Abstract
The Homeostatic Model Assessment for Triglyceride Glucose Index (TyG) and its related indices, including triglyceride glucose-waist circumference (TyG-WC), triglyceride glucose-waist-to-height ratio (TyG-WHtR) and triglyceride glucose-body mass index (TyG-BMI), has emerged as a practical tool for assessing insulin resistance in metabolic disorders. However, limited studies have explored the connection between TyG, TyG-related indices and osteoporosis. This population-based study, utilizing data from the National Health and Nutrition Examination Survey 2011–2018, involved 5456 participants. Through weighted multivariate linear regression and smoothed curve fitting, a significant positive correlation was found between TyG, TyG-related indices and total bone mineral density (BMD) after adjusting for covariates [β = 0.0124, 95% CI (0.0006, 0.0242), P = 0.0390; β = 0.0004, 95% CI (0.0003, 0.0004), P < 0.0001; β = 0.0116, 95% CI (0.0076, 0.0156), P < 0.0001; β = 0.0001, 95% CI (0.0001, 0.0001), P < 0.0001]. In subgroup analysis, race stratification significantly affected the relationship between TyG and total BMD. Additionally, gender and race were both significant for TyG-related indices. Non-linear relationships and threshold effects with inflection points at 9.106, 193.9265, 4.065, and 667.5304 (TyG, TyG-BMI, TyG-WHtR, TyG-WC) were identified. Saturation phenomena were observed between TyG-BMI, TyG-WC and total BMD with saturation thresholds at 314.177 and 1022.0428. These findings contributed to understanding the association between TyG, TyG-related indices and total BMD, offering insights for osteoporosis prevention and treatment.
Similar content being viewed by others
Introduction
Osteoporosis (OP) is a systemic degenerative skeletal disorder characterized by diminished bone mineral density (BMD) and impaired skeletal microarchitecture, leading to increased skeletal fragility and fractures1,2,3. An epidemiological investigation estimated that approximately 200 million individuals worldwide were affected by OP, which is increasing yearly4. As the global population continues to age, the implications of OP are expected to escalate. Projections indicate that by 2025, the direct costs associated with this condition will reach a staggering $25.3 billion annually5. This will inevitably place a considerable strain on both the healthcare system and the broader social economy6. Undoubtedly, OP has emerged as an imperative global public health concern.
As a major determinant of bone health, the measurement of BMD is a commonly used operational tool for the diagnosis of OP7. Relevant studies have shown that metabolic syndrome (MetS), characterized by abdominal obesity and metabolic dysfunction, has emerged as a new risk factor for BMD reduction8. Insulin resistance (IR) refers to the inability of peripheral tissues to respond to insulin and is the main feature of metabolic syndrome9,10. IR is a potential factor affecting bone density. However, current studies investigating the association between IR and OP have produced inconsistent results. Earlier study reported that the relationship between lumbar spine BMD and IR was negative11. In contrast, a recent research showed that IR was a protective factor for OP in a specific range12. There's also a evidence that reveals the connection between IR and OP risk in type 2 diabetes mellitus (T2DM) patients is significantly affected by gender13. A more precise comprehension of the correlation between IR and BMD could offer novel insights into the prevention and treatment of OP.
Hyperinsulinemic-euglycemic clamp (HECT) is widely considered the gold standard for evaluating IR14. Another prevalent method for assessing IR is the homeostasis model assessment of insulin resistance (HOMA-IR)15. However, both of the above two methods are limited in application scenarios, particularly because fasting insulin levels are infrequently measured in routine clinical settings and large-scale epidemiological studies. In contrast, the triglyceride glucose index (TyG) offers a simpler and more cost-effective way to assess IR. Numerous studies have shown that the accuracy of TyG is on par with that of HECT and HOMA-IR for estimating IR16,17. Moreover, it is imperative to note that patients with high IR often experience obesity18, which is also a significant factor affecting BMD19. In recent years, many researchers have reported that combining TyG and obesity-related indices [body mass index (BMI), waist-to-height ratio (WHtR) and waist circumference (WC)] into TyG-related indices (TyG-BMI, TyG-WHtR and TyG-WC) can enhance the ability to identify IR20,21. Additionally, the latest study discovered that TyG-related indices were generally more effective at predicting the progression of coronary artery calcification and identifying fatty liver than TyG22,23. Despite this, the evidence regarding the superiority of TyG and TyG-related indices in assessing BMD is inconclusive.
Therefore, the present study aimed to analyze the correlation between TyG, TyG-related indices and total BMD through a large-scale cross-sectional study based on the National Health and Nutrition Examination study database (NHANES).
Methods
Data source and study population
The NHANES is a large, ongoing cross-sectional study conducted in the U.S. designed to provide objective health statistics and address emerging public health issues among the general population (http://www.cdc.gov/nchs/nhanes/)24. All participants consented to the use of their data in the research, and the National Center for Health Statistics Institutional Review Board approved the survey techniques.
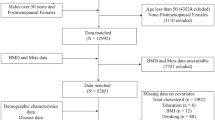
This study employed NHANES data from 2011 to 2018. In total, 39,156 participants completed the demographic survey, laboratory examination, and health condition questionnaire. The exclusion criteria were as follows: (1) Missing total BMD data (N = 21,282); (2) Missing data on fasting triglycerides (N = 11,259) and fasting glucose (N = 7); (3) Missing data on BMI (N = 22) and waist circumference (N = 43); (4) Patients with thyroid disease (N = 249), cancer or malignancy (N = 156) and nephropathy (N = 84); (5) Postmenopausal women (N = 462); (6) Missing data on total calcium (N = 38), low-density lipoproteins (N = 69), creatine phosphokinase (N = 15), total bilirubin (N = 5) and glycohemoglobin (N = 9). Ultimately, 5456 participants were enrolled in the research (Fig. 1). The accompanying NHANES variable codes and their utilization details are outlined in Supplementary Table S1.
BMD examination
Total BMD was measured using dual-energy X-ray absorption (DXA) on a Hologic QDR 4500A fan-beam densitometer25. The screening tool is commonly used internationally to assess fragility-fracture risk. Radiologic technologists were certified and trained to administer DXA examinations. In the survey, DXA scans were available to all survey participants aged 8 and older, but they were not suitable for individuals weighing over 300 pounds, pregnant women, or those who had taken radiographic contrast material in the past 7 days.
Definitions of TyG and TyG-related indices
The indices in the study were defined as follows: WHtR is defined as WC divided by body height26; TyG = ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2]27; TyG-BMI = TyG × BMI28; TyG-WHtR = TyG × WHtR23; TyG-WC = TyG × WC28.
Covariates
Data on demographics, examination, and laboratory are covariates. Demographics data covered age (years), gender (male and female), race/ethnicity (Mexican American, non-Hispanic white, non-Hispanic black, other race/ethnicity), level of education (less than high school, high school or equivalent, college or above) and family income-poverty ratio (family PIR). Examination data included weight (kg), height (cm), body mass index (BMI, kg/m2) and waist circumference (WC, cm). Finally, laboratory data consisted of fasting glucose (mg/dL), fasting triglyceride (mg/dL), alkaline phosphatase (ALP, IU/L), blood urea nitrogen (BUN, mg/dL), creatine phosphokinase (CPK, IU/L), creatinine (mg/dL), phosphorus (mg/dL), total calcium (mg/dL), uric acid (mg/dL), total bilirubin (mg/dL), glycohemoglobin (%), total cholesterol (mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), low-density lipoprotein cholesterol (LDL-C, mg/dL) and 25OHD2 + 25OHD3 (nmol/L).
Statistical analysis
Statistical analysis for this study was conducted using R (http://www.r-project.org) and EmpowerStats (http://www.empowerstats.com), setting a significance level at P < 0.05. In accordance with NCHS analytical guidelines, we employed sample weights in all estimates to better represent the non-institutionalized civilian population in the USA. To investigate correlations between research variables and total BMD, participants were categorized into quartiles based on their total BMD values, which ranged from 0.670 to 1.728 g/cm2. Univariate analyses and chi-square tests were then conducted to examine these correlations. Weighted multiple linear regression analysis analyses were utilized to explore the linear associations between TyG, TyG-related indices and total BMD. The multiple linear regression analysis were structured into three distinct models: Model 1 served as the baseline and involved no adjustments for any variables; Model 2 included adjustments for age, gender and race/ethnicity; Model 3 adjusted for a variety of factors, including age, gender, race/ethnicity, level of education, family PIR, ALP, BUN, CPK, creatinine, phosphorus, total calcium, uric acid, total bilirubin, glycohemoglobin, total cholesterol, HDL-C, LDL-C and 25OHD2 + 25OHD3. Subgroup analyses were also conducted, focusing specifically on variations across gender and race. In the final stage of our statistical analysis, smoothing curve fitting techniques and threshold effects evaluations were employed to assess any non-linear relationships between TyG, TyG-related indices and total BMD respectively.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All analyses were based on data of the National Health and Nutrition Examination Survey (NHANES). The study was approved by the ethics review board of the National Center for Health Statistics. The detailed information located on the NHANES website. Written informed consent was obtained from each participant before their inclusion on the NHANES database. Detailed information on the ethics application and written informed consent are provided on the NHANES website.
Results
Baseline characteristics
After applying inclusion and exclusion criteria, 5456 participants were included in the study with a mean age of 30.33 ± 13.55 years. The sample comprised 55.65% males and 44.35% females, along with 17.19% Mexican–American people, 32.04% non-Hispanic white people, 21.13% non-Hispanic black people and 29.64% individuals from other racial backgrounds.
As shown in Table 1, the clinical characteristics of the participants are listed in stratified groups based on the four categories of total BMD. Compared to the bottom quartile, those in the top total BMD quartile were more likely to be male and older, had a higher proportion of non-Hispanic white and non-Hispanic black, exhibited a higher PIR and education level, and showed increased levels of TyG, TyG-BMI, TyG-WHtR, TyG-WC, weight, height, BMI, WC, fasting glucose, fasting triglyceride, BUN, CPK, creatinine, uric acid, total bilirubin, glycohemoglobin, total cholesterol, LDL-C and 25OHD2 + 25OHD3. Conversely, they demonstrated lower levels of ALP, phosphorus, total calcium and HDL-C (P < 0.05).
Association between TyG, TyG-related indices and total BMD
To investigate the relationships between TyG, TyG-related indices and total BMD, multiple linear regression models ware used. Table 2 presents the outcomes for three distinct models. All models exhibited a positive correlation between TyG, TyG-related indices and total BMD, TyG-BMI, TyG-WHtR and TyG-WC demonstrated a statistically correlated with total BMD (P < 0.0001). After adjusting for all confounding variables, one-unit increments in TyG, TyG-BMI, TyG-WHtR and TyG-WC were linked with 0.0124 g/cm2, 0.0004 g/cm2, 0.0116 g/cm2, and 0.0001 g/cm2 increase in total BMD, respectively. When TyG-BMI, TyG-WHtR and TyG-WC were grouped into quartiles, this association persisted statistically significant (P < 0.0001).
Subgroup analysis
A subgroup analysis was conducted to evaluate the stability of the relationship between TyG, TyG-related indices and total BMD across various demographic contexts. As illustrated in Tables 3, 4, 5 and 6, positive correlations between TyG-related indices and total BMD were significantly affected by gender stratification (P < 0.05 for interactions). Racial differences in absolute effect sizes were statistically significant in racially stratified subgroup analyses of TyG and TyG-related indices (P < 0.05 for interactions). Furthermore, positive correlations between TyG-BMI, TyG-WC and total BMD were significant not only unadjusted but also fully adjusted (P < 0.0001).
Non-linearity and saturation effect analysis between TyG, TyG-related indices and total BMD
As a result of smooth curve fitting, the non-linear association and saturation phenomenon between TyG, TyG-related indices, and total BMD have been delineated (Fig. 2). For TyG, TyG-BMI, TyG-WHtR and TyG-WC, the thresholds were 9.106, 193.9265, 4.065 and 667.5304, respectively (Tables 7, 8, 9, 10). The regression coefficient increases when TyG exceeds the threshold. By contrast, when TyG-BMI, TyG-WHtR and TyG-WC exceed their thresholds, the regression coefficient decreases accordingly. Furthermore, TyG-BMI and TyG-WC displayed saturation effects on total BMD, with saturation thresholds of 314.177 and 1022.0428, respectively.
Association between TyG, TyG-related indices and total BMD. Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. Gender, race, age, education level, family PIR, ALP, BUN, CPK, creatinine, phosphorus, total calcium, uric acid, total bilirubin, glycohemoglobin, total cholesterol, HDL-C, LDL-C and 25OHD2 + 25OHD3 were adjusted.
Supplementary analysis
To ensure the stability of the TyG and TyG-related indices data in NHANES, we performed supplementary correlation analyses between TyG and TyG-related indices and HOMA-IR as well as fasting insulin. The results revealed a positive correlation between TyG and TyG-related indices with both HOMA-IR and fasting insulin. This observation aligns with the findings of previous studies, affirming the consistency of our results with established research in the field (Supplementary Table S2–S3).
To ensure the relative stability of experimental results, missing data for Family PIR were retained, introducing potential bias in the outcomes of Model 3. To address this potential bias, additional subgroup analyses were conducted using all covariates except Family PIR (Supplementary Table S4–S7). In this subgroup analysis, no data were missing in the study population, revealing only a minor deviation compared to Model 3, and indicating no substantive impact on the experimental conclusions.
Discussion
To our knowledge, this is the first cross-sectional study in the United States to examine correlations between TyG, TyG-related indices and BMD, revealing the close relationship between IR and bone metabolism. It was found that IR evaluated by TyG and TyG-related indices was significantly positively correlated with BMD among Americans. This is consistent with previous studies, such as an ongoing prospective study that found TyG-BMI was positively related to BMD and geometry in non-diabetic middle-aged and elderly Chinese individuals29. Moreover, a single-center retrospective study discovered that IR was positively correlated with BMD in postmenopausal women, as well as a protective factor for OP12. As per another study, higher IR values were associated with higher BMD values in non-diabetic older adults30.
Currently, the underlying mechanisms that explain the observed positive correlation between TyG, TyG-related indices and total BMD remain incompletely understood. Several interconnected pathways could contribute to this association. Firstly, IR stimulates the secretion of insulin, and hyperinsulinemia leads to increased BMD. Insulin acts as a promoter of osteoblast proliferation and an inhibitor of osteoclast activity, thus playing an anabolic role in bone. In the state of IR, the body compensates for resistance in skeletal muscle, adipose tissue, and liver by increasing insulin secretion, leading to hyperinsulinemia. This elevated insulin level further boosts bone mass31. Secondly, IR may also influence bone metabolism through its impact on inflammatory responses and estrogen levels. Inflammatory mediators such as TNF-α, IL-1, and IL-9 have been shown to promote osteoclasts differentiation or inhibit the osteogenic differentiation of bone marrow mesenchymal stem cells via the activation of the RANKL/RANK/OPG and Wnt signaling pathways32. Estrogen exerts a direct influence on osteocytes, osteoblasts, and osteoclasts, while also inhibiting osteoclast activation either directly or through osteoblasts and T-cells. The ultimate effect of estrogen on the skeletal system is a reduction in bone remodeling and resorption, while simultaneously preserving bone formation33. Finally, the relationship between IR and bone metabolism is bidirectional, suggesting the potential for a cycle: IR impacts bone metabolism, and in turn, the state of bone health influences glucose metabolism34,35,36,37,38.
Furthermore, our study revealed thresholds between TyG, TyG-related indices and total BMD. As TyG exceeds the threshold, the regression coefficient increases. Conversely, when TyG-related indices exceed their thresholds, the regression coefficient decreases. The discrepancy may be caused by two factors. On the one hand, as IR progresses, insulin sensitivity significantly decreases, resulting in more insulin release39. Insulin is a critical anabolic hormone in osteoblasts40. Insulin action in osteoblasts stimulates mitosis, inhibits apoptosis, and prevents hyperglycemia's deleterious effects on bone formation40,41. Research have demonstrated that the stimulation of insulin receptors promotes proliferation and differentiation of osteoblasts in immature mice42. Mature osteoblasts in culture also express insulin receptors43. Osteoblasts are affected by insulin through multiple signaling pathways, thus de-repressing proliferation44, blocking the pro-apoptotic effect45,46, and promoting anabolism47. On the other hand, the involvement of obesity in IR prevents IGF-1 from binding to insulin receptors on osteoblasts, leading to dysfunctional insulin signaling, which negatively affects bone remodeling48,49,50. Obesity is also associated with the loss of Dock 7 protein and silence of Thy-1 expression, which results in higher bone resorption and higher levels of adipogenesis, leading to impaired bone formation48,51,52,53.
In addition to the threshold effect, TyG-BMI and TyG-WC had a saturation effect on total BMD with saturation thresholds of 314.177 and 1022.0428. Saturation might be caused by genetic determinism and co-leadership interactions. Early in life, bone growth trajectories and peak bone mass were determined54,55, which might account for the plateau in BMD values observed in adults after a limited increase. Pocock et al. explored the influence of genetics on BMD in 38 pairs of monozygotic twins and 27 pairs of dizygotic twins, revealing that genetics played a pivotal role in adult bone mass56. It was determined that genetic factors accounted for 75% of the variance in BMD, regardless of gender57,58. Additionally, acquired environmental factors such as calcium intake, estrogen intake, and physical exercise also had limitations in terms of increasing adult BMD59,60,61. Furthermore, bone mass was also dominated by a complex interplay of multiple factors. Research has demonstrated a connection between adipose and bone tissues within the body, with numerous bioactive molecules maintaining bone homeostasis62. This bone-adipose axis may offer an explanation for the observed saturation effects. It has been found that adipose and bone tissues share a common stem cell precursor and are in a competitive relationship, with excessive fat accumulation resulting in decreased bone mass63,64. Meanwhile, excessive fat accumulation has been linked to decreased bone mass, as confirmed by animal models on high-fat diets65,66,67. According to other studies, adipose tissue also secretes inflammatory mediators such as interleukin-1 and tumor necrosis factor-α68, which inhibit BMD via the RANK/RANKL/OPG pathway69. In summary, the saturation effects observed in TyG-BMI and TyG-WC could be attributable to a variety of factors. Despite these findings, the existing body of direct experimental evidence remains limited, necessitating further in-depth research.
Indeed, excessive IR and obesity were detrimental to the entire population. Firstly, excessive IR and obesity were associated with a number of bone-related disorders, such as increased bone fragility70,71 and increased risk of fractures19,30. In addition, IR and obesity can lead to a variety of chronic diseases and complications, such as cardiovascular disease72,73, T2DM74,75, gallbladder disease76,77 and fatty liver78,79. More seriously, high IR and excessive obesity might increase cancer risk80,81 and cancer-related mortality82,83. Therefore, we believe that maintaining TyG and TyG-related indices at reasonable values might contribute to maintaining optimal BMD and reducing the risk of related diseases and complications.
In subgroup analysis, the racial subgroup showed significant differences in the effects of TyG and TyG-related indices on BMD, which might indicate racial differences in bone and mineral metabolism. This may be due to differences in insulin sensitivity and the relationship to triglyceride concentration among the different race/ethnic groups. According to one research84, African Americans and non-Hispanic Whites have greater insulin sensitivity. Moreover, African Americans have lower triglyceride levels than non-Hispanic Whites at a given level of IR. Intriguingly, a divergent study with a similar focus on adults, highlighting a negative correlation between TyG and BMD85, introduces a notable contrast to our findings. It's crucial to acknowledge that our study included children but excluded postmenopausal females, potentially creating unique subsets within age and gender subgroups. In addition, gender had a significant effect on BMD related to TyG-related indices. This may be related to estrogen, which is an important regulator of body weight, body fat distribution and IRn86,87,88,89. Given these intriguing disparities observed across racial and gender subgroups, further investigation is warranted. A thorough exploration of the underlying mechanisms driving these differences could offer valuable insights into the complex interplay between insulin resistance and bone health across diverse populations.
In summary, this was a successful large cross-sectional study that revealed a positive association between IR and BMD through TyG and TyG-related indices. Primarily, the utilization of a nationally representative cohort ensured that the results summarized the heterogeneity of the overwhelming majority of demographics in the USA. Furthermore, the large sample size enabled us to conduct subgroup analyses, classifying participants by race, thereby enhancing the generalizability of our findings. However, several limitations of this study merit cautious interpretation of the conclusions. Firstly, the cross-sectional design of the study precludes the establishment of a definitive causal link between TyG, TyG-related indices and BMD. Moreover, database constraints meant that comprehensive data on participants' lifestyle, dietary habits and bone metabolism indices, which might have impacted BMD. The inability to gather BMD data from various skeletons prevented the investigation of the consistency of the outcomes across skeletons. Finally, the absence of fracture data in our database meant we could not determine whether participants with lower TyG or TyG-related indices had a higher incidence of fractures compared to the general population.
Conclusion
This study revealed a positive association between TyG, TyG-related indices and total BMD. There was also a saturation phenomenon between TyG-BMI, TyG-WC and total BMD. TyG and TyG-related indices can serve as effective indicators for the prevention and treatment of OP. Maintaining optimal levels of TyG and TyG-related indices is critical for managing bone metabolism effectively.
Data availability
The survey data are publicly available on the Internet for data users and researchers throughout the world http://www.cdc.gov/nchs/nhanes/.
Abbreviations
- ALP:
-
Alkaline phosphatase
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- BUN:
-
Blood urea nitrogen
- CPK:
-
Creatine phosphokinase
- DXA:
-
Dual-energy X-ray absorption
- Family PIR:
-
Ratio of family income to poverty
- HDL-C:
-
High-density lipoprotein cholesterol
- HECT:
-
Hyperinsulinemic-euglycemic clamp
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- MetS:
-
Metabolic syndrome
- NHANES:
-
National Health and Nutrition Examination study database
- OP:
-
Osteoporosis
- T2DM:
-
Type 2 diabetes mellitus
- TyG:
-
Triglyceride glucose index
- TyG-BMI:
-
Triglyceride glucose-body mass index
- TyG-WC:
-
Triglyceride glucose-waist circumference
- TyG-WHtR:
-
Triglyceride glucose-waist-to-height ratio
- WC:
-
Waist circumference
- WHtR:
-
Waist-to-height ratio
References
Kanis, J. A., Cooper, C., Rizzoli, R. & Reginster, J. Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 30, 3–44. https://doi.org/10.1007/s00198-018-4704-5 (2019).
Kanis, J. A., Delmas, P., Burckhardt, P., Cooper, C. & Torgerson, D. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos. Int. 7, 390–406. https://doi.org/10.1007/bf01623782 (1997).
Qaseem, A. et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: A clinical practice guideline update from the American College of Physicians. Ann. Intern. Med. 166, 818–839. https://doi.org/10.7326/m15-1361 (2017).
Kushchayeva, Y., Pestun, I., Kushchayev, S., Radzikhovska, N. & Lewiecki, E. M. Advancement in the treatment of osteoporosis and the effects on bone healing. J. Clin. Med. https://doi.org/10.3390/jcm11247477 (2022).
Dempster, D. W. Osteoporosis and the burden of osteoporosis-related fractures. Am. J. Manag. Care 17(Suppl 6), S164-169 (2011).
Khosla, S. & Hofbauer, L. C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 5, 898–907. https://doi.org/10.1016/S2213-8587(17)30188-2 (2017).
Xue, S. et al. Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005–2014. Bone 131, 115163. https://doi.org/10.1016/j.bone.2019.115163 (2020).
von Muhlen, D., Safii, S., Jassal, S. K., Svartberg, J. & Barrett-Connor, E. Associations between the metabolic syndrome and bone health in older men and women: The Rancho Bernardo Study. Osteopor. Int. 18, 1337–1344. https://doi.org/10.1007/s00198-007-0385-1 (2007).
Haffner, S. M. et al. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 41, 715–722. https://doi.org/10.2337/diab.41.6.715 (1992).
Grundy, S. M. Metabolic syndrome update. Trends Cardiovasc. Med. 26, 364–373. https://doi.org/10.1016/j.tcm.2015.10.004 (2016).
Kalimeri, M. et al. Association of insulin resistance with bone strength and bone turnover in menopausal Chinese–Singaporean women without diabetes. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph15050889 (2018).
Gu, P. et al. The metabolic score of insulin resistance is positively correlated with bone mineral density in postmenopausal patients with type 2 diabetes mellitus. Sci. Rep. 13, 8796. https://doi.org/10.1038/s41598-023-32931-8 (2023).
Wang, X., Jiang, L. & Shao, X. Association analysis of insulin resistance and osteoporosis risk in Chinese patients with T2DM. Ther. Clin. Risk Manag. 17, 909–916. https://doi.org/10.2147/tcrm.S328510 (2021).
Pacini, G. & Bergman, R. N. MINMOD: A computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput. Methods Prog. Biomed. 23, 113–122. https://doi.org/10.1016/0169-2607(86)90106-9 (1986).
Bloomgarden, Z. T. Measures of Insulin Sensitivity. Clin. Lab. Med. 26, 611–633. https://doi.org/10.1016/j.cll.2006.06.007 (2006).
Simental-Mendía, L. E., Rodríguez-Morán, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 6, 299–304. https://doi.org/10.1089/met.2008.0034 (2008).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic–hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351. https://doi.org/10.1210/jc.2010-0288 (2010).
Suren Garg, S., Kushwaha, K., Dubey, R. & Gupta, J. Association between obesity, inflammation and insulin resistance: Insights into signaling pathways and therapeutic interventions. Diabetes Res Clin Pract 200, 110691. https://doi.org/10.1016/j.diabres.2023.110691 (2023).
Palermo, A. et al. BMI and BMD: The potential interplay between obesity and bone fragility. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph13060544 (2016).
Lim, J., Kim, J., Koo, S. H. & Kwon, G. C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS One 14, e0212963. https://doi.org/10.1371/journal.pone.0212963 (2019).
Er, L. K. et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One 11, e0149731. https://doi.org/10.1371/journal.pone.0149731 (2016).
Cho, Y. K. et al. Triglyceride glucose-waist circumference better predicts coronary calcium progression compared with other indices of insulin resistance: A longitudinal observational study. J. Clin. Med. https://doi.org/10.3390/jcm10010092 (2020).
Malek, M., Khamseh, M. E., Chehrehgosha, H., Nobarani, S. & Alaei-Shahmiri, F. Triglyceride glucose-waist to height ratio: A novel and effective marker for identifying hepatic steatosis in individuals with type 2 diabetes mellitus. Endocrine 74, 538–545. https://doi.org/10.1007/s12020-021-02815-w (2021).
Fain, J. A. NHANES. Diabetes Educ. 43, 151. https://doi.org/10.1177/0145721717698651 (2017).
Ouyang, Y. et al. Saturation effect of body mass index on bone mineral density in adolescents of different ages: A population-based study. Front. Endocrinol. (Lausanne) 13, 922903. https://doi.org/10.3389/fendo.2022.922903 (2022).
Nevill, A. M., Stewart, A. D., Olds, T. & Duncan, M. J. A new waist-to-height ratio predicts abdominal adiposity in adults. Res. Sports Med. 28, 15–26. https://doi.org/10.1080/15438627.2018.1502183 (2020).
Kim, J. A. et al. Association of the triglyceride and glucose index with low muscle mass: KNHANES 2008–2011. Sci. Rep. 11, 450. https://doi.org/10.1038/s41598-020-80305-1 (2021).
Khamseh, M. E. et al. Triglyceride glucose index and related parameters (triglyceride glucose-body mass index and triglyceride glucose-waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab. Syndr. Relat. Disord. 19, 167–173. https://doi.org/10.1089/met.2020.0109 (2021).
Wen, Z. et al. Triglyceride glucose-body mass index is a reliable indicator of bone mineral density and risk of osteoporotic fracture in middle-aged and elderly nondiabetic Chinese individuals. J. Clin. Med. https://doi.org/10.3390/jcm11195694 (2022).
Napoli, N. et al. Effect of insulin resistance on BMD and fracture risk in older adults. J. Clin. Endocrinol. Metab. 104, 3303–3310. https://doi.org/10.1210/jc.2018-02539 (2019).
Conte, C., Epstein, S. & Napoli, N. Insulin resistance and bone: A biological partnership. Acta Diabetol. 55, 305–314. https://doi.org/10.1007/s00592-018-1101-7 (2018).
Ponzetti, M. & Rucci, N. Updates on osteoimmunology: What’s new on the cross-talk between bone and immune system. Front. Endocrinol. (Lausanne) 10, 236. https://doi.org/10.3389/fendo.2019.00236 (2019).
Cauley, J. A. Estrogen and bone health in men and women. Steroids 99, 11–15. https://doi.org/10.1016/j.steroids.2014.12.010 (2015).
Wei, J. et al. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J. Clin. Investig. 124, 1–13. https://doi.org/10.1172/jci72323 (2014).
Lee, N. K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469. https://doi.org/10.1016/j.cell.2007.05.047 (2007).
Li, Z. et al. Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology 157, 4094–4103. https://doi.org/10.1210/en.2016-1583 (2016).
Zoch, M. L., Abou, D. S., Clemens, T. L., Thorek, D. L. & Riddle, R. C. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res. 4, 16004. https://doi.org/10.1038/boneres.2016.4 (2016).
Huang, S. et al. Decreased osteoclastogenesis and high bone mass in mice with impaired insulin clearance due to liver-specific inactivation to CEACAM1. Bone 46, 1138–1145. https://doi.org/10.1016/j.bone.2009.12.020 (2010).
Shanik, M. H. et al. Insulin resistance and hyperinsulinemia: Is hyperinsulinemia the cart or the horse?. Diabetes Care 31, S262–S268. https://doi.org/10.2337/dc08-s264 (2008).
Thrailkill, K. M., Lumpkin, C. K. Jr., Bunn, R. C., Kemp, S. F. & Fowlkes, J. L. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am. J. Physiol. Endocrinol. Metab. 289, E735-745. https://doi.org/10.1152/ajpendo.00159.2005 (2005).
Mieczkowska, A. et al. Alteration of the bone tissue material properties in type 1 diabetes mellitus: A Fourier transform infrared microspectroscopy study. Bone 76, 31–39. https://doi.org/10.1016/j.bone.2015.03.010 (2015).
Thrailkill, K. M. et al. Bone formation is impaired in a model of type 1 diabetes. Diabetes 54, 2875–2881. https://doi.org/10.2337/diabetes.54.10.2875 (2005).
Thomas, D. M., Hards, D. K., Rogers, S. D., Ng, K. W. & Best, J. D. Insulin receptor expression in bone. J. Bone Miner. Res. 11, 1312–1320. https://doi.org/10.1002/jbmr.5650110916 (1996).
Uchida, T. et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat. Med. 11, 175–182. https://doi.org/10.1038/nm1187 (2005).
Fang, X. et al. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 18, 6635–6640. https://doi.org/10.1038/sj.onc.1203076 (1999).
White, M. F. Insulin signaling in health and disease. Science 302, 1710–1711. https://doi.org/10.1126/science.1092952 (2003).
Campos Pastor, M. M., López-Ibarra, P. J., Escobar-Jiménez, F., Serrano Pardo, M. D. & García-Cervigón, A. G. Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: A prospective study. Osteoporos. Int. 11, 455–459. https://doi.org/10.1007/s001980070114 (2000).
Imerb, N., Thonusin, C., Chattipakorn, N. & Chattipakorn, S. C. Aging, obese-insulin resistance, and bone remodeling. Mech. Ageing Dev. 191, 111335. https://doi.org/10.1016/j.mad.2020.111335 (2020).
Rosen, C. J. & Motyl, K. J. No bones about it: Insulin modulates skeletal remodeling. Cell 142, 198–200. https://doi.org/10.1016/j.cell.2010.07.001 (2010).
Cao, J. J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 6, 30. https://doi.org/10.1186/1749-799X-6-30 (2011).
Barker, T. H. & Hagood, J. S. Getting a grip on Thy-1 signaling. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1793, 921–923. https://doi.org/10.1016/j.bbamcr.2008.10.004 (2009).
Motyl, K. J. et al. Altered thermogenesis and impaired bone remodeling in Misty mice. J. Bone Miner. Res. 28, 1885–1897. https://doi.org/10.1002/jbmr.1943 (2013).
Paine, A. et al. Thy1 is a positive regulator of osteoblast differentiation and modulates bone homeostasis in obese mice. FASEB J. 32, 3174–3183. https://doi.org/10.1096/fj.201701379R (2018).
Jones, G. & Dwyer, T. Birth weight, birth length, and bone density in prepubertal children: Evidence for an association that may be mediated by genetic factors. Calcif. Tissue Int. 67, 304–308. https://doi.org/10.1007/s002230001148 (2000).
Weiler, H. A., Yuen, C. K. & Seshia, M. M. Growth and bone mineralization of young adults weighing less than 1500 g at birth. Early Hum. Dev. 67, 101–112. https://doi.org/10.1016/s0378-3782(02)00003-8 (2002).
Pocock, N. A. et al. Genetic determinants of bone mass in adults. A twin study. J. Clin. Investig. 80, 706–710. https://doi.org/10.1172/jci113125 (1987).
Flicker, L. et al. Bone density determinants in elderly women: A twin study. J. Bone Miner. Res. 10, 1607–1613. https://doi.org/10.1002/jbmr.5650101102 (1995).
Videman, T. et al. Heritability of BMD of femoral neck and lumbar spine: A multivariate twin study of Finnish men. J. Bone Miner. Res. 22, 1455–1462. https://doi.org/10.1359/jbmr.070606 (2007).
Tønnesen, R., Schwarz, P., Hovind, P. H. & Jensen, L. T. Physical exercise associated with improved BMD independently of sex and vitamin D levels in young adults. Eur. J. Appl. Physiol. 116, 1297–1304. https://doi.org/10.1007/s00421-016-3383-1 (2016).
Watts, N. B., Nolan, J. C., Brennan, J. J. & Yang, H.-M., Group, E. S. O. S. Esterified estrogen therapy in postmenopausal women. Relationships of bone marker changes and plasma estradiol to BMD changes: A two-year study. Menopause 7(6), 375–382. https://doi.org/10.1097/00042192-200011000-00002 (2000).
Hirsch, C. ACP Journal Club Review: Dietary or supplemental calcium increase BMD by ≤ 1.8% in persons > 50 years of age. Ann. Intern. Med. 164, Jc5. https://doi.org/10.7326/acpjc-2016-164-2-005 (2016).
Gómez-Ambrosi, J., Rodríguez, A., Catalán, V. & Frühbeck, G. The bone-adipose axis in obesity and weight loss. Obes. Surg. 18, 1134–1143. https://doi.org/10.1007/s11695-008-9548-1 (2008).
Gregoire, F. M., Smas, C. M. & Sul, H. S. Understanding adipocyte differentiation. Physiol. Rev. 78, 783–809. https://doi.org/10.1152/physrev.1998.78.3.783 (1998).
Akune, T. et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 113, 846–855. https://doi.org/10.1172/jci19900 (2004).
Halade, G. V., El Jamali, A., Williams, P. J., Fajardo, R. J. & Fernandes, G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp. Gerontol. 46, 43–52. https://doi.org/10.1016/j.exger.2010.09.014 (2011).
Iwaniec, U. T. et al. Body mass influences cortical bone mass independent of leptin signaling. Bone 44, 404–412. https://doi.org/10.1016/j.bone.2008.10.058 (2009).
Halade, G. V., Rahman, M. M., Williams, P. J. & Fernandes, G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J. Nutr. Biochem. 21, 1162–1169. https://doi.org/10.1016/j.jnutbio.2009.10.002 (2010).
Abrahamsen, B., Bonnevie-Nielsen, V., Ebbesen, E. N., Gram, J. & Beck-Nielsen, H. Cytokines and bone loss in a 5-year longitudinal study–hormone replacement therapy suppresses serum soluble interleukin-6 receptor and increases interleukin-1-receptor antagonist: The Danish Osteoporosis Prevention Study. J. Bone Miner. Res. 15, 1545–1554. https://doi.org/10.1359/jbmr.2000.15.8.1545 (2000).
Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 142, 5050–5055. https://doi.org/10.1210/endo.142.12.8536 (2001).
Dolan, E., Swinton, P. A., Sale, C., Healy, A. & O’Reilly, J. Influence of adipose tissue mass on bone mass in an overweight or obese population: Systematic review and meta-analysis. Nutr. Rev. 75, 858–870. https://doi.org/10.1093/nutrit/nux046 (2017).
Cipriani, C. et al. The interplay between bone and glucose metabolism. Front. Endocrinol. (Lausanne) 11, 122. https://doi.org/10.3389/fendo.2020.00122 (2020).
Kim, M. S. et al. Association between adiposity and cardiovascular outcomes: An umbrella review and meta-analysis of observational and Mendelian randomization studies. Eur. Heart J. 42, 3388–3403. https://doi.org/10.1093/eurheartj/ehab454 (2021).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766. https://doi.org/10.1016/j.metabol.2021.154766 (2021).
Scheen, A. J. & Van Gaal, L. F. Combating the dual burden: Therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2, 911–922. https://doi.org/10.1016/s2213-8587(14)70004-x (2014).
Taylor, R. Type 2 diabetes: Etiology and reversibility. Diabetes Care 36, 1047–1055. https://doi.org/10.2337/dc12-1805 (2013).
Cortés, V. A., Barrera, F. & Nervi, F. Pathophysiological connections between gallstone disease, insulin resistance, and obesity. Obes. Rev. 21, e12983. https://doi.org/10.1111/obr.12983 (2020).
Nervi, F. et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J. Hepatol. 45, 299–305. https://doi.org/10.1016/j.jhep.2006.01.026 (2006).
Fabbrini, E., Sullivan, S. & Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 51, 679–689. https://doi.org/10.1002/hep.23280 (2010).
Marušić, M., Paić, M., Knobloch, M. & Liberati Pršo, A. M. NAFLD, insulin resistance, and diabetes mellitus type 2. Can. J. Gastroenterol. Hepatol. 2021, 6613827. https://doi.org/10.1155/2021/6613827 (2021).
Chiefari, E. et al. Insulin resistance and cancer: In search for a causal link. Int. J. Mol. Sci. https://doi.org/10.3390/ijms222011137 (2021).
Iyengar, N. M., Gucalp, A., Dannenberg, A. J. & Hudis, C. A. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J. Clin. Oncol. 34, 4270–4276. https://doi.org/10.1200/jco.2016.67.4283 (2016).
Majumder, K., Gupta, A., Arora, N., Singh, P. P. & Singh, S. Premorbid obesity and mortality in patients with pancreatic cancer: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 14, 355–368.e; quiz e332. https://doi.org/10.1016/j.cgh.2015.09.036 (2016).
Farooki, A. & Schneider, S. H. Insulin resistance and cancer-related mortality. J. Clin. Oncol. 25, 1628–1629; author reply 1629–1630, https://doi.org/10.1200/jco.2007.10.9637 (2007).
Raygor, V. et al. Impact of race/ethnicity on insulin resistance and hypertriglyceridaemia. Diabetes Vasc. Dis. Res. 16, 153–159. https://doi.org/10.1177/1479164118813890 (2019).
Zhan, H., Liu, X., Piao, S., Rong, X. & Guo, J. Association between triglyceride-glucose index and bone mineral density in US adults: A cross sectional study. J. Orthop. Surg. Res. 18, 810. https://doi.org/10.1186/s13018-023-04275-6 (2023).
Brussaard, H. E., Gevers Leuven, J. A., Frölich, M., Kluft, C. & Krans, H. M. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia 40, 843–849. https://doi.org/10.1007/s001250050758 (1997).
Brown, L. M. & Clegg, D. J. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 122, 65–73. https://doi.org/10.1016/j.jsbmb.2009.12.005 (2010).
Brown, L. M., Gent, L., Davis, K. & Clegg, D. J. Metabolic impact of sex hormones on obesity. Brain Res. 1350, 77–85. https://doi.org/10.1016/j.brainres.2010.04.056 (2010).
Meyer, M. R., Clegg, D. J., Prossnitz, E. R. & Barton, M. Obesity, insulin resistance and diabetes: Sex differences and role of oestrogen receptors. Acta Physiol. (Oxf.) 203, 259–269. https://doi.org/10.1111/j.1748-1716.2010.02237.x (2011).
Funding
This work was supported by the Elderly Health Research Project of Jiangsu Commission of Health (LKZ2022008), the Natural Science Foundation of Nanjing University Of Chinese Medicine (XZR2021060), and the Foundation of The Second Affiliated Hospital of Nanjing University of Chinese Medicine (SEZ202003).
Author information
Authors and Affiliations
Contributions
N.T. collected data. N.T., S.C., H.W.H., and J.J. organized the study and performed the statistical analysis. Z.W.L. drafted the manuscript, to which all authors contributed, and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, N., Chen, S., Han, H. et al. Association between triglyceride glucose index and total bone mineral density: a cross-sectional study from NHANES 2011–2018. Sci Rep 14, 4208 (2024). https://doi.org/10.1038/s41598-024-54192-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54192-9
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.