Abstract
The subfamily Polygonoideae encompasses a diverse array of medicinal and horticultural plants that hold significant economic value. However, due to the lack of a robust taxonomy based on phylogenetic relationships, the classification within this family is perplexing, and there is also a scarcity of reports on the chloroplast genomes of many plants falling under this classification. In this study, we conducted a comprehensive analysis by sequencing and characterizing the complete chloroplast genomes of six Polygonoideae plants, namely Pteroxygonum denticulatum, Pleuropterus multiflorus, Pleuropterus ciliinervis, Fallopia aubertii, Fallopia dentatoalata, and Fallopia convolvulus. Our findings revealed that these six plants possess chloroplast genomes with a typical quadripartite structure, averaging 162,931 bp in length. Comparative chloroplast analysis, codon usage analysis, and repetitive sequence analysis demonstrated a high level of conservation within the chloroplast genomes of these plants. Furthermore, phylogenetic analysis unveiled a distinct clade occupied by P. denticulatum, while P. ciliinrvis displayed a closer relationship to the three plants belonging to the Fallopia genus. Selective pressure analysis based on maximum likelihood trees showed that a total of 14 protein-coding genes exhibited positive selection, with psbB and ycf1 having the highest number of positive amino acid sites. Additionally, we identified four molecular markers, namely petN-psbM, psal-ycf4, ycf3-trnS-GGA, and trnL-UAG-ccsA, which exhibit high variability and can be utilized for the identification of these six plants.
Similar content being viewed by others
Introduction
Subfamily Polygonoideae Eaton is one of the most species-rich subfamilies within the Polygonaceae family, containing plants from genera such as Persicaria (L.) Mill, Polygonum L, and Fallopia Adans1. Modern pharmacological studies have demonstrated that many plants from the Subfam. Polygonoideae possess significant medicinal value. For instance, Pteroxygonum denticulatum (C. C. Huang) T. M. Schust. & Reveal has anti-inflammatory, anti-gastric ulcer, and antibacterial properties2, while Pleuropterus multiflorus (Thunb.) Nakai exhibits hypolipidemic3 and antidiabetic effects4. These medicinal plants are primarily found in East Asia, specifically in China, Japan, and Korea, and have a long history of use in traditional Chinese medicine.
Compared to other plant families, identification of Polygonaceae is generally straightforward. However, distinguishing them within the family itself poses challenges due to significant morphological variations. Consequently, taxonomic arrangements have been subject to discrepancies and the proliferation of names5. The tribe Persicarieae Dumort., belonging to the Subfam. Polygonoideae, has encountered such issues. For instance, the 1998 edition of FRPS (Flora Reipublicae Popularis Sinicae) reported approximately 20 species within the genus Fallopia, including Fallopia multiflora (synonymous with Pleuropterus multiflorus) and Fallopia denticulata (synonymous with Pteroxygonum denticulatum)1. In 2015, Schuster et al.6 screened three barcodes (nrITS, matK, trnL-trnF) and analyzed 199 species of Polygonaceae using maximum likelihood and Bayesian methods. Their results showed that P. denticulatum clustered with Pteroxygonum giraldii Damm. et Diels as a branch and that Fallopia denticulata (Huang) A. J. Li was revised to Pteroxygonum denticulatum (C. C. Huang) T. M. Schust. & Reveal. Furthermore, the “Catalogue of Life China: 2023 Annual Checklist” reassigned Genus Fallopia, as documented in the Flora of China, into three genera: Pteroxygonum (including P. denticulatum and P. giraldii), Pleuropterus (including P. multiflorus and Pleuropterus ciliinervis Nakai), and Fallopia (including Fallopia aubertii (L. Henry) Holub, Fallopia convolvulus (L.) Á. Löve, Fallopia cynanchoides (Hemsl.) Harald., Fallopia dentatoalata (F. Schmidt) Holub, and Fallopia dumetorum (Linnaeus) Holub)7. Although the classification of Polygonaceae plants is continuously being updated, the latest plant revision names have not been widely promoted. For instance, most recent studies on P. multiflorus still use its synonym F. multiflora8,9,10.
Chloroplasts play a crucial role in energy conversion and photosynthesis in plants. They are also involved in developmental processes, secondary metabolic activities11, and facilitate gene expression coordination between organelles and the nuclear genome12. Chloroplasts are found in land plants, algae, and certain protozoa. In plants, the chloroplast genome constitutes one of the three genetic systems, alongside the nucleus and mitochondria. These genetic systems contain both eukaryotic introns and prokaryotic operons13. Furthermore, chloroplasts are semi-autonomous organelles, possessing their own transcription and transport systems, as well as independent genomes14. In addition to synthesizing sugars through photosynthesis, chloroplasts also participate in the synthesis of complex organic substances such as amino acids and fatty acids15.
The advancement of high-throughput sequencing technologies has significantly enhanced our understanding of chloroplast genomes. Modern chloroplast genomes exhibit common structural features, typically ranging in size from 107 to 218 kb, characterized by compactness and containing approximately 100–120 genes16. A typical chloroplast genome follows a cyclic pattern and consists of four components: a large single-copy (LSC) region, a small single-copy (SSC) region, and two inverted repeat (IR) regions17,18. The variation in chloroplast genomes among different plant species mainly stems from the contraction and expansion of the IR region19,20. Chloroplast genomes exhibit high conservation in terms of organization, gene order, and content, ensuring homology across evolutionary groups. Moreover, these genomes are effectively haploid, ensuring genetic homogeneity among species. As a result, chloroplast genomes serve as an ideal model for investigating species identification and evolution. For instance, Yu and Ye21,22 conducted sequencing of the chloroplast genomes of Polygonum chinense L. and Polygonum cuspidatum Siebold et Zucc., classifying the plants based on the obtained results. Guo et al.23 sequenced and assembled the chloroplast genomes of four species from genus Polygonum, followed by phylogenetic analysis, revealing the relationships between them. Additionally, Chen et al.24 analyzed chloroplast genomes of Reynoutria japonica Houtt. from different regions, performing phylogenetic analyses that highlighted R. japonica's close association with P. multiflorus. Furthermore, Chinese R. japonica could be further divided into two distinct main groups.
Chloroplast genomes offer a valuable genetic resource for investigating genetic and evolutionary relationships among species in the Polygonaceae family. However, studies focusing on chloroplast genomes of Polygonaceae species remain limited. To address this knowledge gap, this study pursued the following objectives: (i) sequencing and assembling the chloroplast genomes of six plants from Subfam. Polygonoideae (P. denticulatum, P. multiflorus, P. ciliinervis, F. aubertii, F. dentatoalata, F. convolvulus) to contribute to the field of chloroplast research, (ii) reconstructing the phylogeny by integrating the sequencing results with available chloroplast genomes of other published Polygonaceae plants to provide robust evidence for the classification of species from Subfam. Polygonoideae, and (iii) developing molecular markers based on highly variable regions within the chloroplast genome.
Materials and methods
Sample collection, DNA extraction, and sequencing
The collection of fresh leaves from the six Subfam. Polygonoideae species was conducted in accordance with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora, with permission obtained from the local authorities at the collection sites (Fig. 1). The samples were identified as Pteroxygonum denticulatum (C. C. Huang) T. M. Schust. & Reveal, Pleuropterus multiflorus (Thunb.) Nakai, Pleuropterus ciliinervis Nakai, Fallopia aubertii (L. Henry) Holub, Fallopia dentatoalata (F. Schmidt) Holub and Fallopia convolvulus (L.) Á. Löve by Professor Yu-Lin Lin of the Institute of Medicinal Plants, Chinese Academy of Medical Sciences, and voucher specimens were stored in the Herbarium of the same institute with the codes CMPB57201, CMPB57202, CMPB57203, CMPB57204, CMPB57205 and CMPB57206. All fresh leaves underwent thorough washing with distilled water, followed by drying. The dried leaves were then carefully wrapped in tin foil, labeled, and rapidly frozen in liquid nitrogen. Subsequently, the samples were placed in a transport box filled with dry ice to maintain their frozen state. To ensure preservation, the samples were stored at −80 °C in an ultra-low temperature refrigerator, facilitating subsequent DNA extraction and other experimental studies.
The extraction of plant genome utilized the Plant Genome DNA Extraction Kit (Tiangen Biochemical Technology (Beijing) Co., Ltd.). Subsequently, the DNA's quality and concentration were assessed through 1% (w/v) agarose gel electrophoresis and Qubit 3.0 (Thermo Fisher Scientific, USA). For sequencing, the Illumina technology platform was employed, and a library with a 400 base pairs (bp) insert size was constructed using 500 ng of DNA. The sequencing process followed a double-end (2 × 150 bp) sequencing strategy as per the manufacturer's instructions for the Hiseq platform (Illumina Inc, USA).
Chloroplast genome assembly and annotation
The clean data was assembled on a Linux operating system using the Get Organelle software25. To validate the accurate assembly of chloroplast genes, raw reads were mapped to the clean data using BOWtie2 software (v2.0.1)26. This mapping process assessed the coverage of chloroplast genome sequences and confirmed the integrity of individual contig junctions. Following assembly, the six species were subjected to annotation using the CPGAVAS2 online platform27, with P. multiflorus (NC_041239) serving as the reference. Manual adjustment and correction of gene boundary positions were performed using the Apollo software28. Visualization of the annotated results was accomplished through the CHLOROPLOT (https://irscope.shinyapps.io/Chloroplot/) online platform29.
Genome comparison
The fundamental characteristics of the assembled and annotated chloroplast genomes of the six plant species were examined. Geneious v11.1 (https://www.geneious.com) was employed to determine the total length, gene count, base content, gene length, and repetitive gene count for each species' chloroplast genomes. The Blast tool30 and the "Find Repeats regions" script were utilized to identify the large single copy (LSC) and small single copy (SSC) regions within the plant chloroplast genome, along with the two inverted repeats that separate them. Through a comparison of the tetrameric regions' genome lengths and base contents, the total genome lengths and base contents of the six plants were derived. Furthermore, the genes annotated in the genomes of each species were categorized based on their functional classification, and the differences in functional genes were quantified.
To assess differences in the boundaries of the four regions (IRa, IRb, LSC, SSC) within the chloroplast genome of the six species, the online visualization tool IRscope31 was utilized. The occurrence of gene rearrangement was examined using Mauve32, and collinearity analysis was conducted on the chloroplast genomes of the six species. Furthermore, a genome-wide comparative analysis was performed utilizing the mVISTA (http://genome.lbl.gov/vista/mvista/submit.shtml) online platform33,34 to explore discrepancies among the six sequences.
Analysis of base composition and codon usage
The base composition differences and the relative usage of synonymous codons among the six chloroplast genomes were examined. For this analysis, the Geneious v11.1 was employed to determine the base composition of the six chloroplast genome sequences. Furthermore, all protein-coding sequences were extracted, exported to fasta format, and consolidated into a single fasta file, using Geneious v11.1 software. To assess the distribution of codon usage, the CodonW with relative synonymous codon usage (RSCU) ratios was utilized for the final analysis.
Repeated Sequence and Simple Sequence Repeat Analysis
The identification of long fragment repeats within the chloroplast genome sequences was performed using the online tool REPuter35. The parameters were configured as follows: Forward repeat, Reverse repeat, Complement repeat, and Palindrome repeat. The minimum length for a repeat unit was set to 30 bp, and the maximum number of detected repeat sequences was limited to 1000. The similarity threshold for repeat sequences was set at 90%, while the Hamming distance was specified as 3.
The detection of simple sequence repeats (SSRs) sequences within the chloroplast genome sequences was accomplished using the MISA script, developed in the Perl programming language36. The parameter settings were as follows: mononucleotide repeats with a minimum of 10 repeats, dinucleotide repeats with a minimum of 8 repeats, trinucleotide repeats with a minimum of 4 repeats, and tetranucleotide, pentanucleotide, and hexanucleotide repeats with a minimum of 3 repeats37.
Phylogenetic analysis
To investigate the phylogeny of Polygonaceae, a phylogenetic analysis was conducted using the chloroplast genomes of 29 species from the family Polygonaceae and two outgroups (Myricaria prostrata NC_046761.1, Tamarix taklamakanensis NC_054218.1). A total of 23 sequences were obtained from the NCBI database38, while the remaining six sequences were obtained through sequencing in this study. The phylogenetic tree was constructed using the Maximum Likelihood (ML), Maximum Parsimony (MP), and Neighbor-Joining (NJ) methods. The coding sequences (CDS) of the entire genome and the full-length chloroplast sequences were separately utilized for the tree construction.
The CDS from the chloroplast genome sequences of 31 species were extracted using the "Extract" function in PhyloSuite39. The sequence type was set to "Chloroplast Genome". Multi-copy genes were screened, and the selected coding sequences were saved in fasta format. The "MAFFT" function40 in PhyloSuite was utilized to perform multiple gene alignment of the CDS sequences. The alignment mode was set to "Normal", with the standard code and an auto strategy (-auto). The aligned CDS sequences were then concatenated using the "Concatenate Sequence" function in PhyloSuite. The resulting concatenated sequences were saved in fasta, phy, and nex formats. For constructing the ML tree, the "IQ-TREE" function in PhyloSuite41 was employed. The concatenated phy format file was imported, with M. prostrata and T. taklamakanensis selected as the outgroup. The default parameters were utilized. To build the MP trees, the "Construct/Test Maximum Parsimony Tree(s)" function in MEGA-X software42 was utilized. The concatenated fasta file obtained earlier was imported, selecting "Nucleotide Sequences" as the data type and "Standard" as the gene model. The phylogenetic test was performed using the bootstrap method with 1000 replicates, while the remaining parameters were set to their default values. The NJ tree was constructed using the "Construct/Test Neighbor-Joining Tree(s)" function in MEGA-X software42. The concatenated fasta format file was imported, selecting "Nucleotide sequence" as the data type and "Standard" as the gene mode. The phylogeny test was conducted using the bootstrap method with 1000 replicates, and the model/method selected was "p-distance". The default values were used for the remaining parameters.
Non-synonymous and synonymous substitution rate analysis
To identify the genes under selection, we scanned the chloroplast genomes of 29 species from the family Polygonaceae and two outgroups (Myricaria prostrata NC_046761.1, Tamarix taklamakanensis NC_054218.1) using the software EasyCondeML43. This tool facilitated the computation of non-synonymous (dN) and synonymous (dS) substitution rates, as well as their ratios (ω = dN/dS). Selective pressure analyses were carried out on the maximum likelihood (ML) tree of these 31 species, formatted in Newick. Each single-copy CDS sequence was aligned based on its amino acid sequence. We employed a site-specific model with five site models (M0, M1a & M2a, M7 & M8) to pinpoint adaptation signatures across chloroplast genomes. This model allowed the ω ratio to vary among sites while maintaining a fixed ω ratio across all branches. Specifically, the site-specific models, M1a (nearly neutral) vs. M2a (positive selection), and M7 (β) vs. M8 (β & ω) were computed to detect positive selection44. Likelihood ratio tests (LRT) comparing M1a vs. M2a and M7 vs. M8 were employed to assess the strength of selection. Subsequently, the Bayes empirical Bayes (BEB) method45 was utilized to calculate posterior probabilities. In the BEB analysis, posterior probabilities exceeding 0.95 and 0.99 indicated sites subject to positive selection and strong positive selection, respectively.
Analysis of highly variable areas
A custom script was developed to identify the most divergent regions. Using this script, the intergenic spacer regions (IGS) of six plant chloroplasts were extracted from the GenBank file, with their respective starting and ending points. The extracted sequences were then compared using the ClustalW2 program (v. 2.0.12) with the parameters "-type = DNA -gapopen = 10 -gapext = 2"46. Pairwise distances were calculated using the K2p evolutionary model implemented in the distmat program of the EMBOSS package (v. 6.3.1)47.
Primer design and PCR validation
To facilitate the development of molecular markers for the six plants, we screened the highly variable regions within their chloroplast genomes. The selected candidate sequences had a length ranging from 300 to 1000 bp, ensuring a high success rate for amplification and sequencing. Moreover, to facilitate the design of universal primers, the candidate sequences were conserved at both ends. Subsequently, the primers were designed using SnapGene software, and the primer sequences were submitted to TsingkeBiotechnology Co, Ltd.
We carefully selected primers that displayed clear, single, and accurate amplification bands across individual samples. Subsequently, PCR amplification was conducted on the total DNA extracted from each of the six plants. The reaction protocol involved an initial denaturation step at 95 °C for 3 min, followed by 32–36 cycles consisting of denaturation at 94 °C for 25 s, annealing at 55–64 °C for 25 s, and extension at 72 °C for 10–15 s. A final extension step was performed at 72 °C for 5 min. The resulting PCR amplification products were visualized by gel electrophoresis using a 1.5% agarose gel stained with SYBR. Gel electrophoresis was carried out under the following conditions: 130 V, 120 mA, 300 W for 45 min. Subsequently, the PCR amplification products were sent to TsingkeBiotechnology Co, Ltd. for further processing. The sequencing peak maps obtained from the sequencing company were analyzed using SnapGene software. The software was used to read the peak maps, perform proofreading, and eliminate low-quality sequences as well as primer regions.
Result
Comparative analysis of chloroplast genomes of six species of Polygonaceae
Table S1 presents the data acquired through sequencing on the Illumina HiSeq platform. The largest total raw read count was 21,319,251 bp for F. aubertii, while the smallest count was 17,329,101 bp for P. ciliinervis.
Chloroplast genome sequence characteristics and gene structure
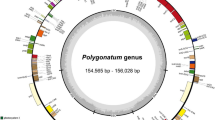
The chloroplast genomes of the six plants analyzed in this study exhibit a typical quadripartite structure consisting of LSC region, SSC region, and two IR regions (Fig. 2). Among the plants studied, P. ciliinervis has the longest chloroplast genome, measuring 163,583 bp, while F. aubertii has the shortest chloroplast genome, measuring 162,393 bp. The average length of the chloroplast genomes in the six studied plants was 162,931 bp, and their differences in length were minimal, with the largest discrepancy being only 1,190 bp. Regarding the LSC, SSC, and IR regions, the LSC region spanned from 87,279 to 88,245 bp, with an average length of 87,730 bp. The SSC region ranged from 13,170 to 13,564 bp, with an average length of 13,453 bp. Finally, the IR region varied from 30,853 to 30,899 bp, with an average length of 30,873 bp.
In the complete chloroplast genome, the GC content ranged from 37.38% to 37.66%. Furthermore, the LSC, SSC, and IR regions exhibited GC contents of 35.39–35.83%, 32.28–32.92%, and 41.21–41.28%, respectively (Table S2). Table 1 provide a comprehensive overview of the chloroplast genomes of these six plants, revealing a total of 133 genes encoded, including 88 protein-coding genes, 37 (Transfer RNA) tRNA genes, and 8 rRNA (Ribosome RNA) genes. Notably, each IR region contained 16 genes, encompassing 4 rRNA genes, 6 tRNA genes, and 6 protein-coding genes. The chloroplast genomes of the six plants analyzed in this study primarily consisted of genes associated with photosynthesis and self-replication. The photosynthetic gene category encompassed subunits of ATP synthase, subunits of photosystem II, and subunits of NADH-dehydrogenase, among others. The self-replicating gene category included the large subunit of ribosome, DNA-dependent RNA polymerase, and small subunit of ribosome. P. denticulatum and F. aubertii each possess 21 genes with introns, while P. multiflorus, F. dentatoalata, and F. convolvulus have 19 genes with introns, and P. ciliinervis has 18 genes with introns. Notably, all these plants share two identical genes, clpP and ycf3, each containing two introns and three exons (Table S3).
Note: Genes outside the circle are transcribed counterclockwise, while genes inside the circle are transcribed clockwise. Genes with different functions are indicated by different colors. The purple inner circle indicates GC content, while the green inner circle indicates AT content.
Comparative IR/SC boundary analysis
In this study, we conducted a comparative analysis of expansion and gene rearrangement at the boundaries of the LSC, SSC, and IR regions in the chloroplast genomes of six species (Fig. 3). Each of the six species possessed a complete LSC region spanning from 87,279 bp to 88,245 bp, an SSC region ranging from 13,170 bp to 13,564 bp, and an IR region in between spanning from 30,853 bp to 30,899 bp. Due to differential expansion and contraction of the LSC/IRb and IRb/SSC regions within the chloroplast genome of these six plants, significant alterations occurred at the boundaries of this region. For instance, the IRa region exhibited loss of the rps19 and ndhF, while IRb region exhibited loss of the ycf1. The results indicated that the distribution and length of genes at the boundary of the chloroplast quadripartite structure were highly consistent among the six plants, yet each underwent varying degrees of expansion and contraction.
Collinearity analysis and sequence variation analysis of the chloroplast genome
The chloroplast genomes of the six species were subjected to collinearity analysis using Mauve software, revealing that gene rearrangements were absent in these plants' chloroplast genomes (Fig. 4). To further elucidate the differences among the chloroplast sequences of the six species, a global sequence alignment was performed using mVISTA. The results revealed minimal variations among the chloroplast sequences of the six species, indicating a high degree of sequence conservation (Fig. 5). Nonetheless, several regions and genes, namely rps16 ~ trnQ-UUG, trnS-GCU ~ trnG-UCC, rpoB ~ trnC-GCA, petN ~ psbM, rps4 ~ trnT-UGU, petD ~ rpoA, and rpl16, exhibited notably higher variation rates within the six chloroplast genomes. These regions and genes hold substantial potential for supporting future studies on phylogeny, genetic diversity, and species identification.
Note: Gray arrows represent genes, purple areas represent exon regions, blue areas represent tRNA regions, red areas represent IGS and intron regions, brown areas represent rRNA regions, vertical scale represents the percentage of identity, ranging from 50 to 100%.
Analysis of codon usage
The codon usage frequencies of the six chloroplast genomes, comprising 27,413 to 27,676 codons (Fig. 6). The most commonly utilized amino acids were arginine (Arg) and leucine (Leu). Conversely, amino acids such as cysteine (Cys), aspartic acid (Asp), histidine (His), methionine (Met), proline (Pro), glutamine (Gln), arginine (Arg), tryptophan (Trp), and tyrosine (Tyr) had less than 2,000 codons. Notably, cysteine (Cys) exhibited the lowest frequency, occurring only 603 times, accounting for 1.29% of the total codons. In terms of stop codons, all six species predominantly employed UAA, which accounted for over 50% of occurrences. Thirty codons in all six species possessed RSCU values greater than 1, with 29 of them ending in A/U, except for UUG encoding leucine (Leu), which deviated from the A/U pattern. On the other hand, 32 codons had RSCU values below 1, with 29 of them ending in C/G, except for AUA for isoleucine (Ile), CUA for leucine (Leu), and UGA for the terminator (TER), which did not end in C/G. Methionine (Met) and tryptophan (Trp) displayed an RSCU value of 1, signifying no particular preference in codon usage.
Repeated sequence and simple sequence repeat analysis
Table 2 reveals that P. denticulatum exhibited the highest number of SSRs, amounting to 62. Among these, the mononucleotide SSRs were predominantly composed of thymine (T) and adenine (A) repeats, while the dinucleotide SSRs primarily consisted of TA repeats. Notably, the guanine (G) repeat type was only detected in F. convolvulus. Upon further examination and comparison of the size and position of the different SSR units, it was found that T and A repeats were the most abundant SSR types across all six species.
To identify the presence of long repeat sequences in the chloroplast genomes, the online tool REPuter was employed, and the distribution of these repeats is illustrated in Figs. 7. Interestingly, P. denticulatum and F. convolvulus lacked complement repeats in comparison to the other species. Among the six chloroplast genomes, palindrome repeats were identified, with the highest number falling within the range of 11–20 bp and 21–30 bp. Furthermore, no sequences exceeding 30 bp exhibited complement or reverse repeats, and there were no sequences surpassing 50 bp with forward or palindrome repeats.
Phylogenetic analysis
To elucidate the phylogenetic position of the six Polygonaceae plants under investigation, a comprehensive phylogenetic analysis was conducted using 31 known chloroplast genome sequences from the Polygonaceae family. Both CDS and complete chloroplast genome sequences were employed in the phylogenetic analyses, and the results are presented in Figs. 8. The ML, MP, and NJ trees based on both CDS and complete chloroplast genome sequences exhibited strong branch support, indicating the reliability of the constructed trees. Significantly, P. multiflorus forms a cluster along with the NCBI publication of P. multiflorus (NC_041239.1), indicating a close relationship with Fallopia sachalinensis. Furthermore, P. ciliinervis, F. aubertii, F. dentatoalata, and F. convolvulus group together, while P. denticulatum holds a distinctive position outside this cluster, implying its divergence from the other five species.
Exploration on the phylogeny of 29 species of Polygonaceae. (A) ML phylogenetic tree based on CDS sequence; (B) MP phylogenetic tree based on CDS sequence; (C) NJ phylogenetic tree based on CDS sequence; (D) ML phylogenetic tree based on complete chloroplast genome; (E) Based on MP phylogenetic tree of the complete chloroplast genome; (F) NJ phylogenetic tree based on the complete chloroplast genome.
Selective pressures analysis
The non-synonymous to synonymous substitution ratio (ω = dN/dS) was examined for all 62 shared protein-coding genes across 31 complete chloroplast genomes in Polygonaceae. According to the M8 model (β & ω > 1), a total of 14 protein-coding genes exhibited positive selection with a posterior probability exceeding 0.95, as determined by the Bayes empirical Bayes (BEB) method (Table 3). Among these genes, psbB displayed the highest count of positive amino acid sites (74), trailed by ycf1 (18) and ycf2 (12). The remaining 11 protein-coding genes—namely atpA, atpB, atpI, ndhA, ndhB, ndhF, ndhK, psbJ, rbcL, rpoA, and rps7—each featured 2, 1, 1, 3, 4, 1, 1, 1, 3, 1, and 1 amino acid sites, respectively, that were conclusively under positive selection.
Analysis of highly variable areas
In this study, we employed the K2p model to compare pairwise intergenic regions (IGS) and identify regions of pronounced differentiation among the six species (Fig. 9). Out of the 115 intergenic regions analyzed, the K2p genetic distances ranged from 0.24 to 81.3. Notably, the IGS regions such as psaI-ycf4, rpl22-rps19, and trnS-GCU-trnG-UCC exhibited the highest K2p genetic distances of 81.3, 52.25, and 20.25, respectively. These intergenic regions hold significant potential for the development of molecular markers in future studies.
Primer design and PCR validation
In order to differentiate among the six species, we employed SnapGene to screen 115 regions known for high variability. From this analysis, we identified 16 intergenic regions that could potentially serve as molecular markers (Table S4). PCR amplification of total DNA extracted from samples of the six species confirmed the specificity of petN-psbM, psal-ycf4, ycf3-trnS-GGA, and trnL-UAG-ccsA to these six species, yielding distinct and well-defined bands on agarose gels (Fig. S1). Subsequently, DNA fragments were extracted from each strip and subjected to Sanger sequencing, revealing variant loci among the different plants (Fig. 10). As a result, these four molecular markers, either individually or in combination, facilitate the identification of the six plants based on these variant loci.
Disscussion
This study aimed to sequence and analyze the chloroplast genomes of six species belonging to Subfam. Polygonoideae. For the first time, we obtained the complete chloroplast genomes of P. denticulatum, F. aubertii, F. dentatoalata, and F. convolvulus. The chloroplast genomes of these six species exhibit a typical quadripartite structure, which is consistent with other plants of the Polygonaceae family, such as Calligonum L.48, Polygonum L.23, F. sachalinensis49, and Reynoutria japonica Houtt.24. This phenomenon is also widely observed in other angiosperm chloroplast genomes50,51,52.
The lengths of the chloroplast genomes in these six species do not differ significantly, and the lengths of the tetrad regions are consistent with other Polygonaceae plants, including Fagopyrum Mill.53, Muehlenbeckia Meisn.54, and Persicaria chinensis (L.) H. Gross21. Furthermore, the GC content of the chloroplast genomes in these six plants is lower than the AT content, which is also observed in other Polygonaceae plants such as Fagopyrum dibotrys (D. Don) Hara55 and R. japonica56. The higher GC content in the IR region compared to the LSC and SSC regions is worth noting, possibly attributed to the abundance of rRNA within the IR region. These aforementioned plants belong to distinct genera within the Polygonaceae classification, and their geographical distribution is scattered. This indicates that the chloroplast genomes of most Polygonaceae plants share common structural and length characteristics.
Introns, non-coding regions found in RNA transcripts or DNA encoding RNA, play a vital role in gene expression regulation57. They can significantly enhance the expression of exogenous genes at specific times and locations in plants, thereby controlling gene expression levels across various spatial and temporal contexts58. In our study, we identified genes with introns in all six plant species examined, with the number of introns ranging from 18 to 21. Notably, we observed a consistent pattern wherein both clpP and ycf3 genes in each plant contained two introns and three exons. This finding differs from the results reported by Guo et al.23 for Polygonum, which also belongs to the Polygonaceae family. Guo et al. showed that four Polygonum plants possessed only one gene (rps12) with three exons, while two genes (ycf3 and clpP) contained two introns. Furthermore, they observed that the 5' end of the rps12 gene in Polygonum was located in the LSC region, with its 3' end duplicated in the IRs region, whereas in all plants examined in our study, the rps12 gene was exclusively situated in the IR region. Although the occurrence and loss of introns in plant chloroplast genomes are observable, the precise regulatory mechanisms governing them in plants remain unclear23. Moreover, there exists a research gap concerning the study of introns in Polygonaceae plants, highlighting the need for future investigations to gain a deeper understanding of their functions.
The presence of the IR region is a recurring feature in most chloroplast genomes16, and the size of the IR region, containing rRNA genes, varies significantly across different biological clades59. Four junctions, namely JLA, JSA, JSB, and JLB, exist between the two IRs and the SC region60. The successive expansions of the IR have led to floating JLAs and JLBs, which hold evolutionary significance61. However, the mechanism behind IR region expansion and contraction, primarily governed by the double-strand break repair (DCBR) theory62, remains a subject of controversy. In our study, we observed similar levels of IR boundary contraction and expansion among all six Polygonoidea plants. Specifically, rps19 and ndhF straddle the JLB and JSB boundaries, respectively, while ycf1 is exclusively present in the IRa region. This consistency aligns with Polygonum23. Based on these findings, it is reasonable to speculate that all or some of the biological lineages within Polygonoidea share the same IR boundary feature. To confirm this hypothesis, further analysis of chloroplast genomes in Polygonoidea plants is warranted.
Codons, also known as the genetic code, serve as a crucial link between nucleic acids and proteins. They play a pivotal role in recognizing and transmitting biological genetic information, contributing significantly to genetic processes and variations in living organisms63. Due to variations in protein translation processes across different species, there is a tendency to utilize specific synonymous codons during translation, which is referred to as codon usage bias (CUB)64, which plays an important role in cellular metabolic processes such as mRNA translation and DNA transcription65. In the chloroplast genomes of the six plants examined in this study, Arg and Leu were found to be more frequently used. Among amino acids with a total codon count below 2,000, Cys exhibited the lowest frequency, aligning with patterns observed in terrestrial plants like Allium mongolicum Regel66 and Psammosilene tunicoides W.C. Wu et C.Y. Wu67. All six species displayed a preference for UAA as a termination codon, and each species exhibited 30 codons with RSCU values greater than 1. Remarkably, 29 out of these 30 codons concluded with A/U, demonstrating strong consistency with the observed phenomenon in Polygonum, which also belongs to Polygonoidea23.
Variable regions of the chloroplast genome are usually associated with many repetitive sequences, and most of them are located in intergenic regions or on the same gene68. Simple repeat sequences (SSRs), also known as Microsatellites, are widely distributed in chloroplast genomes and are extremely variable, and therefore are often used as molecular markers to study chloroplast genome evolution and population genetics69,70. In the six selected plant chloroplast genomes analyzed in this study, the SSRs primarily consist of single nucleotides, aligning with observations in Polygonum. However, it is noteworthy that the total number of SSRs in the studied plants is significantly lower than that observed in Polygonum. Moreover, highly variable regions play a crucial role in resolving phylogenies and distinguishing closely related plant species71. In this study, we employed a combined approach of highly variable region analysis and PCR amplification analysis to identify four chloroplast gene regions suitable as molecular markers, namely petN-psbM, psal-ycf4, ycf3-trnS-GGA, and trnL-UAG-ccsA. Single-nucleotide polymorphisms within intergenic regions may directly impact the structural conformation or expression levels of proteins, depending on their specific locations. This could potentially influence the morphology and genetic mechanisms of plants72. Prior research has successfully employed molecular markers derived from petN-psbM to differentiate between five Alpinia Roxb. species73. The variable hotspot region of ycf3-trnS-GGA has been utilized for assessing interspecific differentiation in Dipsacales species. It is proposed as a candidate DNA barcode for species within Adoxaceae and Caprifoliaceae74. Similarly, this region has been employed for identifying three Salvia L. species75.
Previous studies have utilized chloroplast genomic techniques to conduct phylogenetic analysis of Polygonaceae plants, shedding light on the issue of taxonomic confusion within this plant family6,23,24. In this study, we employed both CDS and whole genome data to reconstruct the phylogenetic relationships among 29 Polygonaceae plants. Remarkably, the phylogenetic trees constructed using these two data types exhibited high consistency, validating the reliability of utilizing chloroplast genomes for phylogenetic reconstruction. Notably, our findings revealed a distinct clustering of F. sachalinensis with P. multiflorus within the same clade, while P. ciliinervis displayed a closer relationship with Genus Fallopia. These results diverge from the current classification wherein P. multiflorus and P. ciliinervis are grouped within the same genus7. Additionally, our results consistently support the conclusion that P. denticulatum does not belong to Genus Fallopia and that F. dentatoalata is more closely related to F. convolvulus in Genus Fallopia, aligning with previous studies6,76. However, due to the unavailability of fresh leaves from P. giraldii and the subsequent lack of chloroplast genomic data for this species, we were unable to ascertain the phylogenetic relationship between P. denticulatum and P. giraldii based on chloroplast genomes. This calls for more comprehensive sampling and further investigation in future studies.
Positive selection is considered pivotal in the adaptation of organisms to various environments77, while negative (purifying) selection stands as a prevalent evolutionary force accountable for genomic sequence conservation over extensive evolutionary periods78. The ratio (ω = dN/dS) has served as a widely employed metric for gauging selective pressure79,80,81. The ω ratio > 1 represents positive selection, whereas ω < 1 indicates purifying selection80. In this investigation, we identified 14 genes harboring positive selection sites. Within these genes featuring amino acid positive sites, it was observed that the psbB, ycf1, and ycf2 genes exhibited a higher count (74, 18, 12 respectively) of positive amino acid sites among Polygonaceae species, suggesting a potentially significant role of the psbB gene in the adaptive evolution of Polygonaceae species. Additionally, a gene (rps7) encoding ribosomal subunit protein was found under positive selection, which is deemed crucial for chloroplast biogenesis and function, implying that Polygonaceae plants might enhance evolutionary adaptability by modulating the encoding of ribosomal subunit protein in chloroplasts82. Furthermore, nine photosynthesis-related genes, namely atpA, atpB, atpI, psbB, psbJ, ndhA, ndhB, ndhF, ndhk, and rbcL, were also identified with positive selection sites in the present study. Recent investigations have indicated the prevalence of these 14 genes exhibiting positive selection across certain angiosperms. For instance, ndhF has been documented as undergoing positive selection in Aroideae species79; ndhA, ndhB, psbB, rbcL, rps7, ycf1, and ycf2 have been reported as undergoing positive selection in Zingiber species83,84; while atpA, atpB, atpI have been identified as undergoing positive selection in Chrysosplenium species85. Polygonaceae represents a cosmopolitan plant family widely distributed across the northern temperate regions and occasionally found in tropical regions86. Consequently, Polygonaceae species are likely subjected to various environmental stresses in their ecological habitats, and these 14 genes undergoing positive selection may exert crucial roles during the evolution and adaptation of Polygonaceae species to their respective ecological habitats.
Polygonaceae encompasses a diverse array of medicinal and horticultural plants that possess significant economic value6,87. However, certain species within the Polygonaceae family exhibit a highly invasive nature in certain countries and regions, leading to severe ecological damage5. The absence of a robust taxonomy, grounded in an understanding of phylogenetic relationships, poses obstacles to the advancement of cash crops within Polygonaceae and the effective management of invasive species. Thus, it is imperative to undertake comprehensive investigations into the phylogeny of Polygonaceae plants in order to address these concerns. This paper focuses on three genera within Subfam. Polygonoidea, namely Pteroxygonum, Pleuropterus, and Fallopia. For the first time, we have obtained the complete chloroplast genome data of P. denticulatum, P. ciliinervis, F. aubertii, F. dentatoalata, and F. convolvulus. These newly acquired chloroplast genome data serve as a valuable resource for understanding the taxonomic, phylogenetic, and evolutionary history of Polygonoidea.
Data availability
The complete chloroplast genomes were generated in the NCBI database with Accession Number MZ618350, MZ579703, MZ618351, MZ618352, OK040956, OK040957.
References
Li, A., Gao, Z., Mao, Z. & Liu, Y. Flora Reipublicae Popularis Sinicae Vol. 25(1), 360 (Science Press, 1998).
Liu, Y. Studies on the chemical constituents and anti-inflammatory activity in vitro from the root of Fallopia denticuta of Yunnan Master thesis. Kunming University of Science and Technology (2019).
Li, F. et al. Inflammation inhibition and gut microbiota regulation by TSG to combat atherosclerosis in ApoE−/− mice. J. Ethnopharmacol. 247, 112232. https://doi.org/10.1016/j.jep.2019.112232 (2020).
Yang, J.-B. et al. Dianthrone derivatives from Polygonum multiflorum Thunb: Anti-diabetic activity, structure-activity relationships (SARs), and mode of action. Bioorg. Chem. 135, 106491. https://doi.org/10.1016/j.bioorg.2023.106491 (2023).
Schuster, T., Wilson, K. & Kron, K. Phylogenetic relationships of Muehlenbeckia, Fallopia, and Reynoutria (Polygonaceae) investigated with chloroplast and nuclear sequence data. Int. J. Plant Sci. 172, 1053–1066. https://doi.org/10.1086/661293 (2011).
Schuster, T. M., Reveal, J. L., Bayly, M. J. & Kron, K. A. An updated molecular phylogeny of Polygonoideae (Polygonaceae): Relationships of Oxygonum, Pteroxygonum, and Rumex, and a new circumscription of Koenigia. TAXON 64, 1188–1208. https://doi.org/10.12705/646.5 (2015).
Liu, B. Catalogue of Life China: 2023 Annual Checklist (ed Biodiversity Committee of Chinese Academy of Sciences) (Beijing, 2023).
Yang, Z. Y. et al. Identification and functional characterization of three phenylalanine ammonia-lyase genes from Fallopia multiflora (Thunb.) Harald. Russ. J. Bioorg. Chem. 49, 655–663. https://doi.org/10.1134/s1068162023030263 (2023).
Zhao, Y. J. et al. The first chromosome-level Fallopia multiflora genome assembly provides insights into stilbene biosynthesis. Hortic. Res. 10, 047. https://doi.org/10.1093/hr/uhad047 (2023).
Yu, H. et al. Chemical constituents of the roots of Fallopia multiflora. Chem. Nat. Compd. 58, 125–128. https://doi.org/10.1007/s10600-022-03637-6 (2022).
Wicke, S., Schneeweiss, G. M., dePamphilis, C. W., Müller, K. F. & Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 76, 273–297. https://doi.org/10.1007/s11103-011-9762-4 (2011).
Woodson, J. D. & Chory, J. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 9, 383–395. https://doi.org/10.1038/nrg2348 (2008).
Bhattacharyya, D. & Chakraborty, S. Chloroplast: The Trojan horse in plant-virus interaction. Mol. Plant pathol. 19, 504–518. https://doi.org/10.1111/mpp.12533 (2018).
Clegg, M. T., Gaut, B. S., Learn, G. H. & Morton, B. R. Rates and patterns of chloroplast DNA evolution. Proc. Natl. Acad. Sci. 91, 6795–6801. https://doi.org/10.1073/pnas.91.15.6795 (1994).
Yao, X. H. et al. The first complete chloroplast genome sequences in actinidiaceae: Genome structure and comparative analysis. PLOS ONE 10, e0129347. https://doi.org/10.1371/journal.pone.0129347 (2015).
Daniell, H., Lin, C. S., Yu, M. & Chang, W. J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 17, 134. https://doi.org/10.1186/s13059-016-1004-2 (2016).
Aldrich, J., Cherney, B., Merlin, E., Williams, C. & Mets, L. Recombination within the inverted repeat sequences of the Chlamydomonas reinhardii chloroplast genome produces two orientation isomers. Current genetics 9, 233–238. https://doi.org/10.1007/bf00420317 (1985).
Yue, F., Cui, L., dePamphilis, C. W., Moret, B. M. & Tang, J. Gene rearrangement analysis and ancestral order inference from chloroplast genomes with inverted repeat. BMC Genomics 9(Suppl 1), S25. https://doi.org/10.1186/1471-2164-9-s1-s25 (2008).
Chang, C. C. et al. The chloroplast genome of Phalaenopsis aphrodite (Orchidaceae): Comparative analysis of evolutionary rate with that of grasses and its phylogenetic implications. Mol. Biol. Evol. 23, 279–291. https://doi.org/10.1093/molbev/msj029 (2006).
Guisinger, M. M., Kuehl, J. V., Boore, J. L. & Jansen, R. K. Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: Rearrangements, repeats, and codon usage. Mol. Biol. Evol. 28, 583–600. https://doi.org/10.1093/molbev/msq229 (2011).
Yu, B., Liu, J., Liu, X., Lan, X. & Qu, X. The complete chloroplast genome sequence of Polygonum chinense L. Mitochondrial DNA Part B 5, 2139–2140. https://doi.org/10.1080/23802359.2019.1693931 (2020).
Ye, X. et al. The complete chloroplast genome of the medicinal plant Polygonum cuspidatum (Polygonaceae) and its phylogenetic implications within the subfamily Polygonoideae. Mitochondrial DNA Part B 6, 1563–1565. https://doi.org/10.1080/23802359.2021.1917313 (2021).
Guo, S. et al. A comparative analysis of the chloroplast genomes of four polygonum medicinal plants. Front. Genet. 13, 764534. https://doi.org/10.3389/fgene.2022.764534 (2022).
Chen, J. H. et al. A comparative phylogenetic analysis on the chloroplast genome in different Reynoutria japonica populations. Genes 13, 1979 (2022).
Jin, J. J. et al. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21, 241. https://doi.org/10.1186/s13059-020-02154-5 (2020).
Langdon, W. B. Performance of genetic programming optimised Bowtie2 on genome comparison and analytic testing (GCAT) benchmarks. BioData Min. 8, 1. https://doi.org/10.1186/s13040-014-0034-0 (2015).
Shi, L. et al. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 47, W65-w73. https://doi.org/10.1093/nar/gkz345 (2019).
Firtina, C. et al. Apollo: A sequencing-technology-independent, scalable and accurate assembly polishing algorithm. Bioinformatics 36, 3669–3679. https://doi.org/10.1093/bioinformatics/btaa179 (2020).
Zheng, S., Poczai, P., Hyvönen, J., Tang, J. & Amiryousefi, A. Chloroplot: An online program for the versatile plotting of organelle genomes. Front. Genet. 11, 576124. https://doi.org/10.3389/fgene.2020.576124 (2020).
Mount, D. W. Using the basic local alignment search tool (BLAST). CSH Protoc. 2007, pdb.top17. https://doi.org/10.1101/pdb.top17 (2007).
Amiryousefi, A., Hyvönen, J. & Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 34, 3030–3031. https://doi.org/10.1093/bioinformatics/bty220 (2018).
Darling, A. C., Mau, B., Blattner, F. R. & Perna, N. T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14, 1394–1403. https://doi.org/10.1101/gr.2289704 (2004).
Brudno, M. et al. LAGAN and Multi-LAGAN: Efficient tools for large-scale multiple alignment of genomic DNA. Genome Res. 13, 721–731. https://doi.org/10.1101/gr.926603 (2003).
Frazer, K. A., Pachter, L., Poliakov, A., Rubin, E. M. & Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 32, W273-279. https://doi.org/10.1093/nar/gkh458 (2004).
Kurtz, S. et al. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 29, 4633–4642. https://doi.org/10.1093/nar/29.22.4633 (2001).
Beier, S., Thiel, T., Münch, T., Scholz, U. & Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 33, 2583–2585. https://doi.org/10.1093/bioinformatics/btx198 (2017).
Raman, G., Park, K. T., Kim, J. H. & Park, S. Characteristics of the completed chloroplast genome sequence of Xanthium spinosum: Comparative analyses, identification of mutational hotspots and phylogenetic implications. BMC Genomics 21, 855. https://doi.org/10.1186/s12864-020-07219-0 (2020).
Sayers, E. W. et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 49, D10-d17. https://doi.org/10.1093/nar/gkaa892 (2021).
Zhang, D. et al. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 20, 348–355. https://doi.org/10.1111/1755-0998.13096 (2020).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. https://doi.org/10.1093/molbev/mst010 (2013).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. https://doi.org/10.1093/molbev/msu300 (2015).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Gao, F. et al. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 9, 3891–3898. https://doi.org/10.1002/ece3.5015 (2019).
Yang, Z. & Nielsen, R. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol. Biol. Evol. 19, 908–917. https://doi.org/10.1093/oxfordjournals.molbev.a004148 (2002).
Yang, Z., Wong, W. S. W. & Nielsen, R. Bayes empirical Bayes inference of amino acid sites under positive selection. Mol. Biol. Evol. 22, 1107–1118. https://doi.org/10.1093/molbev/msi097 (2005).
Thompson, J. D., Gibson, T. J. & Higgins, D. G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. https://doi.org/10.1002/0471250953.bi0203s00 (2002).
Rice, P., Longden, I. & Bleasby, A. EMBOSS: The European molecular biology open software suite. Trends Genet. 16, 276–277. https://doi.org/10.1016/s0168-9525(00)02024-2 (2000).
Song, F., Li, T., Burgess, K. S., Feng, Y. & Ge, X. J. Complete plastome sequencing resolves taxonomic relationships among species of Calligonum L. (Polygonaceae) in China. BMC Plant Biol. 20, 261. https://doi.org/10.1186/s12870-020-02466-5 (2020).
Raman, G., Park, K. T., Nam, G. H., Kwak, M. & Park, S. Characterization of the complete chloroplast genome sequence of the giant knotweed, Fallopia sachalinensis from the volcanic island Dokdo, Republic of Korea. Mitochondrial DNA Part B 4, 2972–2973. https://doi.org/10.1080/23802359.2019.1663769 (2019).
Feng, Z. et al. The chloroplast genomes comparative analysis of Taihangia rupestris var. rupestris and Taihangia rupestris var. ciliata, two endangered and endemic cliff plants in Taihang Mountain of China. South Afr. J. Bot. 148, 499–509. https://doi.org/10.1016/j.sajb.2022.05.022 (2022).
Feng, Z. et al. Complete chloroplast genome of Gentianopsis barbata and comparative analysis with related species from Gentianaceae. Genome 65, 363–375. https://doi.org/10.1139/gen-2021-0080%M35531903 (2022).
Zheng, Y., Li, J., Chen, H. & Huang, L. The complete chloroplast genome sequence of Hemerocallis fulva. Mitochondrial DNA Part B Resour 5, 3543–3544. https://doi.org/10.1080/23802359.2020.1829126 (2020).
Wang, C. L. et al. Comparative analysis of four buckwheat species based on morphology and complete chloroplast genome sequences. Sci. Rep. 7, 6514. https://doi.org/10.1038/s41598-017-06638-6 (2017).
Maurin, K. J. L., Smissen, R. D. & Lusk, C. H. A dated phylogeny shows Plio-Pleistocene climates spurred evolution of antibrowsing defences in the New Zealand flora. New Phytol. 233, 546–554. https://doi.org/10.1111/nph.17766 (2022).
Wang, X., Zhou, T., Bai, G. & Zhao, Y. Complete chloroplast genome sequence of Fagopyrum dibotrys: Genome features, comparative analysis and phylogenetic relationships. Sci. Rep. 8, 12379. https://doi.org/10.1038/s41598-018-30398-6 (2018).
Candassamy, S. V. et al. The complete chloroplast genome of medicinal herb Reynoutria japonica Houtt. (Polygonaceae). Mitochondrial DNA Part B 5, 1983–1985. https://doi.org/10.1080/23802359.2020.1756962 (2020).
Xu, J. et al. Panax ginseng genome examination for ginsenoside biosynthesis. GigaScience 6, 1–15. https://doi.org/10.1093/gigascience/gix093 (2017).
Wei, X. et al. Complete chloroplast genomes of Achnatherum inebrians and comparative analyses with related species from Poaceae. FEBS Open Bio 11, 1704–1718. https://doi.org/10.1002/2211-5463.13170 (2021).
Wang, R. J. et al. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol. Biol. 8, 36. https://doi.org/10.1186/1471-2148-8-36 (2008).
Sugiura, M. The chloroplast chromosomes in land plants. Annu. Rev. Cell Biol. 5, 51–70. https://doi.org/10.1146/annurev.cb.05.110189.000411 (1989).
Hansen, D. R. et al. Phylogenetic and evolutionary implications of complete chloroplast genome sequences of four early-diverging angiosperms: Buxus (Buxaceae), Chloranthus (Chloranthaceae), Dioscorea (Dioscoreaceae), and Illicium (Schisandraceae). Mol. Phylogenet. Evol. 45, 547–563. https://doi.org/10.1016/j.ympev.2007.06.004 (2007).
Machour, F. E. & Ayoub, N. Transcriptional regulation at DSBs: Mechanisms and consequences. Trends Genet. 36, 981–997. https://doi.org/10.1016/j.tig.2020.01.001 (2020).
Zheng, Y. T. Construction of multifunctional codon analysis and optimization platform and its application study Master thesis, Zhejiang University (2020).
Hershberg, R. & Petrov, D. A. Selection on codon bias. Annu. Rev. Genet. 42, 287–299. https://doi.org/10.1146/annurev.genet.42.110807.091442 (2008).
Lyu, X., Yang, Q., Zhao, F. & Liu, Y. Codon usage and protein length-dependent feedback from translation elongation regulates translation initiation and elongation speed. Nucleic Acids Res. 49, 9404–9423. https://doi.org/10.1093/nar/gkab729 (2021).
Wang, Y. Y. & Yang, M. Q. Analysis of the codon usage bias in the chloroplast genome of Allium mongolicum Regel. Mol. Plant Breeding 19, 1084–1092 (2021).
Gao, Y. F., Liu, Y. Y., Yang, C. W., Li, G. D. & Qian, Z. G. Analysis on structure and phylogeny of complete chloroplast genomes in Psammosilene tunicoides. Chin. Tradit. Herbal Drugs 50, 5532–5536 (2019).
Jansen, R. K. et al. Methods in Enzymology Vol. 395, 348–384 (Academic Press, 2005).
Pauwels, M. et al. Nuclear and chloroplast DNA phylogeography reveals vicariance among European populations of the model species for the study of metal tolerance, Arabidopsis halleri (Brassicaceae). New Phytologist 193, 916–928. https://doi.org/10.1111/j.1469-8137.2011.04003.x (2012).
Dong, W. P., Xu, C., Cheng, T., Lin, K. & Zhou, S. L. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of saxifragales. Genome Biol. Evol. 5, 989–997. https://doi.org/10.1093/gbe/evt063 (2013).
Dong, W., Liu, J., Yu, J., Wang, L. & Zhou, S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLOS ONE 7, e35071. https://doi.org/10.1371/journal.pone.0035071 (2012).
Ferreira, A. O., Cardoso, H. G., Macedo, E. S., Breviario, D. & Arnholdt-Schmitt, B. Intron polymorphism pattern in AOX1b of wild St John’s wort (Hypericum perforatum) allows discrimination between individual plants. Physiol. Plantarum 137, 520–531. https://doi.org/10.1111/j.1399-3054.2009.01291.x (2009).
Yang, H. et al. Phylogenetic analysis and development of molecular markers for five medicinal Alpinia species based on complete plastome sequences. BMC Plant Biol. 21, 431. https://doi.org/10.1186/s12870-021-03204-1 (2021).
Li, P. et al. Comparison of the complete plastomes and the phylogenetic analysis of Paulownia species. Sci. Rep. 10, 2225. https://doi.org/10.1038/s41598-020-59204-y (2020).
Du, Q. et al. Comparative genomics and phylogenetic analysis of the chloroplast genomes in three medicinal salvia species for bioexploration. Int. J. Mol. Sci. 23, 12080 (2022).
Desjardins, S. D., Bailey, J. P., Zhang, B., Zhao, K. & Schwarzacher, T. New insights into the phylogenetic relationships of Japanese knotweed (Reynoutriajaponica) and allied taxa in subtribe Reynoutriinae (Polygonaceae). PhytoKeys 220, 83–108. https://doi.org/10.3897/phytokeys.220.96922 (2023).
Moseley, R. C. et al. Conservation and diversification of circadian rhythmicity between a model Crassulacean acid metabolism plant Kalanchoë fedtschenkoi and a model C3 photosynthesis plant Arabidopsis thaliana. Front. Plant Sci. 9, 1757. https://doi.org/10.3389/fpls.2018.01757 (2018).
Cvijović, I., Good, B. H. & Desai, M. M. The effect of strong purifying selection on genetic diversity. Genetics 209, 1235–1278. https://doi.org/10.1534/genetics.118.301058 (2018).
Li, B. et al. Complete chloroplast genome sequences of three aroideae species (Araceae): Lights into selective pressure, marker development and phylogenetic relationships. BMC Genomics 23, 218. https://doi.org/10.1186/s12864-022-08400-3 (2022).
Jiang, H. et al. Comparative and phylogenetic analyses of six Kenya Polystachya (Orchidaceae) species based on the complete chloroplast genome sequences. BMC Plant Biol. 22, 177. https://doi.org/10.1186/s12870-022-03529-5 (2022).
Yu, J., Fu, J., Fang, Y., Xiang, J. & Dong, H. Complete chloroplast genomes of Rubus species (Rosaceae) and comparative analysis within the genus. BMC Genomics 23, 32. https://doi.org/10.1186/s12864-021-08225-6 (2022).
Lee, K., Leister, D. & Kleine, T. Arabidopsis mitochondrial transcription termination factor mTERF2 promotes splicing of group IIB introns. Cells 10, 315. https://doi.org/10.3390/cells10020315 (2021).
Li, D. M., Ye, Y. J., Xu, Y. C., Liu, J. M. & Zhu, G. F. Complete chloroplast genomes of Zingiber montanum and Zingiber zerumbet: Genome structure, comparative and phylogenetic analyses. PLoS ONE 15, e0236590. https://doi.org/10.1371/journal.pone.0236590 (2020).
Jiang, D. et al. Complete chloroplast genomes provide insights into evolution and phylogeny of Zingiber (Zingiberaceae). BMC Genomics 24, 30. https://doi.org/10.1186/s12864-023-09115-9 (2023).
Wu, Z. et al. Analysis of six chloroplast genomes provides insight into the evolution of Chrysosplenium (Saxifragaceae). BMC Genomics 21, 621. https://doi.org/10.1186/s12864-020-07045-4 (2020).
Fan, D.-M. et al. Molecular phylogeny of Koenigia L. (Polygonaceae: Persicarieae): Implications for classification, character evolution and biogeography. Mol. Phylogenet. Evol. 69, 1093–1100. https://doi.org/10.1016/j.ympev.2013.08.018 (2013).
Chai, X. et al. A new triterpene and phenolic compounds from the roots of Pteroxygonum giraldii. Helvet. Chim. Acta 95, 127–133. https://doi.org/10.1002/hlca.201100267 (2012).
Acknowledgements
We gratefully acknowledge the CAMS Innovation Fund for Medical Sciences (CIFMS, 2022-I2M-1-017), Beijing Natural Scientific Foundation (7242244) and the Open Fund of State Key Laboratory of Southwestern Chinese Medicine Resources (SCMR2022015).
Author information
Authors and Affiliations
Contributions
Z.F.: Supervision, Writing—original draft, Writing – review & editing. Y.Z.: Investigation, Methodology, Writing—original draft. Y.J.: Methodology. J.P.: review & editing. L.H.: Writing – review & editing, Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, Z., Zheng, Y., Jiang, Y. et al. Phylogenetic relationships, selective pressure and molecular markers development of six species in subfamily Polygonoideae based on complete chloroplast genomes. Sci Rep 14, 9783 (2024). https://doi.org/10.1038/s41598-024-58934-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58934-7
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.