Abstract
Constipation is a highly prevalent gastrointestinal disorder in patients with chronic kidney disease (CKD). However, our understanding of its epidemiology and management in CKD is limited. We aimed to explore real-world data on constipation and laxative use in patients with CKD in a nationwide population-based cohort from the Korean Health Insurance Review and Assessment-National Patient Sample database. This study analyzed retrospective health claims data in Korea from 2012 to 2017 that were transformed into the Observational Medical Outcomes Partnership Common Data Model. The pooled proportion of constipation diagnoses was 30.5% in all patients with CKD and 15.9%, 16.5%, 17.4%, 29.9%, and 43.3% in patients with CKD stages 1–5, respectively, suggesting a higher prevalence in advanced CKD. Patients receiving peritoneal dialysis or hemodialysis had the highest prevalence of constipation, while transplant recipients showed a prevalence comparable to that of patients with early CKD. Patients with CKD had a significantly higher risk of constipation than age- and sex-matched non-CKD individuals (range of odds ratio [OR]:1.66–1.90). Laxative prescribing patterns differed by CKD severity. Osmotic agents were prescribed in more than half of patients with advanced CKD, while magnesium salts and bulking agents were prescribed less frequently. The CKD patients with constipation were more likely to be prescribed constipation-inducing medications, including antipsychotic and neurological medications. Our findings provide real-world constipation and laxative prescription status in the Korean CKD population, revealing a significantly higher risk of constipation and different laxative prescribing patterns in patients with CKD.
Similar content being viewed by others
Introduction
Constipation is one of the leading gastrointestinal symptoms commonly encountered in the general population, particularly among older adults1. Constipation significantly impairs the health-related quality of life (QoL) of affected individuals and poses considerable economic and healthcare burdens2. Recent evidence suggests a close association between constipation and clinical outcomes such as cardiovascular disease, chronic kidney disease (CKD) progression, and mortality3,4,5,6. In particular, numerous observational studies have reported a higher prevalence of constipation in patients with CKD. Although the global prevalence of constipation in the general population has been estimated at approximately 14%, the prevalence in patients with CKD is reported to be much higher7. A recent metaanalysis reported a constipation prevalence of 38.8% in patients with advanced non-dialysis CKD8. Patients with end-stage kidney disease (ESKD) have been shown to have a substantially higher constipation prevalence, with some studies reporting over 50%9. CKD is associated with constipation both clinically and pathophysiologically. Dietary and fluid restrictions, high pill burdens, comorbidities, gut dysbiosis, and uremic milieu may all contribute to chronic constipation10.
Despite these observations, most research topics on constipation in patients with CKD remain unexplored. The exact prevalence and incidence rates of constipation in patients with CKD remain undetermined; most studies involved a relatively small number of participants, often less than 1008. The detailed characteristics and epidemiological data on constipation in patients with CKD are largely unknown. Furthermore, the clinical impacts and outcomes of constipation in patients with CKD have rarely been investigated. Patients with CKD are often managed for constipation in primary care. However, there are no specific guidelines for managing constipation in patients with CKD. The general guidelines for CKD from the Kidney Disease Improving Global Outcomes do not cover the management of constipation11. Given the growing interest in patient-reported outcomes in managing patients with CKD, constipation is a major component of improving the quality of care12,13. In addition, as recent studies have highlighted the gut microbiota as a therapeutic target for CKD and gut motility is associated with dysbiosis, constipation management is no longer a negligible issue14,15. Generally, CKD-specific cohorts have no or limited data on gastrointestinal symptoms. Most well-designed cohort studies of CKD have no detailed information on constipation or laxative use. To address this knowledge gap, we conducted a nationwide population-based study using the Korean Health Insurance Review and Assessment-National Patient Sample (HIRA-NPS) database. Our data analysis was based on the common data model (CDM), using standardized structures and vocabularies for reliable evidence. We aimed to provide real-world evidence of the epidemiology of constipation and laxative use in patients with CKD.
Methods
Data source
We used the HIRA-NPS database, which is a nationwide standard cohort based on claims data from 2012 to 2017. In Korea, national health insurance covers almost 98% of the national population16; HIRA-NPS data are representative datasets generated during the reimbursement for healthcare services, sampling approximately 3% of the total population. HIRA-NPS was sampled using a proportionate stratified sampling method by dividing total population into 32 strata (2 gender strata and 16 age strata)17. Estimates from HIRA-NPS demonstrates a high level of representativeness for the total population17. The Korean Health Insurance Review and Assessment (HIRA) and HIRA-NPS data have enabled diverse real-world data analyses in previous publications18,19,20,21. HIRA-NPS data from 2012 to 2017 were transformed into the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM, version 5.0) developed by the Observational Health Data Sciences and Informatics (OHDSI) network with standard vocabulary and ontology22.
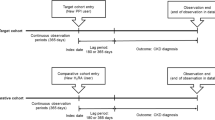
Study design and cohort definition
Definitions of the concept sets used are presented in Supplementary Table 1. Descendant concepts of each concept set were also used in the analysis. CKD concepts were defined according to CKD stages. The CKD stage was determined by the highest CKD stage code among patient condition occurrences. We only included patients with 2 or more inpatient or outpatient ICD codes for each condition, similar to other studies using HIRA database23,24. Patients who underwent kidney transplantation (KT) were investigated separately from the total CKD population. Patients undergoing peritoneal dialysis (PD) and hemodialysis (HD) were identified using procedure-occurrence concepts. Constipation medications included 8 types of laxatives (psyllium, polycarbophil, polyethylene glycol [PEG], magnesium salts, lactulose, lactitol, bisacodyl, and docusate). Some constipation drugs, such as PEG, are often used for bowel preparation for colonoscopy; thus, cases with a drug era length of less than 3 days were excluded. Over-the-counter drugs such as senna and non-benefit drugs were not included in the analysis. This study was conducted in accordance with the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Inha University Hospital (IRB No. 2023–08-034). The requirement for informed consent from the study subjects was waived by the IRB of Inha University Hospital due to the retrospective study design. All CDM-converted data used in the analysis were fully anonymized.
Statistical analysis
Data analysis was performed using an interactive analysis tool ATLAS (version 2.12.0) provided by the FEEDER-NET platform (https://feedernet.com) 25. We used the characterization, cohort pathways, and estimation functions of ATLAS and R (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria). Fisher’s exact test was used to compare differences in drug prescriptions between CKD patients with and without constipation. The risk of constipation and laxative use was evaluated in patients with CKD compared to age- and sex-matched individuals without CKD using logistic regression. The cohort entry event was the initial occurrence of diagnostic codes, and the time-at-risk was defined as the time between 1 day after cohort start date and cohort end date. Demographic covariates were included in the model fitting, and regularization was applied. KT recipients and patients receiving HD or PD were also compared in the same manner. Patients with stage-unspecified CKD were only included in the analyses where CKD stage and severity were not used. P-values of < 0.05 were considered statistically significant, and the Bonferroni correction was applied for multiple testing.
Results
Characteristics of the study population
A total of 39,293 individuals diagnosed with CKD between 2012 and 2017 were included in this study. Table 1 presents the characteristics of the participants. The overall number of patients with CKD increased noticeably from 2012 to 2017. This trend reflects the age distribution of the Korean population and the recent rapid population aging26. Recent data on CKD and ESKD populations in Korea also provide consistent findings27,28. Patients with CKD stage 1 and 2 were less likely to be captured, possibly due to low rates of screening or follow-up. Patients with CKD stage 5 were more likely to be recruited based on the Korean Classification of Disease (KCD) codes due to a high coverage benefit. Males were 60.1%, and the most common age groups were middle (40–64 years) and old age groups (65–79 years). However, individuals of ≥ 80 years have also increased markedly from 2015. The number of ESKD patients identified using procedure codes also increased over time, mostly attributed to the increasing number of HD patients. Kidney transplantation also increased by 40% during the study period. Patients undergoing PD were reduced from 14.3% to 9.1% of all ESKD patients.
Constipation diagnosis and laxative use in CKD
The proportions of constipation and laxative exposure in patients with CKD are shown in Fig. 1. Overall, patients with higher CKD stages were more likely to be diagnosed with constipation and treated with laxatives. The proportions of constipation and laxative use were similar in CKD stages 1 to 3 but markedly increased in CKD stage 4 or higher. The pooled proportion of constipation during the study period was 32.3% in all patients with CKD and 15.9%, 16.5%, 17.4%, 29.9%, and 43.3% in patients with CKD stages 1–5, respectively (16.8% for CKD stages 1 to 3). Similarly, 30.1% of all patients with CKD and 11.7%, 12.9%, 14.5%, 26.1%, and 44.2% of patients with CKD stages 1–5 were prescribed laxatives in the pooled cohort (13.4% for CKD stages 1 to 3). Patients with ESKD had the highest pooled proportions of constipation and laxative use of 44.5% (HD: 45.0%, PD: 40.5%) and 45.7% (HD: 45.7%, PD: 45.2%), respectively. Constipation diagnosis was slightly higher in HD patients compared to PD patients. However, the proportion of laxative prescription was similar between PD and HD patients. (Figs. 1C and 1D). KT recipients showed relatively lower prevalence of constipation and laxative use compared to those on HD/PD (pooled proportion 13.1% and 16.1%). In the logistic regression analysis, patients with CKD showed significantly higher odds ratios (ORs) for constipation and laxative use than age- and sex-matched non-CKD cohorts (Table 2). The estimated ORs for constipation and laxative use ranged from 1.66 to 1.90 and 2.18 to 2.33, respectively. Patients with advanced CKD had a significantly higher risk of constipation and laxative use compared to age- and sex-matched patients with mild CKD (Supplementary Table 2). In addition, patients receiving HD or PD had significantly higher ORs for constipation and laxative use compared to age- and sex-matched KT recipients (Supplementary Table 3 and 4).
Proportion of constipation and laxative prescriptions in patients with chronic kidney disease (CKD). (A) Constipation diagnosis according to the CKD stages. (B) Laxative use according to the CKD stages. (C) Constipation diagnosis in patients with end-stage kidney disease (ESKD) including kidney transplant (KT) recipients. (D) Laxative use in patients with ESKD including KT recipients.
Treatment pathways for laxative prescriptions
The treatment pathway of laxative prescriptions was examined in the different subgroups (Fig. 2 and Supplementary Fig. 1–5). In individuals without CKD, the commonly prescribed laxatives are lactulose, magnesium salts, and polycarbophil. However, as CKD progressed from the mild to advanced stages, the use of magnesium salts and polycarbophil decreased markedly, while the use of lactulose gradually increased. In a sunburst plot, the outer rings represent the second-line and subsequent therapies in individual patients. Sunburst plots of patients with CKD had a larger second ring area, indicating a greater need for second-line laxatives. In patients with ESKD, lactulose accounts for over 60% of all laxatives, followed by polycarbophil. Magnesium salts are rarely used in patients with ESKD. Sunburst plots of patients on HD showed longer spikes in the third and fourth outer rings, suggesting more frequent changes in the laxatives. Most of the second- or higher-line therapies are osmotic agents, such as lactulose and lactitol. KT recipients were prescribed mainly lactulose and bisacodyl, but the proportion of lactulose was similar to patients with mild CKD.
Treatment pathways for laxatives in patients with (A) non-chronic kidney disease (non-CKD), (B) mild chronic kidney disease (CKD) (CKD stages 1–3), (C) advanced CKD (CKD stages 4–5), (D) peritoneal dialysis (PD), (E) hemodialysis (HD), and (F) kidney transplantation. Sunburst plots are drawn using data in 2017. Plots using data in 2012–2016 are presented in Supplementary Fig. 1–5. PEG = polyethylene glycol.
Differences in drug prescriptions
We evaluated differences in medication prescriptions between CKD patients with and without constipation. Excluding laxatives, the top 10 drug groups sorted by ORs are shown in Table 3 (individual drugs are presented in Supplementary Table 5). The CKD patients with constipation were more frequently prescribed medications known to cause constipation, such as antipsychotics (risperidone, quetiapine, and haloperidol), antidepressants (nortriptyline, amitriptyline, and escitalopram), antiepileptics (valproate and clonazepam), hypnotics and sedatives (zolpidem), anxiolytics (diazepam and lorazepam), opioids (fentanyl, oxycodone, and tramadol), nonsteroidal anti-inflammatory drugs (celecoxib and diclofenac), and antimuscarinics (solifenacin and propiverine)29. Of note, the findings suggest that drug categories such as antipsychotics may pose the greatest risk for constipation in patients with CKD, and that special caution is needed when prescribing these drugs. In addition, drugs commonly used in patients with CKD have been identified, such as diuretics (furosemide and spironolactone), potassium binders (calcium polystyrene sulfonate), and iron supplements (ferrous sulfate). While most medications with high ORs may be directly related to constipation from their adverse effects, others are possibly linked to specific medical conditions, such as hypokalemia (potassium chloride) and poor oral intake (megestrol).
Discussion
In this study, we present real-world data on constipation and laxative use in patients with CKD in a nationwide cohort based on health insurance claims data. Consistent with previous observations, our data show a high prevalence of constipation in patients with CKD. In particular, constipation and laxative prescriptions were notably higher in the later stages of CKD, with ESKD being the highest. The risks of constipation and laxative use in patients with CKD were also significantly higher than those in age- and sex-matched populations without CKD. Second-line laxative use increases with the progression of CKD. The most frequently prescribed laxative is the osmotic agent lactulose, which accounts for nearly half of all laxative prescriptions for patients with advanced CKD. We also found differences in the prescription patterns of constipation-inducing drugs, including antipsychotic and neurological medications, between CKD patients with and without constipation.
While previous studies on constipation have mostly focused on patients at specific stages of CKD, such as ESKD, this study explored constipation and laxative use across all stages of CKD in a population-based cohort, which is a major strength of our study. The prevalence of constipation largely depends on the diagnostic tools used. Early studies used a self-reported definition of constipation; however, recent studies have favored the more standardized Rome criteria based on defecation symptoms and stool forms. In general, the prevalence based on the Rome criteria was lower than the patient-reported prevalence. To date, only a small number of studies have examined the prevalence of constipation in patients with non-dialysis CKD. A recent meta-analysis reported the pooled prevalence of self-reported and functional constipation in different CKD stages8. The prevalence of self-reported and functional constipation in patients with CKD stage 4–5 was 38.78% and 21.97%, respectively. Therefore, the prevalence of 29.9% (CKD stage 4), and 43.3% (CKD stage 5) in this study was close to the self-reported prevalence. The self-reported prevalence of constipation in CKD stage 3 was 29.75% in metaanalysis, which was mainly driven by two recent studies30,31. However, these studies only included patients with CKD stage 3B (GFR 30–44 ml/min/1.73 m2), while we included all stage 3 patients, resulting in a relatively low prevalence of 17.4%. For patients with CKD stages 1 and 2, small studies with sample sizes of 22 or less have reported a prevalence of 12.5–31.8%32,33. In our study, the estimated prevalence of constipation in CKD stages 1 and 2 was 15.9% and 16.5%, respectively, which was much lower than in advanced CKD.
On the other hand, numerous reports on constipation in patients with CKD have mainly focused on ESKD populations, which show a significantly higher prevalence. The prevalence of constipation has been reported to be higher in HD patients than in PD patients, which may be due to fluid restriction during the interdialytic period and low physical activity34. A study by Yasuda et al. compared the prevalence of constipation between patients on PD (n = 204) and HD (n = 268) and found a prevalence defined using a questionnaire of 28.9% in PD patients and 63.1% in HD patients35. Studies using the Rome criteria and with a sample size of 100 or more have reported a prevalence of 30.5–71.7% in HD patients and 14.2% in PD patients36,37,38. In this study, the pooled prevalence of constipation in HD and PD patients was estimated to be 45.0% and 40.5%, respectively. We found that constipation was more prevalent in patients on HD than those on PD, but the difference was not as pronounced as in previous studies. Notably, patients receiving PD had a higher proportion of laxative use than expected from their constipation diagnosis. This may indicate chronic use of laxatives in patients on PD regardless of the severity of their symptoms. Indeed, constipation can cause catheter dislocation, leading to drainage failure, which can lead clinicians to prescribe more laxatives. Of note, KT recipients showed a substantially lower prevalence of constipation and laxative use compared to patients with advanced CKD or ESKD. The prevalence of self-reported constipation in KT recipients is reported to be 37–43% in recent questionnaire-based studies39,40. However, in contrast to patients with ESKD, diarrhea rather than constipation is the most common and problematic gastrointestinal symptom in KT recipients40. These differences are likely related to immunosuppressive agents such as mycophenolate mofetil, low pill burden, normalization of uremic milieu, and changes in the gut microbiota after KT41.
We found that lactulose was the most frequently prescribed laxative in Korean patients with CKD. Lactulose is a relatively safe laxative, even in patients with advanced CKD. Also, there is some evidence that lactulose has a beneficial effect on gut microbiota in CKD, suppressing uremic toxins42,43. The use of magnesium salts and polycarbophil were greatly reduced in patients with advanced CKD. Magnesium salts act as osmotic agents but are avoided due to the risk of hypermagnesemia in patients with CKD44. Half of the patients on PD taking a polycarbophil as first-line therapy switched to the osmotic agent lactulose. Polycarbophil is a bulk-forming agent that can induce abdominal fullness and have a relatively weaker efficacy than osmotic agents. Moreover, polycarbophil is typically administered in the form of a calcium salt, which produces free calcium ions, posing a burden on calcium pools in patients with CKD45. It remains uncertain which laxatives are more beneficial for patient outcomes. To date, no direct comparison of mortality between laxatives in patients with CKD has been reported. This topic deserves to be explored further in future well-designed prospective studies. In addition, there is no consensus regarding the management of constipation in patients with CKD. The KDIGO guidelines do not address the management of constipation in CKD11, and a recent draft of the KDIGO clinical practice guideline only mentions the role of constipation in terms of hyperkalemia treatment. Recently published algorithms for constipation treatment in patients on HD recommend to avoid magnesium- or phosphate-containing laxatives46. The authors of the algorithm suggested nonpharmacological therapies such as fiber, fluid intake, and exercise, followed by pharmacological therapy using PEG, lactulose, senna, and bisacodyl46.
Constipation in patients with CKD is speculated to be multifactorial9. Fluid and dietary restrictions are among the main causes of constipation. Decreased physical activity and medications commonly used in CKD also affect bowel movements. As expected, our study showed that CKD patients with constipation were frequently prescribed constipation-inducing medications related to their CKD condition, including diuretics, potassium binders, iron supplements, antihistamines for pruritus, and analgesics for chronic pain. However, we also found the greatest risk of constipation in CKD patients who are prescribed psychotropic drugs such as antipsychotics, antiepileptics, and antidepressants. Psychotropic drugs and medications for neurologic disorders such as Parkinson's disease and dementia are known to cause constipation, but the risk of constipation when prescribing these medications in patients with CKD has been relatively unrecognized47,48. Therefore, our data suggest that clinicians should be aware of the potential adverse effects of these drug categories in managing patients with CKD. Constipation could also be attributed to multiple comorbidities that are highly prevalent in CKD, such as diabetes, cardiovascular disease, depression, and insomnia, which is consistent with our findings49,50. Prior to pharmacotherapy, identifying medications and conditions associated with constipation should be prioritized. In addition, several studies have supported that uremic toxins and alterations in the gut microbiome are the main mechanisms responsible for constipation in CKD, but the causal role and clinical implications of dysbiosis remains elusive51,52,53.
Our study has some limitations. We used retrospective data obtained from the health insurance reimbursement process. We determined CKD based on the diagnostic codes of treated patients, not on GFR or albuminuria. There may be some discrepancies between the HIRA data and actual patient conditions. Therefore, we only included patients with 2 or more inpatient or outpatient ICD codes for CKD per year to reduce false positive cases. Given that patients with early-stage CKD have low health care utilization rates, this method could be particularly effective for selecting true mild CKD patients. On the other hand, we were able to identify nearly all ESKD patients with insurance claim codes because all patients receiving HD or PD in Korea are registered with the differential co-payment system of the National Health Insurance Service54. The definition of constipation is also based on diagnostic codes and not clinical diagnostic criteria. Therefore, the estimated prevalence may be higher than that estimated using the Rome criteria. In addition, because data after 2017 were not available, newly developed drugs for constipation, such as serotoninergic agonists and chloride channel activators, were not included. To overcome these limitations, more controlled prospective studies, including recently developed drugs, are needed. Nevertheless, HIRA contains enormous amounts of data, including demographics, diagnoses, prescriptions, and outcomes, extracted from almost the entire Korean population. The findings are not specific to any one region or institution but rather reflect the overall treatment patterns and characteristics of constipation in patients with CKD. To our knowledge, this is the first study to investigate the prevalence of constipation in Korean patients with CKD, including pre-dialysis patients. Therefore, our findings add to the understanding of the epidemiology of constipation in patients with CKD and could serve as a useful reference for future studies.
In conclusion, this study provides real-world evidence on the prevalence of constipation and laxative use according to the CKD stage in a nationwide population-based cohort. We found a significantly higher risk of constipation in patients with CKD than in non-CKD individuals and different prescribing patterns of laxatives.
Data availability
The data generated in this study are included in the published article and Supplementary Information files. Raw data were obtained with the approval of the Health Insurance Review and Assessment Service in Korea and are not publicly available.
References
Camilleri, M. et al. Chronic constipation. Nat Rev Dis Primers. 3, 17095 (2017).
Peery, A. F. et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 162(2), 621–644 (2022).
Sumida, K. et al. Constipation and risk of death and cardiovascular events. Atherosclerosis. 281, 114–120 (2019).
Sundboll, J. et al. Constipation and risk of cardiovascular diseases: A Danish population-based matched cohort study. BMJ Open. 10(9), e037080 (2020).
Salmoirago-Blotcher, E., Crawford, S., Jackson, E., Ockene, J. & Ockene, I. Constipation and risk of cardiovascular disease among postmenopausal women. Am. J. Med. 124(8), 714–723 (2011).
Sumida, K. et al. Constipation and incident CKD. J. Am. Soc. Nephrol. 28(4), 1248–1258 (2017).
Suares, N. C. & Ford, A. C. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am. J. Gastroenterol. 106(9), 1582–1591 (2011).
Ruszkowski, J., Majkutewicz, K., Heleniak, Z., Witkowski, J. M. & Debska-Slizien, A. Prevalence and severity of lower gastrointestinal symptoms amongst non-dialysis chronic kidney disease patients: a systematic review and meta-analysis. J. Clin. Med. 11(21), 6363 (2022).
Sumida, K., Yamagata, K. & Kovesdy, C. P. Constipation in CKD. Kidney Int. Rep. 5(2), 121–134 (2020).
Ikee, R., Yano, K. & Tsuru, T. Constipation in chronic kidney disease: It is time to reconsider. Renal Replacement Therapy. 5(1), 51 (2019).
Group KDIGOKCW. KDIGO. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013(3), 1–150 (2012).
Perrone, R. D., Coons, S. J., Cavanaugh, K., Finkelstein, F. & Meyer, K. B. Patient-reported outcomes in clinical trials of CKD-related therapies: Report of a symposium sponsored by the national kidney foundation and the U.S. Food and Drug Administration. Am. J. Kidney Dis. 62(6), 1046–1057 (2013).
Davison, S. N. et al. Patient-reported outcome measures in the care of in-centre hemodialysis patients. J. Patient Rep. Outcomes 5(Suppl 2), 93 (2021).
Pan, R. et al. Crosstalk between the gut microbiome and colonic motility in chronic constipation: Potential mechanisms and microbiota modulation. Nutrients. 14(18), 3704 (2022).
Krukowski, H. et al. Gut microbiome studies in CKD: Opportunities, pitfalls and therapeutic potential. Nat. Rev. Nephrol. 19(2), 87–101 (2023).
Kim, J. A., Yoon, S., Kim, L. Y. & Kim, D. S. Towards actualizing the value potential of korea health insurance review and assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 32(5), 718–728 (2017).
Kim, L., Kim, J. A. & Kim, S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol. Health 36, e2014008 (2014).
Hwang, S. et al. Changes in acute kidney injury epidemiology in critically ill patients: A population-based cohort study in Korea. Ann. Intensive Care. 9(1), 65 (2019).
Park, S. et al. Disparity in accessibility to and prognosis of kidney transplantation according to economic inequality in South Korea: a widening gap after expansion of insurance coverage. Transplantation. 105(2), 404–412 (2021).
Lim, E. J. et al. Nationwide epidemiological characteristics of chronic fatigue syndrome in South Korea. J. Transl. Med. 19(1), 502 (2021).
Jeong, H., Choi, E., Suh, A., Yoo, M. & Kim, B. Risk of cardiovascular disease associated with febuxostat versus allopurinol use in patients with gout: A retrospective cohort study in Korea. Rheumatol. Int. 43(2), 265–281 (2023).
Hripcsak, G. et al. Observational health data sciences and informatics (OHDSI): Opportunities for observational researchers. Stud. Health Technol. Inf. 216, 574–578 (2015).
Shim, Y. J., Choi, H. G. & Wee, J. H. Association between chronic kidney disease and sudden sensorineural hearing loss: A longitudinal follow-up studies using ICD-10 codes in a National Health Screening Cohort. J. Clin. Med. 12(8), 2861 (2023).
Kim, S. H., Jo, M. W., Go, D. S., Ryu, D. R. & Park, J. Economic burden of chronic kidney disease in Korea using national sample cohort. J. Nephrol. 30(6), 787–793 (2017).
Park, R. W. The distributed research network, observational health data sciences and informatics, and the South Korean Research Network. Korean J. Med. 94(4), 309–314 (2019).
The Lancet Regional Health-Western P. South Korea's population shift: challenges and opportunities. Lancet Reg. Health West Pac. 36, 100865 (2023).
Yoon, S. Y. et al. National trends in the prevalence of chronic kidney disease among Korean adults, 2007–2020. Sci. Rep. 13(1), 5831 (2023).
Hong, Y. A. et al. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean Renal Data System (KORDS). Kidney Res. Clin. Pract. 40(1), 52–61 (2021).
Branch, R. L. & Butt, T. F. Drug-induced constipation. Adverse Drug React. Bull. 257, 987–990 (2009).
Grove, B. E. et al. Patient-reported outcome measures for clinical decision-making in outpatient follow-up: validity and reliability of a renal disease questionnaire. J. Patient-Rep. Outcomes. 5(1), 107 (2021).
Yapa, H. E., Purtell, L., Chambers, S. & Bonner, A. Alterations in symptoms and health-related quality of life as kidney function deteriorates: A cross-sectional study. J. Clin. Nurs. 30(11–12), 1787–1796 (2021).
Ruszkowski, J. et al. Constipation and the quality of life in conservatively treated chronic kidney disease patients: A cross-sectional study. Int. J. Med. Sci. 17(18), 2954–2963 (2020).
Lee, S. J. & Jeon, J. Relationship between symptom clusters and quality of life in patients at stages 2 to 4 chronic kidney disease in Korea. Appl. Nurs. Res. 28(4), e13–e19 (2015).
Kim, J. C., Young Do, J. & Kang, S. H. Comparisons of physical activity and understanding of the importance of exercise according to dialysis modality in maintenance dialysis patients. Sci. Rep. 11(1), 21487 (2021).
Yasuda, G. et al. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am. J. Kidney Dis. 39(6), 1292–1299 (2002).
Zhang, J. et al. Health-related quality of life in dialysis patients with constipation: a cross-sectional study. Patient Prefer. Adher. 7, 589–594 (2013).
Ramos, C. I., Andrade de Lima, A. F., Grilli, D. G. & Cuppari, L. The short-term effects of olive oil and flaxseed oil for the treatment of constipation in hemodialysis patients. J. Ren. Nutr. 25(1), 50–56 (2015).
Dos Santos, R. G. et al. Higher frequency of fruit intake is associated with a lower risk of constipation in hemodialysis patients: a multicenter study. J. Ren. Nutr. 31(1), 85–89 (2021).
Chan, S. et al. Patient-reported gastrointestinal symptoms and the association with quality of life following kidney transplantation. Kidney Int. Rep. 6(1), 138–145 (2021).
Fatly ZA, Betjes MG, van Gestel J, Verschragen M, de Weerd AE. the burden of gastrointestinal complaints in kidney transplant recipients using tacrolimus with and without mycophenolate mofetil: A randomized controlled study. Front. Nephrol. 2, 93395 (2022).
Xiao, J. et al. Organ transplantation and gut microbiota: Current reviews and future challenges. Am. J. Transl. Res. 10(11), 3330–3344 (2018).
Sueyoshi, M. et al. Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats. Clin. Exp. Nephrol. 23(7), 908–919 (2019).
Tayebi-Khosroshahi, H. et al. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease: A randomized clinical trial. J. Renal Inj. Prev. 5(3), 162–167 (2016).
Cho, Y. S. et al. 2022 Seoul consensus on clinical practice guidelines for functional constipation. J. Neurogastroenterol. Motil. 29(3), 271–305 (2023).
Danhof, I. E. Pharmacology, toxicology, clinical efficacy, and adverse effects of calcium polycarbophil, an enteral hydrosorptive agent. Pharmacotherapy 2(1), 18–28 (1982).
Ng, P., Lei, K., Teng, L., Thomas, A. & Battistella, M. Development and validation of a constipation treatment toolkit for patients on hemodialysis. Hemodial. Int. 26(1), 66–73 (2022).
Talley, N. J., Jones, M., Nuyts, G. & Dubois, D. Risk factors for chronic constipation based on a general practice sample. Am. J. Gastroenterol. 98(5), 1107–1111 (2003).
Xu, Y., Amdanee, N. & Zhang, X. Antipsychotic-induced constipation: A review of the pathogenesis, clinical diagnosis, and treatment. CNS Drugs. 35(12), 1265–1274 (2021).
Shirazian, S. et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int. Rep. 2(1), 94–107 (2017).
Tan, L. H. et al. Insomnia and poor sleep in CKD: A systematic review and meta-analysis. Kidney Med. 4(5), 100458 (2022).
Ramos, C. I. et al. Bowel habits and the association with uremic toxins in non-dialysis-dependent chronic kidney disease patients. J. Ren. Nutr. 30(1), 31–35 (2020).
Pereira, N. B. F. et al. Influence of bowel habits on gut-derived toxins in peritoneal dialysis patients. J. Nephrol. 33(5), 1049–1057 (2020).
Vaziri, N. D. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 83(2), 308–315 (2013).
Jin, D. C. & Han, J. S. Renal replacement therapy in Korea, 2012. Kidney Res. Clin. Pract. 33(1), 9–18 (2014).
Acknowledgements
This work was supported by INHA UNIVERSITY Research Grant.
Author information
Authors and Affiliations
Contributions
K.K. designed the study and drafted the original manuscript. J-E.K. performed the formal analyses. J.H.K. performed data curation. S.H.A. and C.Y.J. participated in data analysis. S.D.H. and S.W.L. contributed to the data interpretation, and J.H.S. reviewed and supervised the study. All authors have approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, K., Kim, JE., Kim, J.H. et al. Real-world evidence of constipation and laxative use in the Korean population with chronic kidney disease from a common data model. Sci Rep 14, 6610 (2024). https://doi.org/10.1038/s41598-024-57382-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57382-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.