Abstract
Reproductive tract infections (RTIs) are a persistent public health threat worldwide, particularly among women in low-income countries of Africa, including Ethiopia, where drug resistance is also a growing problem. It is crucial to address this problem to ensure women's health and well-being. A cross-sectional study was carried out among a cohort of 398 women of reproductive age who sought medical attention at the Gynecology Department of the Arba Minch General Hospital, southern Ethiopia, from January to June 2020. They were chosen through systematic random sampling, and a pre-tested structured questionnaire was used to collect the data. The collection of vaginal and/or cervical swabs were done to diagnose bacterial vaginosis (BV) and aerobic vaginitis (AV) using Nugent and AV score analyses, respectively. The swabs were subjected to standard microbiological culture techniques to detect the isolates causing AV and vaginal candidiasis (VC). The susceptibility profiles of the causative agents of AV were checked by the Kirby-Bauer disc diffusion technique. Descriptive and inferential statistical analyses were also done. Aerobic vaginitis was the predominantly diagnosed RTI (n = 122, 30.7%), followed by BV (n = 117, 29.4%) and VC (n = 111, 27.9%). The prominent bacteria of AV were Escherichia coli (n = 36, 34.2%) and Klebsiella pneumoniae (n = 30, 28.5%). The overall rate of multidrug-resistant (MDR) bacteria was 65.71% (n = 69). History of abortion (p = 0.01; AOR = 4.0, 95% CI = 2.1, 7.7) and the habit of using vaginal pH-altering contraceptives (p = 0.01; AOR = 4.7, 95% CI = 2.5, 8.8) have the greatest odds of RTI. The high prevalence of RTIs in our study warrants an urgent intervention to minimize the associated morbidities and complications. The overall rate of MDR bacterial isolates necessitates the implementation of an effective surveillance program in the study setting.
Similar content being viewed by others
Introduction
Infections occurring in the reproductive or genital tracts (RTIs) comprising sexually transmitted diseases, iatrogenic infections, bacterial vaginosis (BV), and vulvovaginal candidiasis (VC) are caused by either exogenous or endogenous agents1,2. The World Health Organization (WHO) states that RTIs pose significant public health threats, especially in developing nations1. Young women are at greater risk of contracting infections during menstruation, pregnancy, and childbirth3. Bacterial vaginosis, aerobic vaginitis (AV), and vaginal candidiasis are indeed the worst hit, with endogenous lower RTIs4.
As per a conceptual model developed to study the pathogenesis of BV, Gardnerella vaginalis is causing the onset of BV, along with other secondary intruders4. Aerobic vaginitis is characterized by an abnormal microflora in the vagina in conjunction with an enhanced local inflammatory reaction and immune response and is often misdiagnosed5. The bacterial aetiology of AV is mainly aerobic in nature, or it can be due to facultative anaerobic commensals or pathogens5. Recently, the WHO enlisted AV into their guidelines, stressing the importance of accurate diagnosis and prompt treatment6. Vaginal candidiasis/moniliasis is the second most common RTIs after BV. The epidemiologic data showed that about 75% of women experienced at least an episode of VC, and 40–50% of them had to face recurrence during later life, which varied significantly across countries, regions, and also among different study populations7,8,9.
Nevertheless, the prevalence of other RTIs in Ethiopia remains the highest in African nations10; evidence is scarce concerning the existing rates of lower RTIs, particularly BV, AV, and VC. Unfortunately, routine surveillance of RTIs is not performed in the country, and thus, the estimation of cumulative prevalence is difficult. A literature survey revealed that studies had not yet been conducted in this context in southern Ethiopia, leading to the present attempt.
Materials and methods
Study area, period, design, population, and eligibility criteria
This research was conducted in Arba Minch General Hospital from January to June 2020 and is a cross-sectional type, including women of reproductive age, suspected of RTIs, who sought medical care from the Gynecology Outpatient ward. The inclusion criteria were: all women ≥ 18 years who were clinically suspected of RTIs (those who fulfilled at least three of Amsel's criteria for BV; red and edematous vaginal appearance, burning sensation, dyspareunia, thick and mucoid yellow discharge connected to AV and peri-vaginal pruritis, erythema and thick and curdy discharge for VC) and who gave consent to involve in the study. The exclusion criteria were: women who received antibiotics/antifungals during the two weeks before sample collection, those who were extremely unwell and hence could not give answers to questions, and also women who were on a catamenial period. After a thorough briefing of all the study-related procedures and associated risk factors, informed consents were obtained from each participant before their enrolment in the study.
Sample size determination and patient recruitment
For determining the sample size, a single population proportion formula was used. A prevalence of 48.6% of RTIs was opted from a previous study conducted elsewhere in Ethiopia11. By applying a confidence interval of 95% (z = 1.96) and a 5% marginal error (d = 0.05), the sample size calculated was 378. A 10% non-response rate (38 subjects) was applied, and the final sample size thus became 416. The study participants were recruited using a systematic random sampling method.
Data collection
After the admission of patients to the gynecology ward, they were thoroughly examined by a gynecologist and recruited in conjunction with the clinical criteria of RTIs. Data on socio-demography, medical history, and other pertinent factors were solicited by a face-to-face interview using a pre-tested structured questionnaire. The clinical data corresponding to each participant were collected after reviewing the medical records.
Sample collection
Samples were meticulously collected from the lateral vaginal wall and posterior fornix using sterile high vaginal cotton swabs moistened with Amie's medium; swabs were placed in Amie's transporting media and quickly taken to the Medical Microbiology and Parasitology Laboratory and stored at 5 °C.
Microbiological diagnosis of bacterial vaginosis, aerobic vaginitis, and vaginal candidiasis
Vaginal swabs were initially smeared onto clean glass slides and subjected to Gram-staining, and each slide was observed under oil emersion light microscopy with a maximum magnification of 100 × and graded as per the standardized quantitative morphological classification of Nugent score analysis12. Briefly mentioning, Gram-stained smears were scored according to a morphotype classification: (a). Lactobacillus morphotypes (score 4 to 0), (b). small Gram-negatives (G. vaginalis, score 0 to 4), (c). curved Gram variable rods (Mobiluncus morphotypes, score 0 to 2). The scoring system (0 to 10) is a sum of all the three morphotypes. The diagnostic criterion for bacterial vaginosis was a score of ≥ 7. The diagnosis of AV is made by an ‘AV’ score analysis (a score of ≥ 3 is diagnostic)6 and is further substantiated by conventional culture techniques. Cervical swabs were also subjected to Gram-staining to detect the presence of any Gram-positive yeast cells so as to diagnose VC.
Culture of bacteria (aerobic vaginitis) and yeast-like fungus (vaginal candidiasis)
Swabs were inoculated onto mannitol salt agar, MacConkey, blood agar (5%), and chocolate agar to isolate non-fastidious aerobic bacteria associated with AV. The inoculated plates were incubated aerobically at 37 °C for 24 to 48 h; colonies were identified by Gram-staining and conventional biochemical tests13. In the case of suspected VC, cervical swabs were inoculated onto Sabouraud dextrose agar, and isolates were characterized by conventional methods13. All the microbiological media used were purchased from HiMedia Laboratories Pvt. Ltd, Mumbai, India.
Antibiotics susceptibility testing
Antimicrobial susceptibility testing of non-fastidious aerobic bacteria was performed by the Kirby-Bauer disc diffusion technique on Mueller Hinton agar (Oxoid, Basingstoke, Hampshire, UK), according to the guidelines set by the Clinical Laboratory Standard Institute (CLSI)14. Multidrug-resistant (MDR) bacteria in this study were extrapolated as those resistant to ≥ 3 classes of antibiotics tested15.
Data quality assurance
The structured questionnaire was pre-tested on 5% of the sample size in Chencha General Hospital. The data collectors and technicians were given sufficient training sessions as per standard procedures. The data were checked daily for accuracy, clarity, and completeness, and any incompleteness and errors found were immediately corrected with utmost confidence. Standard operating procedures (in-house SOP manual) were followed during the collection, transportation, and processing to maintain the highest level of quality. All reagents, culture media, and antibiotic discs were carefully inspected for their shelf life and physical condition and were stored at 2–8 °C. The culture media were incubated at 37 °C overnight to ensure sterility until actual sample processing. The efficacy of the media was determined by inoculating the American Type Culture Collection (ATCC) (S. aureus (ATCC 25923) E. coli (ATCC 25922), P. aeruginosa (ATCC 27853), and Candida albicans (ATCC 10231)).
Statistical analyses
The collected data were coded, cleaned, and entered using Epi-Data version 4.2 and then exported to SPSS version 25 software for further analysis. The IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA) was used for the data analysis, and descriptive statistics, including frequency, mean, and standard deviations, were done. To evaluate the association among variables and RTIs, bivariable and multivariable logistic regression analyses were applied. In the former model, variables with a p value < 0.25 were selected, whereas in the latter case, a p value ≤ 0.05 was considered statistically significant.
Ethical considerations
Ethical clearance was approved by the Institutional Review Board, College of Medicine and Health Science, Arba Minch University (Ref. IRB/150/12, Dated 19-12-2019). This study was in line with the declaration of Helsinki and its later amendments. Before the enrolment, each participant completed and signed a consent form indicating their willingness.
Results
Socio-demographic characteristics
The study included a cohort of 398 participants, with a response rate of 95.67% (out of 416). Most of them were within the age group of 25 to 30 (n = 165, 41.5%), and more than half of them were married (n = 207, 52%). Many of them had a history of previous sexual intercourse, and of these, the majority had only one heterosexual partner (n = 227, 57%) (Table 1).
Obstetrics, clinical, and hygienic characteristics
The majority have the habit of changing their panties daily (n = 290, 72.9%), douching (rinsing) twice a day, and had a history of RTIs/STIs as well as chronic diseases, i.e., 58% (n = 231) and 57.3% (n = 228), respectively. Also, a considerable number of women with chronic diseases had HIV infection (n = 123, 30.9%); most of them (n = 237, 59.5%) used some kind of pH-altering vaginal contraceptives.
Prevalence of reproductive tract infections
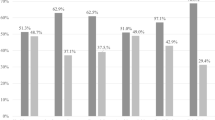
The overall prevalence of RTIs was 49.7% (n = 198) (95% CI 44.7, 54.8). The extent of BV and AV diagnosed by Nugent and AV scoring criteria was 29.4% (n = 117) (95% CI 25, 34.1) and 30.7% (n = 122) (95% CI 26.2, 35.4), respectively. However, compared to the AV score, the number of positive cases detected by the culture technique was slightly lower, i.e., 26.4% (n = 105) only. On the other hand, the prevalence of VC was 27.9% (n = 111) (95% CI 23.5, 32.6) (Fig. 1). The rate of co-infections was 11.1% (n = 44) (95% CI 8.1, 14.6) for BV/ AV and 5% (n = 5) (95% CI 3.1, 7.7) for BV /VC.
Bacterial profile of aerobic vaginitis
In total, 105 (n) isolates were detected; most of them were Gram-negative (n = 90, 85.7%), whereas Gram-positives accounted for only a total of 14.2% (n = 15). The predominant Gram-negative isolate was E. coli (n = 36, 34.3%), followed by K. pneumoniae (n = 30, 28.5%). The isolates of S. aureus were the only Gram-positive bacteria (n = 15, 14.2%) detected in this study (Fig. 2). Of notice, 2.8% of women were diseased with gonococcal vaginitis, and is an alarming fact.
Antimicrobial susceptibility profiles of aerobic vaginitis
The overall susceptibility profiles of bacteria against sixteen antibiotics were summarized in Table 2. More than half of the isolates of E. coli were resistant against amoxicillin-clavulanic acid (66.6%) and sulfamethoxazole-trimethoprim (58.3%). The isolates of K. pneumoniae also showed a higher extent of resistance against meropenem (70%) and ceftriaxone (60%), whereas 90% of them were susceptible to ciprofloxacin. The isolates of P. aeruginosa were exclusively susceptible to ciprofloxacin, gentamicin, amikacin, piperacillin, and norfloxacin. On the other hand, 50% of these isolates were resistant to a pair of antibiotics, namely meropenem and cefepime. All the isolates of S. aureus were resistant to cefoxitin (100%) and sulfamethoxazole-trimethoprim (100%). Invariably, all S. aureus isolates were 100% susceptible to gentamicin and clindamycin (Table 2); out of the entire isolates obtained 69 (65.71%) were MDR. The MDR patterns correspond to 100% of Proteus sp., 100% of S. aureus, and 75% of E. coli (Table 3).
Associated factors of reproductive tract infections
During the bivariable logistic regression analysis, variables such as age, educational level, marital status, occupation, number of sexual partners, family income, history of RTI, chronic diseases, parity, pregnancy, history of abortion, smoking habit, history of antibiotics, consumption of corticosteroids and also the usage of vaginal pH-altering contraceptives were statistically significant with respect to RTIs. However, in multivariable logistic regression analyses, only two variables, namely the history of abortion (p = 0.01; AOR = 4.09, 95% CI = 2.16, 7.73) and the usage of vaginal pH-altering contraceptives (p = 0.01; AOR = 4.71, 95% CI = 2.51, 8.83) showed statistical association with RTIs (Table 4).
Discussions
This is the first study done among women of reproductive age suspected of RTIs in Arba Minch, which underscores the importance of research and its impact on their health. The overall prevalence of RTIs was 49.7%, highlighting the necessity of serious management of the issue. The types of RTIs and the aetiological agents found by us are different from those reported in recent literature. In other words, each study is unique, and the scenario and results obtained need not be an exact replica of previous studies. For instance, recent studies on RTIs carried out in other parts of the country showed a wider prevalence range, 15.6—50%, corresponding to varied aetiology16,17. The higher prevalence found in our study warrants an urgent intervention so that associated morbidities and complications can be minimized. It is well recognized that RTIs are a persisting problem, particularly in low-income countries of Africa. A failure in prompt diagnosis and treatment can lead to various pregnancy-related complications as well as congenital infections. Recently, it has been shown that RTIs can also increase the chances of HIV transmission18. Ensuring routine screening for and treatment of RTIs in women is crucial for the prevention of complications and for maintaining economic productivity and quality of life. Focusing on the high-risk group must be done but by giving equal importance to regular screening for all women of reproductive age. In general, the prevalence of AV is 7–12%, and it is less than that of BV19. However, in our study, as per the scoring criteria, AV was the most frequently diagnosed RTIs (30.7%). The positivity rate was higher than the results reported in an earlier study done in another part of the country (22.9%)16, as well as in Belgium (7.9%)12. Vulvovaginal candidiasis is one of the trivial reasons for medical, nursing, and pharmacist consultations, and this substantiates our findings that vaginal candidiasis was the second most common RTI affecting 27.9%; this result tally with earlier findings from Nepal (25%)20; VC is often linked to the recent use of antibiotics, progesterone-containing oral contraceptives, or immunosuppression21. The exact cause behind the excessive growth of endogenous microbes is still being investigated; prompt diagnosis and treatment can avoid the risk of recurrence. A recently published article thoroughly reviewed the epidemiology and risk factors linked to BV22. We found that BV was the third most frequently diagnosed RTI, with a prevalence as high as 26.3%, which is even higher than that reported earlier from Bahir Dar (2.8%)17. On the other hand, the prevalence of BV now found in Arba Minch is substantially below the extent reported in other cities of the country, Gondar and Addis Ababa (35.5–48.6%)11,16, and also Gabon (62.8%)23. The prevalence of BV can fluctuate widely due to varying diagnostic criteria, alterations in the characteristics of clinical populations enrolled, and some mismatching factors in the study populations. It is envisaged that severe infections are usually poly-microbial in nature, whereas milder infections are generally mono-microbial. In our study, 11% of cases correspond to AV and BV co-infection.
The pathogenic microbiota in the vagina of AV patients is very complex, and precise detection of aetiological agents is challenging but vital for the better management of the cases. The Gram-negative isolates were the prominent group of causative agents of AV, as in the case of previous studies from Gondar17 and Addis Ababa11. The dominant one of this kind found in our study was E. coli, 34.2% (n = 36), which is at par with the outcome of an earlier study done in the capital city11. Klebsiella pneumoniae was the second most frequently isolated bacteria, as is the case in an earlier study from Addis Ababa11. The frequently isolated Gram-positive bacteria in the current study was S. aureus, which supports the notion that many species of bacteria can colonize both the gastrointestinal and reproductive tract and can become the source or reservoir24; however, their clinical relevance is yet to be elucidated.
We found that 85% of Gram-negative bacteria were susceptible to ciprofloxacin, whereas 81.2% were susceptible to chloramphenicol. This is in agreement with a couple of studies from Bahir Dar17,25; the latter drug is generally not recommended for the treatment of RTIs. The overall resistance rates of Gram-negative bacteria against sulfamethoxazole-trimethoprim, ceftriaxone, and amoxicillin-clavulanic acid were above 50% and are likely to shoot up further if ignored, resonating with the outcome of a previous study from the country17. Among the aerobic Gram-negative bacteria, E. coli was the most resistant organism; the resistance was very high against amoxicillin-clavulanic acid (66.6%) and sulfamethoxazole-trimethoprim (58.3%) and is in line with the outcome of a couple of studies conducted in the country11,17. Also, the susceptibility profiles of K. pneumoniae to ciprofloxacin and chloramphenicol are similar to the results of earlier work conducted in Ethiopia itself16.
A dubious finding of our study is related to the higher resistance of K. pneumoniae against meropenem compared to ceftriaxone, although the former drug is not in regular use in our study setting in the case of RTIs; perhaps the unscrupulous usage of this drug in other patients in our hospital would have resulted in this increased resistance. This ambiguity should be cleared and verified by secondary investigations. The variations in the resistance profiles of K. pneumoniae against ceftriaxone in comparison with meropenem appear odd, and to arrive at a convincing conclusion, several future studies are to be conducted continuously, including a larger number of study participants across the country; also, it needs further in-depth studies involving molecular techniques. This phenomenon was also detected in the case of Klebsiella oxytoca (carbapenem-resistant but ceftriaxone and cefepime-sensitive) in a study done in the USA26.
Isolates of S. aureus showed the highest extent of resistance (100%) against penicillin and sulfamethoxazole-trimethoprim and matched well with the results of an earlier study done in Addis Ababa11. Alarmingly, all these isolates were found to be methicillin-resistant too, which is quite contrary to a few studies done in the nation earlier11,16. Although Ethiopia is registered with the Global Antimicrobial Resistance Surveillance System, nationwide studies are sorely lacking. The most alarming observation in this study is that 65.71% of the isolates were MDR, which is much higher than the values reported in a couple of cities in the country16,27. A higher level of multidrug resistance was also observed, particularly in the case of isolates of S. aureus, Proteus sp. (each 100%), E. coli (75%), and K. pneumoniae (70%); this matches with the findings described in a systematic review which concluded that these isolates are highly resistant to the most of the frequently used antibiotics27. Endogenous vaginal infections are often misdiagnosed as STIs, such as trichomoniasis, gonorrhea, and chlamydia leading to the unwanted prescription of antibiotics. The high rate of MDR observed in our study setting could be due to the excessive reliance on empirical therapy, which can be modified by taking into account the current sets of results.
In our setting, ciprofloxacin and ceftriaxone are the first and second-line drugs of treatments for AV, respectively. The antibiogram profile revealed that 11.4 and 52.2% of the bacteria were resistant to these antibiotics, respectively, making the latter drug less effective for an empirical therapy. These results are quite relevant and can be considered to tailor the local antibiotic policy from time to time. Our results also bring a notion that women who are colonized with MDR pathogens are at greater risk of contracting nosocomial infections, highlighting the need for further extensive studies.
Abortion and the usage of vaginal contraceptives were only statistically significant, as noted in some of the preceding studies10,28. We observed that women with a history of abortion are four times more prone to develop RTIs, and this can be attributed to the fact that operative procedures render women more susceptible to infections. Similarly, women who were using vaginal contraceptives were four times more prone to develop RTIs because of fluctuation of pH, which increases the risk of developing VC or BV. To mitigate this issue, modern contraceptive methods, including oral, injectable (medroxyprogesterone), skin implant (Nexplanon), and skin patch (progestin and estrogen), may be promoted.
While precisely enlisting the impact of this research, we stress that enlightening women about the seriousness of RTIs and encouraging them to seek immediate medical assistance is very important. Additionally, there must be augmented vigilance from the side of clinicians in distinctly diagnosing the cases of AV and BV and prescribing the exact regimen. Also, they should instruct the patients to maintain a high level of alert post-abortions as well as while using pH-altering vaginal contraceptives.
Limitations
The molecular characterization of bacterial and fungal isolates could not be performed due to the inadequacy of advanced techniques; pathogenicity and virulence factors of bacteria also were not studied. In addition, the identification of fastidious organisms and antifungal susceptibility testing of C. albicans was not performed due to the lack of infrastructure/ facilities. We have employed only conventional techniques for the diagnosis of RTIs; therefore, alternate diagnostic tools are required to figure out the reasons for the negative results obtained. Additionally, the study population was hospital-based, and hence, the results may be skewed to a certain extent. A comprehensive multicentric study is required to confirm some of the current findings. We are unable to establish a clinical correlation between the aetiology of AV with the isolated pathogens. Another limitation is the lack of resistant gene analysis of K. pneumoniae against ceftriaxone and meropenem. Finally, we add that data on differential diagnosis are not included.
Conclusions
This is the first report on the prevalence of RTIs among women of reproductive age attending the Arba Minch General Hospital in Ethiopia. The AV, VC, and BV were found to be the common RTIs among them. Isolates of E. coli, K. pneumoniae, and S. aureus were the predominant bacteria causing AV; an alarming finding is that 65.71% of bacterial isolates causing AV were MDR. It is better to initiate an antimicrobial stewardship program aiming at optimizing the selection of drugs in our study setting to prevent untoward consequences. The history of abortions and the usage of pH-altering vaginal contraceptives were statistically associated. Women in the reproductive age group must be enlightened to reduce the burden of infections. The stakeholders and policymakers should give due attention to the management of RTIs by reinforcing the prevention and control strategies through an early promotion of screening and the initiation of treatment if required.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
World Health Organisation. Integrating STI/RTI Care for Reproductive Health—Sexually Transmitted and Other Reproductive Tract Infections: A Guide to Essential Practice (WHO, Geneva, 2005).
Wasserheit, J. N. & Holmes, K. K. Reproductive tract infections: Challenges for international health policy, programs, and research. In Reproductive Tract Infections: Global Impact and Priorities for Women’s Reproductive Health (eds Germain, A. et al.) 7–33 (Plenum Press, New York, 1992).
Jejeebhoy, S. J. Addressing women’s reproductive health needs: Priorities for the family welfare program. Econ. Polit. Wkly. 32, 475–484 (1997).
Schwebke, J. R., Muzny, C. A. & Josey, W. E. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: A conceptual model. J. Infect. Dis. 210(3), 338–343. https://doi.org/10.1093/infdis/jiu089 (2014).
Donders, G. G. G., Bellen, G. & Rezeberga, D. Aerobic vaginitis in pregnancy. BJOG 118(10), 1163–1170. https://doi.org/10.1111/j.1471-0528.2011.03020.x (2011).
Sherrard, J., Wilson, J., Donders, G., Mendling, W. & Jensen J. S. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int. J. STD AIDS. 29(13), 1258–1272. https://doi.org/10.1177/0956462418785451 (2018).
Sobel, J. D. Vulvovaginal candidosis. Lancet 369(9577), 1961–1971 (2007).
Bitew, A. & Abebaw, Y. Vulvovaginal candidiasis: Species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health. 18(1), 94. https://doi.org/10.1186/s12905-018-0607-z (2018).
Røttingen, J.-A., William Cameron, D. & Garnett, G. P. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: How much really is known?. Sex Transm. Dis. 28(10), 579–587 (2001).
Center for disease prevention and control (CDC). Reproductive tract infections reproductive; Reproductive Health Epidemiology Series—Module 3, 2003. https://www.cdc.gov/reproductivehealth/productspubs/pdfs/epi_module_03a_tag508.pdf
Bitew, A., Abebaw, Y., Bekele, D. & Mihret, A. Prevalence of bacterial vaginosis and associated risk factors among women complaining of genital tract infection. Int. J. Microbiol. 2017, 4919404. https://doi.org/10.1155/2017/4919404 (2017).
Nugent, R. P., Krohn, M. A. & Hillier, S. L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29(2), 297–301. https://doi.org/10.1128/jcm.29.2.297-301.1991 (1991).
Collee, J. G., Marmion, B. P., Fraser, A. G. & Simmons, A. In Practical Medical Microbiology: Editors: 14th Edition (eds Mackie, T., & McCartney) (Elsevier, A division of Reed Elsevier India Private Limited, 2012).
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed (CLSI supplement M100 Clinical and Laboratory Standards Institute, 2019).
Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18(3), 268–281 (2012).
Yasin, J., Ayalew, G., Dagnaw, M., Shiferaw, G. & Mekonnen, F. Vulvovaginitis prevalence among women in Gondar, Northwest Ethiopia: Special emphasis on aerobic vaginitis causing bacterial profile, antimicrobial susceptibility pattern, and associated factors. Infect. Drug Resist. 14, 4567–4580. https://doi.org/10.2147/IDR.S337205 (2021).
Mulu, W., Yimer, M., Zenebe, Y. & Abera, B. Common causes of vaginal infections and antibiotic susceptibility of aerobic bacterial isolates in women of reproductive age attending at Felegehiwot Referral Hospital, Ethiopia: A cross-sectional study. BMC Womens Health. 15, 42. https://doi.org/10.1186/s12905-015-0197-y (2015).
Reproductive tract infections. A Set of factsheets (Population Council, Bangkok, 1999).
Donders, G. G. G., Bellen, G., Grinceviciene, S., Ruban, K. & Vieira-Baptista, P. Aerobic vaginitis: No longer a stranger. Res. Microbiol. 168(9–10), 845–858 (2017).
Bohara, M. S., Joshi, A. B., Lekhak, B. & Gurung, G. Reproductive tract infections among women attending gynaecology outpatient department. Int. J. Infect. Microbiol. 1(1), 29–33 (2012).
Gonçalves, B. et al. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 42(6), 905–927 (2016).
Abou Chacra, L., Fenollar, F. & Diop, K. Bacterial vaginosis: What do we currently know?. Front. Cell. Infect. Microbiol. 11, 672429. https://doi.org/10.3389/fcimb.2021.672429 (2022).
Bignoumba, M. et al. Vaginal infections’ etiologies in South-Eastern Gabon—an overview. Int. J. Womens Health 14, 505–515 (2022).
Tumuhamye, J. et al. Vaginal colonisation of women in labour with potentially pathogenic bacteria: A cross sectional study at three primary health care facilities in Central Uganda. BMC Infect. Dis. https://doi.org/10.1186/s12879-020-4821-6 (2020).
Bitew, A., Mengist, A., Belew, H., Aschale, Y. & Reta, A. The prevalence, antibiotic resistance pattern, and associated factors of bacterial vaginosis among women of the reproductive age group from Felege Hiwot Referral Hospital, Ethiopia. Infect. Drug Resist. 14, 2685–2696. https://doi.org/10.2147/IDR.S305329 (2021).
Yang, S. et al. Unusual carbapenem-resistant but ceftriaxone and cefepime susceptible Klebsiella oxytoca isolated from a blood culture: Case report and whole-genome sequencing investigation. IDCases 11, 9–11. https://doi.org/10.1016/j.idcr.2017.11.007 (2017).
Yalew, G. T. et al. Prevalence of bacterial vaginosis and aerobic vaginitis and their associated risk factors among pregnant women from northern Ethiopia: A cross-sectional study. PLoS One 17(2), e0262692. https://doi.org/10.1371/journal.pone.0262692 (2022).
Bhilwar, M., Lal, P., Sharma, N., Bhalla, P. & Kumar, A. Prevalence of reproductive tract infections and their determinants in married women residing in an urban slum of North-East Delhi, India. J. Nat. Sci. Biol. Med. 6(Suppl 1), S29-34. https://doi.org/10.4103/0976-9668.166059 (2015).
Acknowledgements
The authors would like to thank the Research Directorate Office (GOV/AMU/TH-15/CMHS/MeLS/02/12), College of Medicine and Health Sciences, Arba Minch University, and Arba Minch General Hospital. The authors extend their appreciation to the Researchers supporting project number (RSPD2024R543), King Saud University, Riyadh, Saudi Arabia. Thanks are extended to Prof. Dr K. R. Sabu for English-language editorial work.
Author information
Authors and Affiliations
Contributions
A.A., M.W., A.M., M.Y designed research; A.A., M.W., A.M., G.K., M.Y. conduct the study; A.A., M.W., A.M., G.K., M.Y, R.M.A., G.R, and A.I. conducted the review and editing the original draft and A.A. and A.M. reviewed and wrote the paper. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aklilu, A., Woldemariam, M., Manilal, A. et al. Aerobic vaginitis, bacterial vaginosis, and vaginal candidiasis among women of reproductive age in Arba Minch, southern Ethiopia. Sci Rep 14, 9813 (2024). https://doi.org/10.1038/s41598-024-58654-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58654-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.