Abstract
We examined potato rhizosphere bacterial and fungal communities across three regions: Cheongju, Pyeongchang, and Gangneung. These regions have varying soil and climate conditions, resulting in different yields. We found that precipitation was the main limiting factor in our study while soil physiochemical factors affect bacterial and fungal microbiota in correlation with yield. Both bacterial and fungal microbiota showed distinct patterns according to the regions. ASVs positively correlated with yield were predominantly found in the Pyeongchang region which also produced the highest yields, while ASVs negatively correlated with yield were associated with Gangneung where the lowest yields were observed. The greatest bacterial and fungal diversity was detected in Pyeongchang consisting of Propionibacteriales, Burkholderiales, and Vicinamibacteriales. Gangneung, on the other hand primarily belong to Sordariales, Mortierellales, Cystofilobasidiales, and Tremellales. The putative yield-negative ASVs detected in Gangneung may have been influenced by drought stress. This work has highlighted key bacterial and fungal taxa as well as core taxa that may potentially be associated with high and low yields of potato in relation to metadata which includes soil chemical and physical parameters as well as weather data. Taken together we suggest that this information can be used to assess site suitability for potato production.
Similar content being viewed by others
Introduction
Potato (Solanum tuberosum L.) is cultivated worldwide and is an important food crop providing basic nutritional requirements such as carbohydrates and dietary fibre and additional health benefits from antioxidants, vitamins, β-carotene, polyphenols, and minerals1. Potatoes are the sixth highest yielding crop worldwide, with 395 million tonnes harvested in 20202. There are over 5000 potato varieties and many show different characteristics for disease resistance, tuber nutrient content, yield, environmental adaptations and growth period, so different cultivars are grown according to purpose, environment and country3. Potatoes for consumption are cultivated across Korea mainly during the spring and autumn season in plane fields while most seed potatoes are grown in the summer season in highland regions with the cultivar Sumi (Superior), used in this study, being a major cultivar in Korea grown mainly during the spring and summer season4.
Since the introduction of high-throughput sequencing methodologies many microbial community profile studies have been performed, relating crop plant microbiome status to crop production5 and have developed better understanding of plant–microbe interactions under different environmental conditions5. Research suggests that the microbiome associated with the host crop including the rhizosphere, phyllosphere, and endosphere play key roles in plant health and production6. The rhizosphere is the region of soil surrounding roots which is influenced by plant mucilage and root exudates and in which microbes form interactions with the host plants7. There are many factors including soil type, pH, environmental conditions of soil and climate, crop genetic factors and agricultural management (fertilizers, pesticides), cropping systems and tillage which affect microbial community structure8.
Previous research has been performed to elucidate the effect of different regions, cultivars, cropping systems, and developmental stages on potato rhizosphere microbiomes9,10,11. Furthermore, research on tuber storage time and microbiome development has also been reported12. Other studies have compared potato microbiomes under different agricultural amendments, including biocontrol strategies13,14. The results of these potato microbiome studies show consistency with those reported for other crops with niche11,12,14,15, region9,16, and agricultural management10,13 having significant effect on bulk soil, rhizosphere soil, and tuber microbiome profiles.
However, there is a dearth of studies which consider the importance of regional climate zones as well as soil physical and chemical characteristics on potato rhizosphere microbiomes selection and its relationship with potato yield. In addition, most potato microbiome research to date have concentrated mainly on bacterial community structure, with only a few considering fungal11 or both fungal and bacterial microbiomes15,16.
Hence, the purpose of this research is to identify the main bacterial and fungal populations associated with the potato rhizosphere (cv Sumi), their correlation with yield and the main environmental and agricultural drivers that affect these microbiomes across three different regions in Korea. This relationship will allow a better understanding of the management practices required to improve and maintain potato yields to be developed.
Results and discussion
Climate and soil conditions among three regions
The region of Pyeongchang showed the highest temperature and accumulated precipitation during the potato growing season of 2016, with Gangneung receiving the lowest precipitation, although it was exposed to the highest levels of solar radiation (Supplemental Table S1). The preferred climatic conditions for potato cultivation are a temperature range of between 14 and 23 °C and precipitation of between 300 and 450 mm3. The temperature and cumulative solar radiation of all three regions in 2016 were slightly higher than the 20-year average, although the temperatures of all regions were within the optimum range for potato cultivation. Cheongju and Gangneung, and Pyeongchang all received a lower amount of precipitation than the 20-year average with a 30%, 51% and 43% drop respectively, although for the latter this was still within the optimal range for potato cultivation. Temperature and precipitation are important factors for crop cultivation with researchers reporting that temperature has a more significant influence for potato crop yields17.
Incorporating precipitation data alongside soil moisture contents (Supplemental Fig. S3), Gangneung exhibited the lowest soil moisture contents during potato cultivation period, indicating nearly half of the moisture contents compared to other two regions from the flowering stage to harvest. Given that soil moisture contents play a crucial role in tuber bulking4, the low soil moisture contents during that period may have impacted potato yield in Gangneung.
The highest yields were obtained in Pyeongchang among the three regions (Supplemental Fig. S1). During the potato cropping season in 2016, it should be noted that there was no dominating disease pressure in any of the three regions. A large diurnal temperature range, within the optimum temperature range, has been shown to be positively correlated to potato yield18 and as Pyeongchang is at a higher altitude of 766 m, there is a larger diurnal temperature range when compared to the other regions. The higher temperature and greater diurnal temperature range at Pyeongchang may explain the higher yields obtained, although other factors may have contributed to this.
There were different soil conditions among the three chosen regions (Supplemental Fig. S2, Table 1). The results of soil texture analysis showed that a relatively low proportion of sand and higher proportions of both clay and silt were observed in Pyeongchang (classified as sandy clay loam) compared to the other two regions classified as sandy loam. Regarding soil chemical properties: pH, organic matter, available phosphate, potassium, calcium, and magnesium all showed significant differences among the three regions (Table 1). Cheongju recorded the highest pH and calcium values, although the lowest values for organic matter and potassium. Pyeongchang showed the highest value of magnesium and the lowest values for pH, available phosphate, and calcium. Gangneung showed the highest values for organic matter, available phosphate and potassium but the lowest value of magnesium.
According to the optimum soil nutritional requirements for potato cultivation in Korea, 20–30 g/kg of organic matter, 250–350 mg/kg of phosphate, 0.5–0.6 cmol/kg of potassium, 4.5–5.5 cmol/kg of calcium and 1.5–2.0 cmol/kg of magnesium were suggested19. Considering the above values, soil nutrient conditions across the three regions were all within these ranges, supplying the optimal levels or above of each nutrient although there were significant differences between the three regions.
Comparisons of microbiota among the three regions
Microbial community profiling using Qiime2 of both bulk soil and rhizosphere samples identified a total of 10,139 bacterial ASVs and 1758 fungal ASVs. The bacterial rarefaction curves showed that the fewest ASVs were found in Gangneung (GN)—the region with the lowest potato yields (Supplemental Fig. S4A). In contrast, Pyeongchang (PC) which had the highest potato yields, had the least fungal ASVs (Supplemental Fig. S4B). The Venn diagrams also supported this result with the lowest bacterial ASV numbers in Gangneung and the lowest fungal ASV numbers in Pyeongchang (Supplemental Fig. S5).
PERMANOVA analysis and β-diversity showed that bacterial and fungal microbiota were significantly different between regions while there were no significant differences between niches within the same region for either bacterial or fungal microbiotas (Table 2, Supplemental Figs. S6, 7). When the PERMANOVA analysis was conducted separately for region and niche, it showed no significant differences in regions for bacterial rhizosphere soil. From the results of the Supplemental Fig. S6A, there was no clear distribution in rhizosphere soil compared to bulk soil. Some bacterial ASVs in the rhizosphere soil were strongly influenced by potato root and the Venn diagram results showed 3 times higher common bacterial ASVs in rhizosphere soil compared to bulk soils (Supplemental Fig. S5) which were consistent with the PERMANOVA and β-diversity results. Previous research has also demonstrated significant differences in microbial community structure between different regions and an association with different soil types9,16. There are few published papers on potato microbiomes, and most of them are focused on rhizosphere microbiota only, without comparison between bulk soil and rhizosphere soil. Although the effects of niche have been reported on for several crops including potato, maize, soybean, sorghum, and cotton, these previous studies reported no significant differences between the bulk and rhizosphere soil or only showed significant differences in the bacterial community but not in the fungal community20,21,22,23,24. In this study, bulk soils were collected between potato plants at a depth, so that they could be affected by the potato root. This might be one explanation for no significant differences between bulk and rhizosphere ASVs. Alternatively as the fields chosen for this study have had potatoes grown in them over many years, the selective pressure of potato cultivation over this extended period of time, may build up so that the bulk soil is more representative of a potato rhizosphere. A continuous legacy affect, which would not be so pronounced under crop rotations practices.
For the bacterial ASVs, over three times as many core (present in all three regional samples) rhizosphere ASVs (345) than in bulk soil samples (106). However a similar number of core fungal ASVs were detected in both bulk (71 ASVs) and rhizosphere soils (65 ASVs) (Supplemental Fig. S5). Bacterial and fungal diversities were analysed with Shannon and Simpson indices for each region (Fig. 1A–D). Bulk soil communities had a higher mean diversity compared to rhizosphere samples for a given region in both bacterial and fungal communities. The exception being fungal community diversity as interpreted by Simpsons index for Pyeongchang, which was higher in the rhizosphere samples (Fig. 1C). A previous potato microbiome study also reported lower bacterial diversity in rhizosphere soil while slightly higher diversity in the fungal community25. Hence, microbiota comparison between bulk and rhizosphere soils could be influenced by complex factors including soil type, climate, agronomy, sampling methods, and crop variety. More studies are therefore needed to identify the association between bulk and rhizosphere microbiota.
Simpson and Shannon indices of bacterial and fungal ASVs among three regions. (A) Bacterial inversed Simpson index. (B) Bacterial Shannon index. (C) Fungal inversed Simpson index. (D) Fungal Shannon index. CJ_B, Cheongju bulk; CJ_R, Cheongju rhizosphere; PC_B, Pyeongchang bulk; PC_R, Pyeongchang rhizosphere; GN_B, Gangneung bulk; GN_R, Gangneung rhizosphere. Error bar represent standard error. Statistical significance was determined using ANOVA. NS, no significance.
Although there were no significant differences, Pyeongchang and Cheongju showed relatively higher bacterial diversity than Gangneung. For fungal diversity, Pyeongchang showed a significantly higher inversed Simpson index than other regions, though it showed the lowest observed number of ASVs detected (Supplemental Fig. S5, Supplemental Fig. S8) implying a reduced evenness due to the presence of dominant fungal population members. There are different calculation methods between Inversed Simpson and Shannon indices. Both methods consider abundance and evenness, but the Inversed Simpson index is more influenced by ASV proportion data. As such, ASVs with low abundance could exert less influence in Inversed Simpson index than in the Shannon index analysis. Hence, a significantly higher fungal inversed Simpson index in Pyeongchang with no significant difference in the Shannon index might be resulting from the exclusion of low abundant ASVs. This different in fungal structure may be directly related to the increase in yield observed in Pyeongchang and the exclusion of potential pathogen fungal species. The results of relative abundance also corresponded with that of (Fig. 2). Relative abundance described that more ASVs seem to be more equal in Pyeongchang compared to other regions, while some ASVs showed much higher relative abundances in both Cheongju and Gangneung in spite of higher unassigned and low abundant taxa of below 1% on Pyeongchang. The results of the Pielou’s evenness value described that significantly higher value in fungal ASVs of Pyeongchang than the other regions (Supplemental Table S2).
Relative abundance of three regions bacterial (A) and fungal (B) microbiotas. Bacterial and fungal ASVs are described in the class and order levels, respectively. ETC represents the sum of the relative abundances of ASVs that remain unidentified or constitute less than 1% of the total relative abundances.
Relative abundance analysis was conducted on both bacterial and fungal communities in the class and order levels respectively (Fig. 2, Supplemental Fig. S9). The results of bacterial relative abundance showed higher abundances of Nitrososphaeria, Vicnamibacteria, Blastocatellia, and Anaerolineae while lower abundances of Gammaproteobacteria, Acidobacteria, and Thermoleophilia in Cheongju. For Pyeongchang, a higher abundance of Thermoleophilia and a lower abundance of KD4 96 were found. Gangneung showed higher abundances of Actinobacteria, Bacilli, and Ktedonobacteria while lower abundances of Blastocatellia and Vicinamibacteria.
Fungal relative abundance showed different results to bacterial relative abundance with higher diversity in Pyeongchang. For Cheongju, there were higher abundances of Hypocreales, Glomerellales, Cystofilobasidiales, Pezizales, and Microascales while Mortierellales showed lower abundance. For Pyeongchang, there were higher abundances of Capnodiales, Polyporales, Pezizales, Helotiales, Pleosporales, and Eurotiales and a lower abundance of Sordariales. Gangneung showed higher abundances of Sordariales, Mortierellales, and Tremellales while lower abundances of Hypocreales, Glomerellales, and Eurotiales. Arbuscular mycorrhizal (AM) fungi which are commonly observed in soil, have been shown to be common symbionts to plants, conferring many benefits in terms of nutrient uptake and tolerance to biotic or abiotic stress26. Within the fungal phyla Glomeromycota, many AM plant associations have been found and although they represent a very small proportion of fungal ASVs in this study, this group were most abundant in Pyeongchang soils (Supplemental Fig. S10).
Potato yield and microbiome correlation analysis
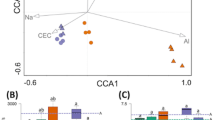
One of the important goals in studying plant microbiota is their potential in stabilising crop production under different abiotic and biotic stresses6. In this research, we attempted to elucidate microbiota which are closely associated to potato yield by analysing how they and environmental factors relate to potato yield. The results of canonical correspondence analysis, which integrated environmental factors including average temperature, precipitation, soil characteristics, bacterial and fungal microbial groups and potato yield, revealed positive and negative correlation among some factors. The results described gradient influences of environmental factors on bacterial and fungal microbiota. Potato yield exhibited strong positive correlation with magnesium, precipitation, and temperature, with exceeding 0.8 of Spearman's correlation coefficients. These factors observed the highest values in Pyeongchang. In the other hand, soil-available phosphate which observed the highest value in Gangneung was negatively correlated with potato yield (Fig. 3, Supplemental Table S3).
The results of canonical correspondence analysis on rhizosphere soil. (A) Bacterial ASVs, yield and climate conditions. (B) Bacterial ASV, yield and chemical properties. (C) Fungal ASVs, yield and climate conditions. (D) Fungal ASVs, yield and soil chemical properties. The distance between points reflects dissimilarities in microbiota. Each point represents each biological sample. Environmental variables are represented by arrows that point in the direction of increasing values of the variable.
Even though solar radiation was positively correlated to potato yield from previous work18, we found a stronger influence of temperature and precipitation on potato yield. Correlations among soil chemical factors were consistent with previous research results, showing a strong positive relation between pH and soil calcium, but except that it was inconsistent in soil chemical factors. Previous studies27 on the relationship between soil fertility and potato tuber nutrients have demonstrated the crucial role of soil pH, which showed a positive correlation with CEC (cation exchange capacity) involving elements such as potassium (K), calcium (Ca), and magnesium (Mg). On the other hand, the effects of nitrogen (N), phosphorus (P), and sulphur (S) on potato nutrients were found to be variable, influenced by factors like cropping systems and management practices. Comparing our findings and previous studies, we observed certain consistent results, particularly regarding the strong positive correlation between pH and calcium content. However, some of our results did not align with previous studies, which can be attributed to differences in the targeted soil. Previous studies collected samples from various soils, exhibiting a diverse range of pH levels and organic matter contents, whereas collected soil samples in our study were under optimum soil fertility for potato cultivation.
There was a negative correlation between yield and soil phosphate level. A previous study reported that phosphate efficiency for potato cropping is largely influenced by soil pH value, and different phosphate applications to potato cropping showed no significant difference in total tuber yield28. For soil magnesium a strong positive relationship to yield was observed as also previously reported by Marketa29. It suggests the possibility that higher soil magnesium could influence the higher microbial diversity in Pyeongchang. Thus, even though there are some positive and negative correlations between yield and soil chemical properties, other factors may be involved and a more cautious consideration is needed. The proportion of silt and clay also show strong positive relationships with yield describing 0.9 of correlation coefficients supported by Jeanne30 who reported that soil texture had a significant impact on potato yield (Supplemental Table S3).
Indicator analysis was also consistent with this result (Fig. 4). Indicator taxa analysis regarding three regions was conducted with the indval function in R program which calculates the indicator value based on relative abundance within clusters. The indicator ASVs with significant differences between regions were selected. Different regional ASVs detected as indicator taxa in bulk soil and rhizosphere soils showed few indicator ASVs were present in both bulk and rhizosphere soil. More indicator taxa were detected in bulk soil than rhizosphere soils for both bacterial and fungal ASVs (Supplemental Table S4). In Pyeongchang which showed the highest yields, fewer bacterial indicator ASVs were detected in the bulk soil, whilst the highest number of bacterial indicator ASVs were detected in rhizosphere soil. Meanwhile, there were the fewest number of fungal indicator ASVs in Pyeongchang in both bulk and rhizosphere soil. On the other hand, the largest number of indicator fungal ASVs were detected in Gangneung which showed the lowest yield. Although there was no significant differences of potato yields between Pyeongchang and Cheongju, no indicator ASVs were detected in across both regions. However, some bacterial and fungal genera were detected as indicator ASVs over all regions including Sphingomonas, Nocardioides, Mortierella, and Penicillium. In rhizosphere soil, the bacterial genera of Sphingomonas and Nocardioides was only detected as an indicator in Cheongju and Pyeongchang, respectively. Meanwhile, the fungal genera of Mortierella and Penicillium were detected in Cheongju and Pyeongchang, but not in Gangneung.
Among the indicator ASVs, all the ASVs positively related to yield were detected in Pyeongchang (Supplemental Table S4) while all the ASVs negatively related to yield were detected in Gangneung. Among 43 bacterial indicator ASVs identified in Pyeongchang, 30 ASVs were positively related to potato yield with over 0.7 of Spearman’s correlation coefficient. Throughout the bulk and rhizosphere soil samples, 8 and 2 ASVs of bacteria and fungi respectively were detected as yield-positive indicator ASVs in Pyeongchang (Supplemental Table S5). Although there were no bacterial indicator yield-negative ASVs detected from both bulk and rhizosphere soil in this region, many indicator ASVs of Gangneung showed a negative correlation with potato yield with 72 fungal yield-negative ASVs from both soil types in Gangneung (Supplemental Table S6).
Overall there were a smaller proportion of indicator ASVs from the bacterial community when compared to the fungal community. However, compared to the other regions, a higher proportion of bacterial indicator ASVs were detected from Pyeongchang consisting of Propionibacteriales, Burkholderiales, and Vicinamibacterales. Other bacterial indicator ASVs were among the Spingomonadales detected in Cheongju, while Chitinophagales and Streptomycetales were detected in Gangneung (Fig. 4A). Fungal indicator ASVs were proportionally far higher than bacteria in Gangneung. The higher proportion of Mortierellales and Pleosporales were identified as indicator taxa in Pyeongchang. Glomerellales showed remarkable proportions of indicator ASVs in Cheongju while higher proportions of Sordariales, Mortirellales, Cystofilobasidiales and Tremellales were detected in Gangneung (Fig. 4B).
Although the clusters defined at the class or phylum level are difficult to identify some functions related to plant health can be inferred. Many bacterial taxa belonging to Vicinamibacterales have been implicated as plant growth promoting bacteria involved in the nitrogen cycle10,31. Species belonging to Burkholderiales have been reported as plant growth promoting bacteria related to phosphorous cycle32. Paraburkholderia, Cupriavidus and Massila of Burkholderiales, which were detected in this research as putative yield-positive ASVs, have all been reported as plant growth promoting bacteria33,34,35, although Massila in this study showed a negative correlation with yield. Rhizobium and Sphingomonas are also well known genera with plant growth promoting functions36. Although there are few studies regarding Gaiellales, it is known that they support efficient soil nutrient cycling by degrading organic matter37.
Putative yield-negative fungal ASVs were associated with the lowest yield region which had reduced accumulated precipitation and drought stress. However, previous research has showed that no significant differences in bacterial and fungal rhizosphere microbiota observed under drought stress compared to control38, though other researchers have shown drought conditions affect soil microbiome either directly or indirectly39,40. More research is therefore required on rhizosphere microbiomes and their relationship to healthy crops, taking into account various factors, including not only climatic conditions but also crop effects such as, root exudates, varieties, plant developmental stages, and soil conditions. Only one paper so far41 has looked at potato microbiomes in relation to water stress. The putative yield-negative ASVs identified in this research should therefore consider whether they are potential plant pathogens, ASVs responding to drought conditions or have other detrimental impacts on plant health i.e. nutrient scavenging etc.
Among 15,706 bacterial ASVs in potato rhizosphere soil, 34 ASVs were positively correlated to potato yield with over 0.7 correlation coefficient, while 19 ASVs were negatively correlated to potato yield below − 0.7 of a correlation coefficient. There were 4,089 fungal ASVs detected in the potato rhizosphere soil, with only 6 ASVs positively correlated to potato yield, while 128 ASVs were negatively correlated to potato yield (Supplemental Fig. S11). A greater number of bacterial ASVs are positively correlated with potato yield than fungal ASVs. In addition, the abundance of putative yield-positive ASVs showed a higher correlation with Pyeongchang which also had the highest potato yield, while the abundance of putative yield-negative ASVs showed a greatest correlation with Gangneung which also generated the lowest potato yield (Figs. 5 and 6). Therefore, the results were consistent with indicator analysis. Most putative yield-negative fungal ASVs were however unable to be identified at the genus level. Within the microbial network, putative yield-negative ASVs exhibited a higher degree of node compared to putative yield-positive ASVs, implying a more intricate relationship within the potato rhizosphere soil microbiome.
Although many fungal ASVs were unable to be identified, the plant pathogen Fusarium was identified as well as Penicillium which has been reported as both a plant pathogen and to have plant growth promoting functions42,43. The putative yield-negative fungus with the highest number of ASVs was Sordariales, and increased in response to drought stress, as did Streptomyces, previously detected under drought conditions44,45. Some known plant pathogens from the orders Pleosporales and Hypocreales46,47, were detected as putative yield-negative ASVs. Additional Mortierella associated putative yield-negative ASVs were detected, however these have previously been were reported as plant growth-promoting fungi in agricultural soils48.
Core ASVs analysis of potato rhizosphere soil
The indicator ASVs described so far are either positively or negatively correlated to yield or other regional or environmental factors across the three regions, they do not therefore necessarily represent the core rhizosphere potato ASVs. The core ASVs have been used widely in microbiome research with various meaning, but generally, the core ASVs indicate the ASVs associated with the host49. Hence, we suggested 17 bacterial ASVs and 21 fungal ASVs as the potato rhizosphere core ASVs in spite of different environmental conditions (Fig. 7).
Even if some ASVs are not identified yet, Pseudarthrobacter, Bacillus, Sphingomonas, Methylotenera, Bradyrhizobium, Nocardioides, Phycicoccus, Terrabacter, Vriovorax and Devosia were detected as core bacterial taxa on rhizosphere soil of potato cv. Sumi. On the other hand, Nectriaceae, Chaetomiaceae, Pleosporaceae, Mortierellaceae, Basidiobaiaceae, Glomerellaceae and Mrakiaceae are described as the core fungal taxa. Although few studies have researched the potato microbiota, potato bacterial and fungal microbiota were clearly distributed depending on the regions, cultivars, and soil types9,16,41,50. Because they did fungal core taxa at the class level, it is difficult to compare to this current research. However, Bradyrhizobium, Sphingomonas, and Devosia, which were detected as core bacterial taxa in this research work, were also described as core bacterial taxa in previous research.
This work has highlighted key bacterial and fungal taxa that are potentially associated with high and low yields of potato in relation to metadata which includes, altitude, soil chemical and physical parameters as well as weather data. Taken together this information can be used to test the field environment prior to as well as during cultivation of this crop in order to guide what agronomic practices should be used to ensure successful agricultural production and to assess whether potatoes are a suitable crop for a given environment. The use of core microbial taxa that show increased or decreased abundance in response to crop yield could be considered as putative target organisms to test system yield potential. Based on the comprehensive analysis of this study, we tentatively propose the possibility that Rhizobium and Sphingomonas may be associated with potato productivity or aspects related to potato productivity in the environment. However, it is important to acknowledge that environmental factors such as soil physiochemical properties and soil moisture contents are crucial influencers of potato yield and soil microbiota. Therefore, additional experiment are needed to identify the direct positive roles of Rhizobium and Sphingomonas groups in enhancing potato yield.
Materials and methods
Potato cultivation in three different regions of Korea
To analyse the relationship between soil microbiota, environmental conditions, and potato growth and yield, potato fields in three different climatic regions of Korea were selected: Cheongju, Pyeongchang, and Gangneung. Cheongju (36.72644559264173, 127.46206046548588) is located in the middle of Korea and potatoes are cultivated during spring from the middle of March to the end of June. Pyeongchang (37.682555689366545, 128.7282038575868) is located in a mountainous area, 700 m altitude, and cultivated from the middle of May to the end of August. Gangneung (37.776176861264474, 128.93208462616758) is located near the East Sea of Korea and potato cultivation is from the end of March to the end of June. The same cultivar and field management were applied to each of the three regions. Two weeks before planting the seed potatoes, compound chemical fertilizer (Nitrogen-Phosphorous-Potassium at 21, 17, and 17%, 500 kg/ha) and compost (20,000 kg/ha) were added to the potato field and rotary tillage was applied. For potato cultivation, the variety Sumi produced by the Highland Agriculture Research Institute was used. Seed potatoes (variety Sumi, supplied by Highland Agriculture Research Institute) were planted at 80 × 25 cm apart distances with black vinyl mulching.
The data of Weather and soil moisture contents collection
Precipitation, solar radiation, and temperature data were collected by the meteorological administration of Korea (https://data.kma.go.kr) and altitude verified with google earth (https://earth.google.com). The average conditions over 20 years were calculated from 1996 to 2015. As the potato cultivation period of each region is slightly different, Cheongju and Gangneung between March to June while Pyeongchang between May to August, the precipitation and solar radiation values were aggregated while average values were applied for temperature during the potato cultivation period for each region (Supplemental Table S1).
The soil moisture contents data were collected by the Rural Development Administration of Korea and courced from https://weather.rda.go.kr. The locations were selected to be as close as possible to each experimental field: Cheongju (code: 365803A001, 36.85385, 127.42977), Gangneung (code: 240813A001, 37.4852, 129.11882) and Pyeongchang (code: 235802A001, 37.20634, 128.9857). Notably, the selected location of Pyeongchang is at an altitude of 700 m, which is similar to the altitude of experimental field in Pyeongchang. Based on potato cultivation periods, soil moisture data were collected from end of March to end of June for spring potato in Cheongju and Gangneung. For Pyeongchang, where summer potato was cultivated, the soil moisture data were collected from mid-May to the end of August. The average soil moisture contents from the flowering stage to harvest were presented in Supplemental Fig. S3. Specifically, the data from 15 May to 30 June were used for spring potato, whereas the data from 15 July to 31 August were used for summer potato.
Soil and rhizosphere sampling
Soils for chemical and textural analysis were collected prior to fertilization and rotary. One experimental field was selected for each region. Five core soil samples were randomly collected with an auger (0–15 cm of depth) for each field and placed into sampling bags. Collected soil samples were mixed and pooled on a tray before air dried for 48 h in the shade. They were then sieved at 2 mm and homogenized before soil chemical and texture analysis.
For microbiome analysis, two different niches, bulk soil and rhizosphere soils were collected at flowering stage. All tools used in the sampling were sterilized with 70% ethanol before sampling. Bulk soils were collected to a depth of 5–20 cm between potato rows using a spade. Five samples of bulk soil were collected into the same bag, and thoroughly mixed. The mixed bulk soil was transferred into 15 ml of sterilized falcon tube for storage. For rhizosphere soil sampling, the potato roots were cut with sterilised scissors and vigorously shaken using a pair of tweezers to remove loosely adhering soil. The root-adhering rhizosphere soil was submerged in 15 ml of sterile water in a 50 ml Falcon tube. Tubes were shaken for 30 s by hand. The soil suspension was centrifuged at 5000 × g for 5 min and supernatant discarded. In this research, microtiota analysis was conducted on three rhizosphere soils and three bulk soils. For bulk soil analysis, DNA extraction was performed three times using homogenized bulk soil collection. Regarding rhizosphere soil analysis, one rhizosphere soil sample was collected from the root of each potato plant. Collected bulk soil and rhizosphere soil were stored at – 70 °C prior to DNA extraction.
Soil analysis for chemical properties and texture
To elucidate soil chemical properties, soil pH, amount of organic matter, available phosphate, exchangeable potassium, calcium, and magnesium were determined. Soil pH was measured using an electrometric method in a 1:5 (w/v) of soil:water ratio with a pH meter (Orion Versa Star Pro, Thermo Scientific, Massachusetts, USA). Soil organic carbon was measured with a C/N analyzer (Vario Max Cube Elementar, Germany). Available phosphate was determined by the Lancaster method51 using a UV/VIS spectrometer (Lamda 25, Perkinelmer Co., Norwalk, CT. USA). Exchangeable cations were measured by inductively coupled plasma spectrometry (Optima 2100DV, PerkinElmer Co., Norwalk, CT, USA) using 1 M of NH4OAc solution soil extract. Soil textures were determined by the Hydrometer method52. Soil texture classification for each region was based on the soil texture triangle classified by USDA53.
Yield investigation
The investigation of potato yield was conducted at the harvest stage, three months after planting. In each experimental field area, three blocks were set up and managed. Ten plants were sampled from each blocks to measure the weight of potato tubers. Based on the investigated data, the yield data was calculated per hectacres (equivalent to 10,000 m2), taking into account the planting distances of 80 × 25 cm. The average value and standard error of three blocks were presented as Supplemental Fig. S1.
Soil microbiota analysis
The DNA from bulk soil or rhizosphere soil was extracted using the ISOIL for beads beating DNA extraction kit (Nippongene, Japan) following the manufacturer’s instructions. Extracted DNA was stored at – 20 °C prior to PCR amplification. For bacterial microbial community profiling, the V4 region of the 16S rRNA gene was amplified using the universal primers: 515F54 (5’-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-GTGCCAGCMGCCGCGGTAA-3’) and 806R55 (5’-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-GGACTACHVGGGTWTCTAAT-3’). For fungal community structure analysis, universal primer pairs for ITS1 spacer region were used: ITS1F (5’-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-CTTGGTCATTTAGAGGAAGTAA-3’) and ITS2R (5’-GTCTCGTGGGCTCGGAGATGTGTATA AGAGACAG-GCTGCGTTCTTCATCGATGC-3’) for ITS1 amplification56.
PCRs were performed using AmpliTaq GOLD (Applied Biosystems; Carlsbad, CA, USA) and PCR conditions were as follows: (1) 95 °C for 10 min, (2) 95 °C for 30 s, (3) 55 °C for 30 s, (4) 72 °C for 1 min and (5) 72 °C for 7 min, repeated (2)–(4) 30 cycles. Illumina sequencing library preparation was performed as per the Illumina, 2013, Illumina Co., California, USA, using Nextera barcodes. Prepared libraries were sequenced by Macrogen Co. (Seoul, Korea) using the MiSeq platform (Illumina Co., California, USA). Sequence data was returned as demultiplexed fastq paired-end files.
Data analysis
Microbial sequence data were analysed using the QIIME2 platform for merging, denoising, and taxonomic assignment to Amplicon Sequence Variants (ASV) with SILVA 138 for bacteria and UNITE v8 databases for fungi. Analyses of the microbiota for α-diversity (Shannon, Simpson, observed ASVs), β-diversity (unweighted and weighted UniFrac, PCoA), permutational multivariate analysis of variance (PERMANOVA), canonical correspondence analysis, indicator taxa analysis, and core taxa analysis were conducted with phyloseq, microbiome, vegan and labdsv packages in R (4.1.1). Relative abundance analysis was calculated and described using Excel and Venn diagrams generated using Venny 2.1. All correlation values were measured with R using Spearman’s correlation. From the results, the data which showed over 0.7 of the absolute value of Spearman’s correlation coefficient were selected for further analysis. Network analysis was conducted with rhizosphere ASVs using Hmisc package in R. Phylogenetic trees for both bacteria and fungi were constructed in iTOL (https://itol.embl.de) using rhizosphere ASVs.
Ethical approval
The research conducted adhered to the guidelines outlined in the IUCN Policy Statement on Research Involving Species at Risk of Extinction and Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Data availability
The datasets generated and/or analysed during the current study are available in the KNB (The Knowledge Network for Biocomplexity) repository. Access link: https://knb.ecoinformatics.org/view/urn%3Auuid%3A7e5e3b7d-83c1-41ac-940c-414ef414faa5.
References
Zaheer, K. & Akhtar, M. H. Potato production, usage, and nutrition—a review. Crc. Rev. Food Sci. 56(5), 711–721 (2016).
FAO, IFAD, UNICEF, WEF &WHO. The stage of food security and nutrition in the world 2022. In Repurposing Food And Agricultural Policies to Make Healty Diets More Affordable. Rome, FAO (2022).
Hijmans, R. J. & Spooner, D. M. Geographic distribution of wild potato species. Am. J. Bot. 88, 2101–2112 (2001).
RDA, National academy of agricultural science, Potato- The agricultural technology. 31, Preprint at https://www.nongsaro.go.kr/portal/ps/psb/psbx/cropEbookLst.ps?menuId=PS65290&stdPrdlstCode=FC&sStdPrdlstCode=FC050501# (in Korean) (2018).
Schlaeppi, K. & Bulgarelli, D. The plant microbiome at work. Mol. Plant MIicrobe 28(3), 212–217 (2015).
Singh, B. K. et al. Crop microbiome and sustainable agriculture. Microbiology 2020, 18 (2020).
Kent, A. D. & Triplett, E. W. Microbial communities and their interactions in soil and rhizosphere ecosystems. Annu. Rev. Microbiol. 56, 211–236 (2002).
Compant, S., Samad, A., Faist, H. & Sessitsch, A. A review on the plant microbiome: Ecology, functions and emerging trends in microbial application. J. Adv. Res. 19, 29–37 (2019).
Barnett, B. A. et al. Site and clone effects on the potato root-associated core microbiome and its relationship to tuber yield and nutrients. Am. J. Potato Res. 92(1), 1–9 (2015).
Zhang, W. et al. Soil bacterial biomass and diversity are affected by different furrow-ridge mulched management systems during potato continuous cropping. BioRxiv 2018, 394551 (2018).
Zimudzi, J. et al. Temporal shifts of fungal communities in the rhizosphere and on tubers in potato fields. Fungal Biol. 122(9), 928–934 (2018).
Buchholz, F. et al. 16S rRNA gene-based microbiome analysis identifies candidate bacterial strains that increase the storage time of potato tubers. Sci. Rep. 11(1), 1–12 (2021).
Akimbekov, N. et al. The effect of leonardite-derived amendments on soil microbiome structure and potato yield. Agriculture 10(5), 147 (2020).
Song, J. et al. Rhizosphere microbiomes of potato cultivated under Bacillus subtilis treatment influence the quality of potato tubers. Int. J. Mol. Sci. 22(21), 12065 (2021).
Hou, Q. et al. Rhizosphere microbial diversity and community dynamics during potato cultivation. Eur. J. Soil Biol. 98, 103176 (2023).
Xiao, H., Bonierbale, M., Williams, M. & Veilleux, R. E. Exploring candidate genes and rhizosphere microbiome in relation to iron cycling in Andean potatoes. https://vtechworks.lib.vt.edu/bitstream/handle/10919/77917/Xiao_H_D_2017.pdf#page=106 (2017).
Bushnell, J. The relation of temperature to growth and respiration in the potato plant. In Technical Bulletin, vol. 34 15–27 (University Farm, St.Paul, 1925).
Kim, Y. U. & Lee, B. W. Effect of high temperature, daylength, and reduced solar radiation on potato growth and yield. Korean J. Agric. For. Meteorol. 18(2), 74–87 (2016).
RDA. National academy of agricultural science, The standards for fertilization for crop cultivation, http://www.pungnong.co.kr/wp-content/uploads/2017/12/unnamed-file.pdf (in Korean) (2010).
Peiffer, J. A. et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. 110(16), 6548–6553 (2013).
Sugiyama, A. et al. Changes in the bacterial community of soybean rhizospheres during growth in the field. PloS One 9(6), e100709 (2014).
Schlemper, T. R. et al. Rhizobacterial community structure differences among sorghum cultivars in different growth stages and soils. FEMS Microbiol. Ecol. 93, 8 (2017).
Kong, X. et al. Maize (Zea mays L Sp) varieties significantly influence bacterial and fungal community in bulk soil, rhizosphere soil and phyllosphere. FEMS Microbiol Ecol. 96, 3 (2020).
Gkarmiri, K. et al. Identifying the active microbiome associated with roots and rhizosphere soil of oilseed rape. Appl. Environ. Microb. 83(22), e01938-e2017 (2017).
Custer, G. F., van Diepen, L. T. A. & Stump, W. An examination of fungal and bacterial assemblages in bulk and rhizosphere soils under Solanum tuberosum in Southeastern Wyoming, USA. Appl. Microbiol. 1(2), 162–176 (2021).
Chifetete, V. W. & Dames, J. F. Mycorrhizal interventions for sustainable potato production in Africa. Front. Sustain. Food Syst. 4, 593053 (2020).
James, N. M. et al. Assessment of soil fertility and potato crop nutrient status in central and eastern highlands of Kenya. Sci. Rep. 10(1), 1–11 (2020).
Ahmed, J. et al. Potato phosphorus response in soils with high value of phosphorus. Agriculture 10(7), 458 (2020).
Marketa, S. M. et al. Tuberosphere and bulk soil microbial communities in fields differing in common scab severity are distinguished by soil chemistry and interactions with pathogens. Plant Soil 468, 259–275 (2021).
Jeanne, T., Parent, S. É. & Hogue, R. Using a soil bacterial species balance index to estimate potato crop productivity. PLoS One 14(3), e0214089 (2019).
Wu, N., Li, Z., Meng, S. & Wu, F. Soil properties and microbial community in the rhizosphere of Populus alba var. pyramidalis along a chronosequence. Microbiol. Res. 250, 126812 (2021).
Ayangbenro, A. S. & Babalola, O. O. Reclamation of arid and semi-arid soils : The role of plant growth-promoting archaea and bacteria. Curr. Plant Biol. 25, 100173 (2021).
Vio, S. A. et al. Paraburkholderia. In Beneficial Microbes in Agro-Ecology 271–311 (Academic Press, 2020).
Tapia-García, E. Y. et al. Plant growth-promoting bacteria isolated from wild legume nodules and nodules of Phaseolus vulgaris L. trap plants in central and southern Mexico. Microbiol. Res. 239, 126522 (2020).
Xiao, X. et al. Interactions of plant growth-promoting rhizobacteria and soil factors in two leguminous plants. Appl. Microbiol. Biotechnol. 101(23), 8485–8497 (2017).
Saharan, B. S. & Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 21(1), 30 (2011).
Lin, Y., Ye, G. & Kuzyakov, Y. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 134, 187–196 (2019).
Liang, Y. et al. Increase in carbohydrate content and variation in microbiome are related to the drought tolerance of Codonopsis pilosula. Plant Physiol. Biochem. 165, 19–35 (2021).
Fuchslueger, L. et al. Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytol. 201, 916–927 (2014).
Chen, Y. et al. Current studies of the effects of drought stress on root exudates and rhizosphere microbiomes of crop plant species. Int. J. Mol. Sci. 23, 2374 (2022).
Faist, H. et al. Potato root-associated microbiomes adapt to combined water and nutrient limitation and have a plant genotype-specific role for plant stress mitigation. Res. Sq. 2021, 1–33 (2021).
Lai, T. et al. The response of growth and patulin production of postharvest pathogen Penicillium expansum to exogenous potassium phosphite treatment. Int. J. Food Microbiol. 244, 1–10 (2017).
Chen, J. et al. The antagonism of Penicillium griseofulvum CF3 against soil-borne fungal pathogens of potato and its growth-promoting effect on the plant. Acta Phytophyl. Sin. 40(4), 301–308 (2013).
Martínez-Arias, C. et al. Changes in plant function and root microbiome caused by flood and drought in a riparian tree. Tree Physiol. 40(7), 886–903 (2020).
Xu, L. & Coleman-Derr, D. Causes and consequences of a conserved bacterial root microbiome response to drought stress. Curr. Opin. Microbiol. 49, 1–6 (2019).
Zhang, Y., Crous, P. W., Schoch, C. L. & Hyde, K. D. Pleosporales. Fungal Divers. 53(1), 1–221 (2012).
Salgado-Salazar, C. et al. Lifestyle, mating type and mitochondrial genome features of the plant pathogen Calonectria hawksworthii (Hypocreales, Nectriaceae) as revealed by genome analyses. Can. J. Plant Pathol. 44(5), 723–736 (2022).
Ozimek, E. & Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 11(1), 7 (2020).
Neu, A. T., Allen, E. E. & Roy, K. Defining and quantifying the core microbiome: Challenges and prospects. Proc. Natl. Acad. Sci. 118(51), e2104429118 (2021).
Pfeiffer, S. et al. Rhizosphere microbiomes of potato cultivated in the High Andes show stable and dynamic core microbiomes with different responses to plant development. FEMS Microbiol. Ecol. 93(2), 242 (2017).
RDA (Rural development administration). Methods of soil chemical analysis. In National Institute of Agricultural Science and Technology, Suwon, Korea (in Korean) (1988).
Gavlak, R., Horneck, D. & Miller, R. O. Particle size analysis: Hydrometer method. In Soil, Plant and Water Reference Methods for the Western Region 128130 (Springer, 2005).
Derek, G. G., Ty, P. A. F., Kelly, R. T. & Amy, K. R. Hydrologic-process-based soil texture classifications for improved visualization of landscape function. Plos One 10(6), e0131299 (2015).
Turner, S., Pryer, K. M., Miao, V. P. W. & Palmer, J. D. Investigating deep phylogenetic relationships among Cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. 46(4), 327–338 (1999).
Apprill, A., McNally, S., Parsons, R. & Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. AME 75, 129–137 (2015).
Gardes, M. & Bruns, T. D. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Mol. Ecol. 2(2), 113–118 (1993).
Acknowledgements
This work was supported by the Rural Development Administration [PJ014866032021]. Rothamsted Research receives strategic funding from the Biotechnology and Biological Sciences Research Council of the United Kingdom. We acknowledge support from the Growing Health (BB/X010953/1) Institute Strategic Programme.
Author information
Authors and Affiliations
Contributions
Y.H.L. and G.R.B. designed the experimental setup and supervised the research process. G.R.B. conducted the experiments, collected samples and gathered data. S.J. and J.T.L. assisted with preparation for of the field experiment. G.R.B., H.K., I.M.C., T.H.M., K.K.L. and V.N.K. collaborated on data analysis and interpretation. G.L. provided specialized expertise in constructing the phylogenetic tree. G.R.B. and H.K. wrote the initial draft of manuscript and GRB integrated inputs from all authors. Y.H.L., I.M.C. and T.H.M. contributed substantially to manuscript revision and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bak, GR., Lee, K.K., Clark, I.M. et al. The potato rhizosphere microbiota correlated to the yield of three different regions in Korea. Sci Rep 14, 4536 (2024). https://doi.org/10.1038/s41598-024-55263-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55263-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.