and random sites were determined using digital landscape models (DLM/ATKIS, scale: 1:10,000)55. For this purpose, the proportions of the prevailing terrestrial land-cover types were calculated within a 1 km radius around the centre of each breeding territory (i.e. nesting site) and random site. The radius corresponds to the large territories of the Oystercatcher used for foraging40. The following seven land-cover types were distinguished: (i) arable land, (ii) agricultural grassland, (iii) forest and shrub, (iv) built-up area, (v) urban lawn, (vi) industrial area, (vii) water body and marshland. These data were further used to calculate the Shannon index (Hʹ) as a measure of landscape diversity56:
with pi = ni/N where N is the overall area of the 1 km buffer and ni is the area of the respective land-cover types in the buffer area.
We also recorded the size, elevation above ground and substrate type of all rooftops occupied by Oystercatchers as well as the flat roofs selected as random sites. In addition, we measured the distance to the nearest available sports pitch for both occupied nesting sites and random sites (i.e. potential nesting sites). Only pitches with natural grass turf were considered. We calculated the Normalised Difference Vegetation Index (NDVI) based on Sentinel-2 satellite imageries to quantify the effect of drought on potential foraging habitats during the breeding season (cf.57). Mean NDVI was calculated for the area of (i) agricultural grasslands and (ii) urban lawns within the 1 km buffer and (iii) the nearest available sports pitch. These calculations were done using the zonal statistics tool in ArcGIS 10.8.1 for early May, June and late July, respectively.
Statistical analyses
Statistical analyses were performed using R 4.1.258. If the data were normally distributed, statistical differences in environmental parameters between successful and unsuccessful breeding pairs of the Oystercatcher and random sites were tested using ANOVA with Tukey’s test as a post-hoc test. Otherwise, we used the Kruskal–Wallis H test with Dunn’s test as a post-hoc test. We used repeated measures ANOVA on ranks followed by the Holm-Sidak test as a post-hoc test to identify differences in the NDVI of potential foraging habitats in Oystercatcher territories. Differences in foraging activity and success in the habitats that were in fact visited to capture prey were tested using the Wilcoxon Signed Rank Test and the Mann–Whitney U test, respectively.
We used multivariable generalised linear models (GLMs) (binomial error structure) to identify environmental factors that explain the occurrence of successful and unsuccessful breeding pairs against random sites, respectively. To identify the most important predictors, all possible combinations of the sampled environmental parameters were tested, resulting in a number of different candidate models. These were ranked based on Akaike’s information criterion for small sample sizes (AICc) using the ‘dredge’ function (R package MuMIn)59. Only top-ranked models with ΔAICc < 2 were used for model averaging60. All predictor variables were scaled prior to analyses to ensure comparability of model coefficients of different parameters. To avoid multicollinearity in the models, Spearman’s rank correlations (rs) were applied prior to the GLM analyses. Combinations of highly intercorrelated variables (|rs| > 0.5) were excluded from the modelling process. In addition, the number of predictors in the different candidate models was limited to three parameters to avoid overfitting.
Results
Breeding habitats
A total of 24 breeding Oystercatcher pairs were recorded in the study area. In 58% of the pairs (N = 14), at least one young successfully fledged; in the remaining pairs (N = 10), breeding failed. Except for one pair with two chicks, breeding pairs reared one chick, resulting in an overall reproductive success of 0.6 fledglings per breeding pair. All Oystercatcher nesting sites were located on flat roofs, the majority of which (88%) were covered with gravel. Breeding was unsuccessful in two of the three pairs nesting on non-gravelled roofs. Whereas no differences were found in the height and size of the occupied rooftops, clear differences were observed in the landscape surrounding the roofs used for nesting (Table 1). The territories of successful breeding pairs were characterised by a larger proportion of industrial areas (mean ± SE: 10.0 ± 2.1%) and urban lawns (mean ± SE: 19.5 ± 5.3%) compared to the random sites (mean ± SE: 3.4 ± 1.3% and 9.8 ± 3.1%, respectively). Their nesting sites were also closer to the nearest sports pitch (mean ± SE: 445 ± 126 m) than potential nesting sites on the randomly selected rooftops ((mean ± SE: 1230 ± 188 m).
Based on the GLM analyses, the distance between the nesting site and the nearest sports pitch was identified as the key factor for successful breeding (Fig. 2, Table 2). By contrast, we did not detect any environmental factors explaining unsuccessful breeding.
Relationship between the occurrence of successful breeding pairs and the distance to the nearest sports pitch based on generalised linear model with binomial error structure (p < 0.05). Successful breeding pairs (N = 14, dark blue) vs. random sites (N = 12, light blue) formed the two categories of the response variable, Regression slope (± 95% confidence interval = CI) fitted based on the GLM output y = 1/(1 + exp − (− 1.144 − 7.49 × distance to sports pitch)), for more details see Table 2a.
Foraging habitats
Among the potential foraging habitats, the NDVI of urban lawns and agricultural grasslands clearly decreased from May to July (Fig. 3). By contrast, the NDVI of sports pitches did not change during this period. Consequently, pitches were characterised by higher NDVI values than urban lawns throughout the whole breeding season. In June and July, they also had higher NDVI values compared to agricultural grasslands. Both foraging activity and foraging success were also higher on sports pitches than on urban lawns during the rearing period (Fig. 4). No foraging activity was observed on agricultural grasslands during this period.
Differences in the Normalized Difference Vegetation Index (NDVI) between potential foraging habitats of the Oystercatcher in the study area: (i) urban lawns (light blue square) and (ii) agricultural grasslands (medium blue triangle) within a 1 km radius around the centre of each breeding territory and (iii) the nearest available sports pitch (dark blue point) during the breeding season of the species in the study area (May to July). Mean ± SE are shown. Different letters indicate statistical significances (p < 0.05) between the groups.
Differences in foraging activity and success of the Oystercatcher between urban lawns (light blue square) and sports pitches (dark blue point) during the rearing period of the species in the study area (June to July). Mean ± SE are shown. Statistical significances between the groups are indicated by asterisks (*p < 0.05).
Discussion
Compared to the very low reproductive success of the Oystercatcher in its coastal habitats in recent years30,43,45, we detected high nesting success of Oystercatchers nesting on urban rooftops in our study. Breeding mainly took place on flat gravel roofs situated in close proximity to foraging habitats. Successful breeding was particularly favoured by a short distance to sports pitches. By contrast, our study did not detect environmental factors that explain unsuccessful breeding in urban areas.
Roof nesting of Eurasian Oystercatchers has been reported from several regions within their range and also occurs in other Oystercatcher species around the world (e.g.26,32,33,41). In general, Oystercatchers are very flexible in their choice of nesting sites40,41,45. As gravel roofs have a similar structure compared to natural breeding habitats such as shingle beaches, it is not surprising that they were used for nesting in our study. However, confirming the study of Dijkstra and Dillerop47, we observed that non-gravelled roofs were also sometimes used for nesting. Although Oystercatcher chicks sometimes can die by falling or jumping from the roofs (e.g.47), our study revealed that the reproductive success of roof-nesting Oystercatchers (0.6 fledglings/pair was far above the average of the reproductive outcome in coastal (~ 0.18 fledglings/pair on islands and ~ 0.11 fledglings/pair on the mainland coast)30, and agricultural habitats (~ 0.11 fledglings/pair)30,32. This is consistent with other authors that highlighted the high reproductive success of Oystercatcher populations in urban areas (e.g.33,34,43).
The main advantage of rooftop nesting is that it provides effective protection from mammalian predators such as the Red Fox (Vulpes vulpes), whose populations have strongly increased in recent decades, contributing to the dramatic decline of many ground-nesting birds (e.g.61,61,63). It is well known that breeding in habitats that cannot be reached by predatory mammals is an effective breeding strategy for many ground-nesting birds38,44,64. This is also true for most shorebirds, such as the Oystercatcher, which currently has very low breeding success throughout most of its range except for some islands that are largely devoid of predatory mammals24,30,65,66. By contrast, several studies have found that the nesting success of ground-nesting birds can be higher in urban habitats due to lower abundance of mammalian predators (e.g.23,67,68). This is especially true for Oystercatchers nesting on rooftops, which are completely inaccessible to predatory mammals and therefore provide effective protection for juvenile birds, provided that they do not leave the rooftop until they fledge (Ref.47, cf.38). Moreover, human disturbance is also known to be detrimental to the breeding performance of the Oystercatcher (e.g.31,40). This especially applies to habitats with high human activity. While it has recently been found that the abundance of shorebirds in urban beach ecosystems increased during the COVID-19 pandemic due to a reduced frequency of people12, roof-nesting Oystercatchers are protected from human disturbance at any time, because there usually is no public access to rooftops.
Nevertheless, eggs and chicks of roof-nesting Oystercatchers can still be preyed upon by avian predators such as corvids or gulls40,43, 45. Although we did not detect any environmental factors that contributed to breeding losses, predation by corvids, which are usually abundant in urban environments and were very common in the study area, was probably one of the reasons for failed breeding in our study (cf.68). In general, the impact of avian predation on the nest success and chick survival of ground-nesting birds is expected to be less severe, particularly for Oystercatchers, which exhibit aggressive defence towards predators (e.g.40,69,70).
Overall, for successful breeding, the characteristics of the flat roofs seem to be less important than those of the surrounding landscape34,47. Territories of successfully breeding Oystercatcher pairs in the study area were surrounded by a larger proportion of industrial areas. Industrial areas in the study area provided a high availability of flat gravel roofs as potential nesting sites71. Furthermore, they were usually also characterised by a large proportion of urban lawns, whose importance for foraging is highlighted below55. The availability of foraging habitats with abundant and easily accessible prey during the breeding season is known to be another important driver of Oystercatcher breeding performance31,41,46. This is especially true for urban areas where potential foraging habitats are generally scarce. Dijkstra and Dillerop47 observed that Oystercatchers on urban rooftops usually breed in proximity to their foraging habitats. In our study, territories of successful breeding pairs had a larger proportion of urban lawns and were closer to sports pitches than the random sites, both of which are used for foraging. Furthermore, the GLM analyses revealed that the distance to the nearest sports pitch was the most important factor for successful breeding in the study area. This can be explained by the outstanding importance of sports pitches as foraging sites for the species. Low-cut turfgrass systems such as sports pitches can be very rich in earthworms72, the main food source for inland breeding Oystercatchers43,45,47. The high abundance of earthworms is especially promoted by intensive management of the pitches. In particular, the frequent mowing with mulching mowers, which leave fine turfgrass clippings on the pitches, favour the food supply for earthworms72. The short turfs and soft soils of sports pitches also make prey easily accessible for probing Oystercatchers.
Although urban lawns and short-turf grasslands can have a similar structure and were widely available in the study area, they only played a minor role in Oystercatcher foraging. This was mainly due to differences in soil moisture, which affects the availability and accessibility of earthworms in the upper soil layer62,73. While decreasing NDVI values in urban lawns and agricultural grasslands indicate an increasing impact of drought over the rearing period, sports pitches were regularly irrigated and thus steadily characterised by a higher NDVI (i.e. higher soil moisture). As a result, they provided a high availability and accessibility of prey throughout the whole breeding season. This may be particularly important during periods of droughts, which have become more frequent in recent years (including the study period) due to climate change and can lead to food shortage for waders62,69,74. It is well known that the risk of breeding loss increases when adults have to expend more effort on foraging and leave their chicks alone for a longer time (Ref.47, cf.37). The fact that nesting sites of Oystercatcher pairs without nesting success were further away from sports pitches emphasises that this foraging habitat is of vital importance for the breeding performance of Oystercatchers in urban areas.
Limitations of the study
Due to the low number of Oystercatcher territories in the study area (N = 24), larger-scale studies in different geographic locations and over multiple years are needed to deepen the results of our study. Although other authors also reported high number of Oystercatchers breeding on urban rooftops throughout Central and Western Europe (e.g.33,34,47), information on breeding success of roof-nesting Oystercatchers is still scarce. Therefore, future studies on breeding performance of Oystercatchers nesting on flat roofs would be very useful to draw more general conclusions for conservation. Our study does not provide direct evidence for the drivers of nest and chick survival. Therefore, detailed nest monitoring is required to shed more light on the causes of breeding losses of roof-nesting Oystercatchers compared with ground nesters in more natural habitats. Since it has recently been found that a lower level of human disturbance during the COVID-19 lockdown has affected the occurrence of ground-nesting shorebirds11,12, it could be questioned to what extent reduced anthropogenic activity has affected the results of our study in an urban environment. However, as people generally have no access to the roofs used for breeding at any time (i.e. also before and after the lockdown), we assume that the effects of COVID-19-related regulations on the results of our study are negligible. This is underlined by our own observations suggesting that some rooftops in the study area have been used for breeding for at least 20 years. In addition, we frequently observed Oystercatchers foraging on sports pitches only a few metres next to people doing sports during the main rearing period in 2020 (i.e. after the strict COVID-19 regulations have been cancelled at the beginning of May 2020). To dispel any doubts, we nevertheless recommend further studies covering different breeding periods, especially because they also could provide important information on how breeding success and foraging habitat use might vary between different geographic locations and years with different weather conditions.
Conclusions and implications for conservation
Our study has shown that urban gravel rooftops can provide well-suited breeding conditions for the Oystercatcher. Although urban roof-nesting Oystercatchers cannot compensate for the large-scale decline of the species in its coastal ranges, they could at least help to maintain viable populations of the species in the long term. This is mainly due to the protection from mammalian predators, which leads to higher reproductive success of the species compared to coastal and agricultural habitats. As gravel and green roofs can also be suitable for nesting of other ground-nesting birds and have other ecological benefits (e.g.37,38,39,75), they should be favoured wherever possible when constructing flat roofs in urban areas. However, the findings of our study also suggest that the surrounding landscape is another important factor for the Oystercatcher34,47. In particular, successful breeding of urban Oystercatchers depends on habitats that provide a large amount of prey that is easily accessible throughout the breeding season. In our study, this was especially true for sports pitches, which provided a high availability of earthworms due to their moist and soft soils. Other irrigated lawns, such as those in urban parks or on golf courses can provide similar conditions, but were scarce in the study area. As long as the soil is moist enough, short-grass pastures, lawns or road verges can also be suitable for foraging. However, they were of minor importance our study due to drought.
Despite the advantages of breeding on urban rooftops, the species’ strong territory fidelity makes it highly sensitive to the rapid environmental changes in urban areas. For instance, increasing drought due to climate change as well as the conversion from natural grass pitches to artificial turf can severely reduce the availability of foraging sites for Oystercatchers in cities. In addition, the use of pesticides to control earthworms on sports turf can severely reduce the abundance of prey62,72. These examples highlight that the value of urban ecosystems for bird conservation should be better integrated into urban planning and habitat management17. A large proportion of urban green space is particularly beneficial for bird conservation in urban areas5. This also applies to low-cut habitats such as sports pitches, golf courses, lawns as well as short-grass pastures and wastelands, which are important for ground-foraging species such as the Oystercatcher41,47,76.
The increasing shift of the species from its original breeding habitats to urban rooftops also highlights the severe challenges for Oystercatcher conservation in rural landscapes. Conservation measures in the wider countryside must therefore include both large-scale actions to improve the quality of breeding habitats for Oystercatchers and predator control that may also help to mitigate the declines in other shorebirds besides the Oystercatcher30,31,61,77.
Data availability
The data that support the findings of this study are available from the authors upon reasonable request.
References
Foley, J. A. et al. Global consequences of land use. Science 309, 570–574. https://doi.org/10.1126/science.1111772 (2005).
Grimm, N. B. et al. Global change and the ecology of cities. Science 319, 756–760. https://doi.org/10.1126/science.1150195 (2008).
Piano, E. et al. Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob. Change Biol. 26, 1196–1211. https://doi.org/10.1111/gcb.14934 (2020).
Simkin, R. D., Seto, K. C., McDonald, R. I. & Jetz, W. Biodiversity impacts and conservation implications of urban land expansion projected to 2050. Proc. Natl. Acad. Sci. 119, e2117297119. https://doi.org/10.1073/pnas.2117297119 (2022).
Aronson, M. F. J. et al. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 15, 189–196. https://doi.org/10.1002/fee.1480 (2017).
Holtmann, L., Philipp, K., Becke, C. & Fartmann, T. Effects of habitat and landscape quality on amphibian assemblages of urban stormwater ponds. Urban Ecosyst. 20, 1249–1259. https://doi.org/10.1007/s11252-017-0677-y (2017).
Jokimäki, J., Suhonen, J. & Kaisanlahti-Jokimäki, M. L. Urban core areas are important for species conservation: A European-level analysis of breeding bird species. Landsc. Urban Plan. 178, 73–81. https://doi.org/10.1016/j.landurbplan.2018.05.020 (2018).
Fuller, R. A., Tratalos, J. & Gaston, K. J. How many birds are there in a city of half a million people? Divers. Distrib. 15, 328–337. https://doi.org/10.1111/j.1472-4642.2008.00537.x (2009).
Turrini, T. & Knop, E. A landscape ecology approach identifies important drivers of urban biodiversity. Glob. Change Biol. 21, 1652–1667. https://doi.org/10.1111/gcb.12825 (2015).
Bates, et al. Global COVID-19 lockdown highlights humans as both threats and custodians of the environment. Biol. Conserv. 26, 109175. https://doi.org/10.1016/j.biocon.2021.109204 (2021).
Manenti, R. et al. The good, the bad and the ugly of COVID-19 lockdown effects on wildlife conservation: Insights from the first European locked down country. Biol. Conserv. 249, 108728. https://doi.org/10.1016/j.biocon.2020.108728 (2020).
Lewis, J., Collison, J. & Pillay, D. Effects of COVID-19 lockdowns on shorebird assemblages in an urban South African sandy beach ecosystem. Sci. Rep. 12, 5088. https://doi.org/10.1038/s41598-022-09099-8 (2022).
Seress, G. et al. Contrasting effects of the COVID-19 lockdown on urban birds’ reproductive success in two cities. Sci. Rep. 11, 17649. https://doi.org/10.1038/s41598-021-96858-8 (2021).
Chace, J. F. & Walsh, J. J. Urban effects on native avifauna: A review. Landsc. Urban Plan. 74, 46–69. https://doi.org/10.1016/j.landurbplan.2004.08.007 (2006).
Rega-Brodsky, C. C. et al. Urban biodiversity: State of the science and future directions. Urban Ecosyst. 25, 1083–1096. https://doi.org/10.1007/s11252-022-01207-w (2022).
Møller, A. P. Successful city dwellers: A comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159, 849–858. https://doi.org/10.1007/s00442-008-1259-8 (2009).
Snep, R. P. et al. Urban bird conservation: Presenting stakeholder-specific arguments for the development of bird-friendly cities. Urban Ecosyst. 19, 1535–1550. https://doi.org/10.1007/s11252-015-0442-z (2016).
Tryjanowski, P., Morelli, F. & Møller, A. P. Urban birds: Urban avoiders, urban adapters, and urban exploiters. In The Routledge Handbook of Urban Ecology 2nd edn (eds Douglas, I. et al.) 399–411 (Routledge, 2020).
Callaghan, C. T. et al. Generalists are the most urban-tolerant of birds: A phylogenetically controlled analysis of ecological and life history traits using a novel continuous measure of bird responses to urbanization. Oikos 128, 845–858. https://doi.org/10.1111/oik.06158 (2019).
Evans, K. L., Chaberlain, D. E., Hatchwell, B. J., Gregory, R. D. & Gaston, K. J. What makes an urban bird? Glob. Change Biol. 17, 32–44. https://doi.org/10.1111/j.1365-2486.2010.02247.x (2011).
Jokimäki, J., Suhonen, J., Jokimäki-Kaisanlahti, M. L. & Carbó-Ramírez, P. Effects of urbanization on breeding birds in European towns: Impacts of species traits. Urban Ecosyst. 19, 1565–1577. https://doi.org/10.1007/s11252-014-0423-7 (2016).
McMahon, B. J., Doyle, S., Gray, A., Kelly, S. B. A. & Redpath, S. M. European bird declines: Do we need to rethink approaches to the management of abundant generalist predators? J. Appl. Ecol. 57, 1885–1890. https://doi.org/10.1111/1365-2664.13695 (2020).
Šálek, M., Marhoul, P., Pintíř, J., Kopecký, T. & Slabý, L. Importance of unmanaged wasteland patches for the grey partridge Perdix perdix in suburban habitats. Acta Oecol. 25, 23–33. https://doi.org/10.1016/j.actao.2003.10.003 (2004).
Wesołowsky, T. & Fuller, R. J. Spatial variation and temporal shifts in habitat use by birds at the European scale. In Birds and Habitat—Relationships in Changing Landscapes (ed. Fuller, R. J.) 63–92 (Cambridge University Press, 2012).
Balmer, D. E. et al. Bird Atlas 2007–2011: The Breeding and Wintering Birds of Britain and Ireland (British Trust for Ornithology, 2013).
Gedeon, K. C., Grüneberg, C., Mitschke, A. & Sudfeldt, C. Atlas Deutscher Brutvogelarten (Stiftung Vogelmonitoring Deutschland and Dachverband Deutscher Avifaunisten, 2014).
Keller, V. et al. European Breeding Bird Atlas 2: Distribution, Abundance and Change (European Bird Census Council & Lynx Editions, 2020).
Grüneberg, C. et al. Die Brutvögel Nordrhein-Westfalens (LWL Museum of Natural History, 2013).
Krüger, T., Ludwig, J., Pfützke, S. & Zang, H. Atlas der Brutvögel in Niedersachsen und Bremen 2005–2008. Naturschutz und Landschaftspflege in Niedersachsen 48 (Niedersächsischer Landesbetrieb für Wasserwirtschaft, Küsten- und Naturschutz, 2014).
Thorup, O. & Koffijberg, K. Breeding Success in the Wadden Sea 2009–2012—A review. Wadden Sea Ecosystem 36 (Common Wadden Sea Secretariat/Joint Monitoring Breeding Bird Group, 2016).
Van de Pol, M. et al. A global assessment of the conservation status of the nominate subspecies of Eurasian oystercatcher (Haematopus ostralegus ostralegus). Int. Wader Stud. 20, 47–61 (2014).
Dijkstra, B. & Dillerop, R. Urbane en agrarische Scholeksters Haematopus ostralegus in en rond Assen in 2009–2012. Drentse Vogels 26, 4–13 (2012).
Duncan, A., Duncan, R., Rae, R., Rebecca, G. W. & Stewart, B. J. Roof and ground nesting Eurasian Oystercatchers in Aberdeen. Scottish Birds 22, 1–8 (2001).
Mitschke, A. Atlas der Brutvögel in Hamburg und Umgebung. Hamburger avifaunistische Beiträge 49 (Arbeitskreis Vogelschutzwarte, 2012).
Munro, C. A. Roof nesting oystercatchers. Bird Study 31, 148. https://doi.org/10.1080/00063658409476833 (1984).
Fisk, E. J. The growing use of roofs by nesting birds. Bird Band. 49, 134–141. https://doi.org/10.2307/4512343 (1978).
Forys, E. A. & Borboen-Abrams, M. Roof-top selection by least terns in Pinellas County, Florida. Waterbirds 29, 501–506. https://doi.org/10.1675/1524-4695(2006)29[501:RSBLTI]2.0.CO;2 (2006).
Kubetzki, U. & Garthe, S. Nests with a view: Distribution, nest habitats and diets of roof-breeding Common Gulls (Larus canus) in northern Germany. Waterbirds 30, 602–608. https://doi.org/10.1675/1524-4695(2007)030[0602:NWAVDN]2.0.CO;2 (2007).
Baumann, N., Catalano, C., Pasta, S. & Brenneisen, S. Improving extensive green roofs for endangered ground-nesting birds. In Urban Services to Ecosystems—Green Infrastructure Benefits from the Landscape to the Urban Scale (eds Catalano, C. et al.) 13–29 (Springer, 2021).
Glutz von Blotzheim, U. N. Handbuch der Vögel Mitteleuropas (Vogelzug-Verlag, 2011).
Ens, B. J. & Underhill, L. G. Synthesis of oystercatcher conservation assessments: General lessons and recommendations. Int. Wader Stud. 20, 5–22 (2014).
BirdLife International. Species Factsheet: Haematopus ostralegus. http://datazone.birdlife.org/species/factsheet/eurasian-oystercatcher-haematopus-ostralegus (Accessed 2 November 2023) (2023).
Safriel, U. N. Diet dimorphism within an Oystercatcher Haematopus ostralegus population—Adaptive significance and effects on recent distribution dynamics. Ibis 127, 287–305. https://doi.org/10.1111/j.1474-919X.1985.tb05071.x (1985).
Møller, A. P., Thorup, O. & Laursen, K. Predation and nutrients drive population declines in breeding waders. Ecol. Appl. 28, 1292–1301. https://doi.org/10.1002/eap.1729 (2018).
Bauer, H.-G., Bezzel, E. & Fiedler, W. Das Kompendium der Vögel Mitteleuropas 2nd edn. (Aula, 2012).
Schwemmer, P., Güpner, F., Adler, S., Klingbeil, K. & Garthe, S. Modelling small-scale foraging habitat use in breeding Eurasian oystercatchers (Haematopus ostralegus) in relation to prey distribution and environmental predictors. Ecol. Model. 320, 322–333. https://doi.org/10.1016/j.ecolmodel.2015.10.023 (2016).
Dijkstra, B. & Dillerop, R. Broedlocaties en broedsucces van urbane Scholeksters Haematopus ostralegus onder de loe. Drentse Vogels 30, 25–33 (2016).
DWD (German Meteorological Service). Climate Data Center. https://opendata.dwd.de/climate_environment/CDC/ (Accessed 25 October 2023) (2023).
Peitzmeier, J. Avifauna von Westfalen. Abhandlungen des Landesmuseums für Naturkunde Münster 41 (LWL Museum of Natural History, 1979).
Bibby, C. J., Burgess, N. D., Hill, D. A. & Mustoe, S. H. Bird Census Techniques 2nd edn. (Academic Press, 2000).
Südbeck, P., Andretzke, H., Fischer, S., Schröder, K. & Sudfeldt, C. Methodenstandards zur Erfassung der Brutvögel Deutschlands (Radolfzell, 2005).
Kämpfer, S., Löffler, F., Brüggeshemke, J. & Fartmann, T. Untangling the role of a novel agro-ecosystem as a habitat for declining farmland birds. Ann. Appl. Biol. 181, 367–378. https://doi.org/10.1111/aab.12789 (2022).
Steenhof, K. & Newton, I. Assessing nesting success and productivity. In Raptor Research and Management Techniques (eds Bildstein, K. & Bird, D. M.) (Hancock House, British Columbia and Blane, 2007).
Löffler, F. & Fartmann, T. The importance of landscape heterogeneity and vegetation structure for the conservation of the Ortolan Bunting Emberiza hortulana. Bird Conserv. Int. 33, e55. https://doi.org/10.1017/S0959270923000023 (2023).
Geobasis, N. R. W. Digital Landscape Model of North Rhine-Westphalia. https://www.bezreg-koeln.nrw.de/geobasis-nrw/produkte-und-dienste/landschaftsmodelle/aktuelle-landschaftsmodelle/digitales-basis (Accessed 25 July 2023) (2022).
Fartmann, T. et al. Landscape-scale effects of Christmas-tree plantations in an intensively used low-mountain landscape—Applying breeding bird assemblages as indicators. Ecol. Indic. 94, 409–419. https://doi.org/10.1016/j.ecolind.2018.07.006 (2018).
White, J. G. et al. Can NDVI identify drought refugia for mammals and birds in mesic landscapes? Sci. Total Environ. 851, 158318. https://doi.org/10.1016/j.scitotenv.2022.158318 (2022).
R Development Core Team. R: A Language and Environment for Statistical Computing. http://www.r-project.org (Accessed 25 October 2023) (2023).
Bartón, K. Multi-model Inference (Package MuMIn: version 1.47.5). https://cran.r-project.org/web/packages/MuMIn/index.html (Accessed 26 September 2023) (2023).
Grueber, C. E., Nakagawa, S., Laws, R. J. & Jamieson, I. G. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 24, 699–711. https://doi.org/10.1111/j.1420-9101.2010.02210.x (2011).
Fletcher, K., Aebischer, N. J., Baines, D., Foster, R. & Hoodless, A. N. Changes in breeding success and abundance of ground-nesting moorland birds in relation to the experimental deployment of legal predator control. J. Appl. Ecol. 47, 263–272. https://doi.org/10.1111/j.1365-2664.2010.01793.x (2010).
Newton, I. Farming and Birds (Harper Collins, 2017).
Roos, S., Smart, J., Gibbons, D. W. & Wilson, J. D. A review of predation as a limiting factor for bird populations in mesopredator-rich landscapes: A case study of the UK. Biol. Rev. 93, 1915–1937. https://doi.org/10.1111/brv.12426 (2018).
Kämpfer, S., Engel, E. & Fartmann, T. Weather conditions determine reproductive success of a ground-nesting bird of prey in natural dune grasslands. J. Ornithol. 163, 855–865. https://doi.org/10.1007/s10336-022-01999-w (2022).
Bell, M. V. & Calladine, J. The decline of a population of farmland breeding waders: A twenty-five-year case study. Bird Study 64, 264–273. https://doi.org/10.1080/00063657.2017.1319903 (2017).
Kämpfer, S. & Fartmann, T. Natural coastal dunes on Wadden Sea islands as a refuge for an endangered wader species. J. Coast. Conserv. 26, 53. https://doi.org/10.1007/s11852-022-00897-w (2022).
Gering, J. C. & Blair, R. B. Predation on artificial bird nests along an urban gradient: Predatory risk or relaxation in urban environments? Ecography 22, 532–541. https://doi.org/10.1111/j.1600-0587.1999.tb01283.x (1999).
Kamp, J. et al. High nest survival and productivity of Northern Lapwings Vanellus vanellus breeding on urban brownfield sites. J. Ornithol. 156, 179–190. https://doi.org/10.1007/s10336-014-1114-0 (2015).
Ausden, M. & Bolton, M. Breeding waders on wet grassland: Factors influencing habitat suitability. In Birds and Habitat—Relationships in Changing Landscapes (ed. Fuller, R. J.) 278–306 (Cambridge University Press, 2012).
Schekkerman, H., Teunissen, W. & Oosterveld, E. Mortality of black-tailed godwit Limosa limosa and Northern Lapwing Vanellus vanellus chicks in wet grasslands: Influence of predation and agriculture. J. Ornithol. 150, 133–145. https://doi.org/10.1007/s10336-008-0328-4 (2009).
Geobasis, N. R. W. Gründachkataster des Landes Nordrhein-Westfalen. https://www.opengeodata.nrw.de/produkte/umwelt_klima/klima/gruendachkataster/ (Accessed 22 October 2023) (2023).
Boyle, P. E., Richardson, M. D., Savin, M. C., Karcher, D. E. & Potter, D. A. Ecology and management of earthworm casting on sports turf. Pest Manag. Sci. 75, 2071–2078. https://doi.org/10.1002/ps.5479 (2019).
Onrust, J., Wymenga, E., Piersma, T. & Olff, H. Earthworm activity and availability for meadow birds is restricted in intensively managed grasslands. J. Appl. Ecol. 56, 1333–1342. https://doi.org/10.1111/1365-2664.13356 (2019).
Eglington, S. M. et al. Managing water levels on wet grasslands to improve foraging conditions for breeding northern lapwing Vanellus vanellus. J. Appl. Ecol. 47, 451–458. https://doi.org/10.1111/j.1365-2664.2010.01783.x (2010).
Partridge, D. R. & Clark, J. A. Urban green roofs provide habitat for migrating and breeding birds and their arthropod prey. PLoS ONE 13, e0202298. https://doi.org/10.1371/journal.pone.0202298 (2018).
Pithon, J. A. et al. Grasslands provide diverse opportunities for bird species along an urban-rural gradient. Urban Ecosyst. 24, 1281–1294. https://doi.org/10.1007/s11252-021-01114-6 (2021).
Eglington, S. M. et al. Restoration of wet features for breeding waders on lowland grassland. J. Appl. Ecol. 45, 305–314. https://doi.org/10.1111/j.1365-2664.2007.01405.x (2008).
Acknowledgements
The authors are grateful to the editor and three anonymous reviewers for helpful comments on a former draft of the manuscript. Open Access publishing was enabled by ‘Projekt DEAL’.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Franz Löffler, Jonas Brüggeshemke, Felix Maximilian Freienstein and Steffen Kämpfer. The first draft of the manuscript was written by Franz Löffler. The first draft was mainly edited by Thomas Fartmann and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Löffler, F., Brüggeshemke, J., Freienstein, F.M. et al. Urban rooftops near sports pitches provide a safe haven for a declining shorebird. Sci Rep 14, 9248 (2024). https://doi.org/10.1038/s41598-024-59693-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59693-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.