Abstract
Sleep disturbances like poor and insufficient sleep are common among medical students in the Middle East and North Africa (MENA) countries; however, the extent of medically defined sleep disorders (SDs) remains unclear. This meta-analysis determines SD prevalence and identifies associated factors among medical students in the MENA. PubMed, Web of Science, Google Scholar, and reference lists of included studies were searched (latest search: June 2022). Meta-analyses included 22 studies and were performed using random-effect models. Included studies used self-reported screening tools for assessing SDs and then estimated the proportion of participants at high risk of developing a SD. Central disorders of hypersomnolence were the most prevalent SD [prevalencepooled range: 30.9% (Jordan) to 62.5% (Saudi Arabia)], followed by insomnia disorders [prevalencepooled range: 30.4% (Jordan) to 59.1% (Morocco)], circadian rhythm sleep–wake disorders [prevalencepooled range: 13.5% (Jordan) to 22.4% (Saudi Arabia)], sleep-related breathing disorders [prevalencepooled range: 12.2% (Jordan) to 22.5% (Pakistan)], sleep-related movement disorders [prevalencepooled range: 5.9% (Egypt) to 30.6% (Saudi Arabia)], and parasomnias [prevalencepooled range: 5.6% (Jordan) to 17.4% (Saudi Arabia)]. Female sex, studying in the latter academic years, having anxiety, excessive internet use, and poor academic performance were significantly associated with SDs. SDs are prevalent among MENA medical students. Implementing student-centered interventions targeting high risk groups in medical schools should be considered to improve students’ health and wellbeing.
Similar content being viewed by others
Introduction
Medically defined sleep disorders (SDs) include insomnia disorders, sleep-related breathing disorders, central disorders of hypersomnolence, circadian rhythm sleep–wake disorders, sleep-related movement disorders, and parasomnias1,2,3,4. SDs increased in the last decade globally5,6 but remain under-diagnosed and under-treated7. SDs affect not only physical and mental functioning and work productivity but are also associated with various psychiatric6,8,9,10 and physical11,12,13,14,15 illnesses, workplace injuries7, and sudden death16. Consequently, SDs’ substantially impact the society7.
Insufficient sleep affects up to one third of the global population17 and has been declared a ‘public health epidemic’18.Worldwide, medical students appear to be more affected by sleep disturbances (e.g. poor sleep quality, insufficient sleep duration, irregular sleep, and insomnia symptoms) than non-medical students6,9,19,20,21,22 or the general population9,19,23,24,25, owing to the large academic load and assigned clinical duties6,26. Hence, we expect a high SD prevalence in this population.
Several recently published systematic reviews (SRs) are focused on sleep disturbances rather than SDs among medical students16,20,22,23,24,27,28,29,30,31 and university students19,23,32,33,34,35,36 Also, the available SRs with data on insomnia were conducted among non-medical university students9,36,37,38 with a focus on one specific country37, and the COVID-19 pandemic period36,37,38. Several primary studies on SDs in the Middle East and North Africa (MENA) medical students have been recently published. Notably, the countries of the MENA region have the highest total number of medical schools in their respective continents (Asia and Africa)39.
To our knowledge, no SR and meta-analysis synthesizing the epidemiology of SDs in MENA medical students has been conducted. The aim of this SR and meta-analysis was to quantify SD prevalence and synthesize the factors associated with SDs among medical students in the MENA countries before and during the COVID-19 pandemic.
Methods
The SR methodology was developed based on the Cochrane Handbook for Systematic Review of Interventions and followed the AMSTAR 2 checklist. The manuscript follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Table S1), the PRISMA checklist for search strategy (Supplementary Table S2)40,41 and Meta-analyses of Observational Studies in Epidemiology (MOOSE) guidelines42. The research protocol was developed a priori and registered prospectively on Open Science Framework (https://doi.org/https://doi.org/10.17605/OSF.IO/2WZJ4).
Literature search strategy
PubMed, Web of Science, and Google Scholar were searched by two independent reviewers for grey and non-grey literature. The database selection and search strategy were developed in consultation with a specialized librarian. The latest search was conducted on June 25, 2022. The search included a combination of controlled vocabulary terms and text words related to SDs and medical or university students. The search strategy is described in Supplementary Box S1. Two independent reviewers also manually searched the reference lists of included primary studies and relevant reviews, as well as the internal literature database we developed, titled ‘Mental Health in University Students’.
Eligibility criteria
Primary outcomes
The primary outcome of interest was the prevalence of any SD. We included any SD listed in the International Classification of Sleep Disorders, third edition (ICSD-3): the most widely used classification system and a key reference for the diagnosis of SDs using ‘International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification1,2,3,4.’ SDs reported in the primary studies were categorized, as per the ICSD-3 recommendations, into seven categories that include: (1) insomnia disorders, (2) sleep-related breathing disorders, (3) central disorders of hypersomnolence, (4) circadian rhythm sleep–wake disorders (CRD), (5) parasomnias, (6) sleep-related movement disorders, and (7) other SDs (not fitting in the previous categories). Specific SDs included in each category as per ICSD-3 are presented in Table S3. SDs identified by clinical diagnosis, or any self-reported tools were included in our SR. Severity levels of SDs were included and categorized into mild, moderate, and severe as per the study and/or instrument definition. Cases of risk of SD were defined as those have abnormal scores (any level) according to the scoring system (cut-offs) recommended by the tool. If not reported, the prevalence of an SD was calculated based on crude data reported in the study. Studies with insufficient information to compute prevalence data of SDs, or those reporting only symptoms related to SDs, were excluded.
Secondary outcome
The secondary outcome of interest was the factors associated to a higher risk of SD. We synthesized any effect measure used to quantify a relationship between the factor and SDs reported in the included studies. Reported effect sizes included risk and mean differences, correlations, attributable proportion, risk ratios, relative risks, and odds ratios.
Population of interest
A study was eligible for inclusion if it included pre-medical or medical students enrolled in a medical school among the 20 MENA countries: Algeria, Bahrain, Djibouti, Egypt, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Sudan, Syria, Tunisia, the United Arab Emirates, and Yemen. The list of MENA countries in this study was based on that developed in a series of published SRs and meta-analyses characterizing the population health MENA43,44,45,46,47,48,49,50,51. Pre-medical and medical students mixed with other university students were included only if data specific to the population of interest was available. We excluded studies on medical science students (nursing, pharmacy, dentistry) unless specified as medical students studying for their Medical Degree (MD or MBBS).
Study design
Any observational study (e.g., cross-sectional study, case–control, or cohort) was included in the SR. Reviews, case reports, letters to editors, commentaries, and clinical trials were excluded.
Multi-stage screening
The Rayyan software (Rayyan Systems, Inc, Cambridge, MA, USA, https://www.rayyan.ai/) was used for duplicate removal and multi-stage screening. Two independent reviewers conducted the title and abstract screening, full-text screening, and data extraction. Discrepancies were resolved with a third reviewer to achieve consensus on study inclusion and data extraction.
For inclusion in the systematic review (SR), a study should correspond to the PICOTS framework criteria52—population, outcome, study design, time of the study, and setting (control and intervention criteria were not applicable since they were not relevant to the SR question). We included studies reported in English, Arabic, French, Spanish and/or Urdu—languages spoken by the authors of this SR.
Data extraction
Data was extracted from the included primary studies for the following variables: (1) study characteristics (e.g. study design, sampling method, data collection period, sample size, (2) setting (3) medical student characteristics including age, sex, year of study, and socio-economic status, (4) prevalence of SDs, including the instrument used to diagnose an SD and its related characteristics, (6) factors for which a difference and/or an association with the risk of having an SD was assessed.
Quality assessment
The risk of bias (RoB) and methodological quality of included studies were appraised independently by two reviewers using a validated RoB tool for prevalence studies53. Briefly, the RoB tool uses an items scale to assess: (1) the external validity of the study, based on selection and nonresponse biases, and (2) the internal validity of the study, based on measurement bias and bias related to the analysis. No summary quality score was computed as per COSMOS-E guidance, which provides guidance on conducting SRs of observational studies of aetiology54. Each included study was assigned a low or high ROB for each assessment item. A synthesis of studies’ quality was based on a summary of low and high risk of bias assessment of each quality domain.
Reporting bias due to missing data was discussed. Discussion on the validity and reliability of our estimates was also performed to assess the confidence in the body of evidence presented in the SR. The certainty assessment method was based on the Grading of Recommendations, The Assessment, Development, and Evaluation (GRADE) approach. The GRADE approach used in our study considers the RoB and reporting biases in a body of evidence, precision of the meta-analysis effect estimates, the consistency of the primary study results, and how directly the body of evidence answers the research question55.
Synthesis
A meta-analysis of the prevalence of SD categories (proportion of participants at a high risk of any ICDS category of SD) (Supplementary Table S3) was conducted using the DerSimonian-Laird random-effects model56. Random effects model with the logit transformation of the proportion was used to conduct the meta-analyses pooling prevalence measures and their 95% confidence intervals (95% CI). The Freeman-Tukey double arcsine transformation was used in the analyses involving the pooling of proportions, using the command sm = “PFT” in R57. Clopper-Pearson confidence intervals were computed for individual prevalence measures. The minimum study sample size required for a study to be included in the meta-analysis was 2558.
Subgroup meta-analyses were conducted by sex (males and females), academic training period (preclinical, clinical, and late clinical), and MENA country. For each category of SDs, prevalence data were pooled by SD severity level (mild, moderate, and severe). Prevalence data on mixed disorder levels, mixed sex, and/or mixed training periods were pooled in a separate group. If not reported, the prevalence of an SD was calculated based on crude data reported in the study. Sensitivity analyses were conducted to assess the impact of SD prevalence during the COVID-19 pandemic lockdown on the pooled estimates when applicable. Factors associated with SDs among MENA medical students (secondary outcome) were synthesized as reported in the included studies.
SD prevalence measures stratified by sex, disorder severity level, SDs category, and academic period were prioritized for inclusion in the meta-analysis rather than the overall measures on the entire study population or any SD. Prevalence measures reported for each academic year were combined and classified into preclinical, clinical, and late clinical training periods, according to the medical school curriculum followed by the country. Multiple SDs under the same ICDS category reporting on the same study population were merged prior to the inclusion in the meta-analysis to ensure independency of observations.
The heterogeneity between studies was assessed using the I2 statistic59 and Cochran’s Q between-subgroups statistic60. Heterogeneity between studies was considered as substantial when I2 > 50%61. The Cochran’s Q between-subgroups statistic was used to test for differences between prevalence estimates across subgroups60, and statistical significance was considered at p value ≤ 0.05. Univariate random-effects meta-regression was used to estimate odds ratios (ORs) and corresponding 95% confidence interval (CI)s measuring the magnitude of relative changes in the pooled SDs prevalence according to study-level factors62.
To further explore heterogeneity between studies, univariate random-effects meta-regression was conducted to evaluate potential associations between SD prevalence and measurable study-level factors, including sampling method, sample size, instrument, and study response rate. Meta regression was used to estimate odds ratios (OR) and corresponding 95% CIs to measure the magnitude of relative changes in the pooled SD prevalence according to the study-level factors62.
For the meta regression analyses, all SDs were considered grouped to increase the statistical power.
Both meta-analyses and meta-regressions analyses were conducted using RStudio software (version 2022.07.1 Build 554).
Methodological quality of the included studies was appraised using the risk of bias (RoB) tool for prevalence studies53. Reporting bias due to missing data was also discussed. Discussion on the validity and reliability of our estimates was also performed to assess the confidence in the body of evidence presented in the SR.
Publication bias was assessed using the Doi plot, a method that allows better visual representation of asymmetry as compared to the conventional funnel plot63,64. In the Doi plot, the effect estimate (X-axis) is plotted against the percentiles converted to a normal quantile (Z-score) for each study (Y-axis). The prevalence of SDs was transformed to the log odds scale for better statistical properties for the meta-analysis64. We also estimated LFK index to detect and quantify symmetry of study effects in the Doi plot. A LFK index of zero indicates a complete symmetry. The closer the value of the LFK index to zero, the more symmetrical the Doi plot would be. LFK index, values beyond − 1 and + 1were deemed consistent with asymmetry and potential publication bias63. Alternatively, for pooled SDs prevalence with identified publication bias, the 95% prediction interval was used to describe the distribution of true outcome measures around the pooled prevalence65,66
Results
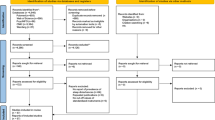
A total of 2046 records were identified through the literature search conducted in PubMed and Web of Science, and 1349 records were identified through Google Scholar, citation hand searching of relevant studies, and other research databases. Twenty-two primary studies were included in the SR and the meta-analysis (Fig. 1). The characteristics of the included studies are described in Table 1. Studies excluded at the full-text screening stage are listed in Supplementary Box S2.
PRISMA flow chart 2020. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71. http://www.prisma-statement.org/.
Characteristics of the included studies
Out of the included studies, 21 were cross-sectional (95%, 21/22) and one study used a longitudinal design. They were conducted in Egypt, Jordan, Morocco, Pakistan, and Saudi Arabia, between 2013 and 2019, and published between 2015 and 2021. The MENA medical students’ mean age ± standard deviation ranged between 20.5 ± 1.67 and 23.1 ± 3.8 years. The total population size varied between 122 and 1,041 medical students (Table 1).
Pooled estimates of sleep disorders
Insomnia disorders
A total of 54 prevalence measures classified under insomnia disorders, including sleep state misperception (paradoxical insomnia), were retrieved from 18 studies conducted in Jordan, Morocco, Pakistan, and Saudi Arabia (Table 1). Following data merging and classification, 30 prevalence measures on any type of insomnia disorder were included in the meta-analysis. Most of the included data (24/30 prevalence measures) were computed from any severity level of insomnia.
The pooled prevalence of insomnia disorders ranged between 30.4% in Jordan and 59.1% in Morocco. A total of three studies67,68,69 were conducted during the COVID-19 pandemic lockdown in Saudi Arabia67,68 and Morocco69. In Saudi Arabia, pooled prevalence computed with and without data during the COVID-19 pandemic lockdown were similar, at 45.9%; 95% CI: 30.2–62.1 and 50.0%; 95% CI: 29.4–70.6 respectively. The pooled prevalence of insomnia disorders, in Saudi Arabia and Pakistan, following the exclusion of the study not reporting the used tool was 42.1% 95% CI: 27.1–57.9 and 36% 95% CI: 23.1–50.0, respectively. All prevalence data identified for Morocco was measured during the COVID-19 pandemic lockdown.
No statistically significant difference in the prevalence of insomnia disorders was identified between MENA countries. In these countries, mild-level of insomnia was reported by 38.4% of medical students, followed by moderate- (22.2%) and severe-levels of insomnia (11.6%) (Table 2). Insomnia disorders were significantly more prevalent among female medical students than among male students (49.9% vs. 26.1%; p value = 0.0027). Although the highest pooled prevalence of insomnia disorders was observed during the late clinical training period (year 7 or more), no statistically significant difference was found between the academic training periods.
Reported factors significantly associated with an increased odds of having insomnia disorders were being a female student69, clinical or late clinical years69, and use of internet for more than 12 h daily70 (Table 3). The reported findings also highlighted the significant negative impact of insomnia70,71,72,73 and sleep state misperception disorder71 (known also as paradoxical insomnia74) on academic performance. Reported data suggests that the impact of anxiety on the risk of insomnia depends on the anxiety severity level70,75.
Sleep-related breathing disorders
A total of 7 prevalence measures classified under sleep-related breathing disorders, including obstructive sleep apnoea (OSA) disorders, were retrieved from 5 studies conducted in Jordan, Pakistan, and Saudi Arabia (Table 1). Following data merging and classification, 6 prevalence measures on any type of sleep-related breathing disorders were included in the meta-analysis.
The pooled prevalence of sleep-related breathing disorders ranged between 12.2% in Jordan and 22.5% in Pakistan (Table 2). Significant differences in the pooled prevalence of these disorders were identified between Jordan, Pakistan, and Saudi Arabia. Only one study67 from Saudi Arabia reported a prevalence of Obstructive Sleep Apnea (OSA) during the COVID-19 pandemic lockdown. Statistically significant difference in the pooled prevalence of sleep-related breathing disorders was also found between sexes (p value = 0.002); however, this observation was based on a limited number of data points. Reported factors significantly associated with an increased odds of having OSA were being a male student70,71, increased age70, progression through the academic years76, use of the internet for more than 4–8 h daily70, and mild and extremely severe anxiety70 (Table 3). Moderate stress was significantly associated with lower odds of having OSA when compared with no stress70.
Central disorders of hypersomnolence
A total of 8 prevalence measures classified under central disorders of hypersomnolence were retrieved from 3 studies, including hypersomnia and narcolepsy disorders, conducted in Jordan and Saudi Arabia (Table 1).
The pooled prevalence of central disorders of hypersomnolence ranged between 30.9% in Jordan and 62.5% in Saudi Arabia (Table 2). Significant differences in the prevalence of central disorders of hypersomnolence were found between these two countries. Only one study reported prevalence data of central disorders of hypersomnolence by sex71, and another one67 during the COVID-19 pandemic lockdown in Saudi Arabia. Reported risk of having narcolepsy was significantly associated with an increased odd of having poor academic performance71 (p value = 0.045) (Table 3). No significant differences were reported for the prevalence of narcolepsy and hypersomnia between males and females71.
Circadian rhythm sleep–wake disorders (CRD)
A total of 5 prevalence measures classified under CRD disorders were retrieved from 3 studies conducted in Jordan and Saudi Arabia (Table 1). Following data merging and classification, 5 prevalence measures on any type of CRD disorder were included in the meta-analysis.
A significant difference in the pooled prevalence of CRD was found between the two countries with available data: Jordan (13.5%) and Saudi Arabia (22.4%) (Table 2). Only one study reported prevalence data of CRD by sex71. and another one67 during the COVID-19 pandemic lockdown in Saudi Arabia. Reported risk of having CRD was associated with an increased odds of having poor academic performance in two studies71,72 (Table 3). No significant difference was reported in the prevalence of CRD between males and females (p value = 0.162)71.
Parasomnias
A total of 8 prevalence measures classified under parasomnias disorders, including nightmares and sleep walking, were retrieved from 2 studies conducted in Jordan and Saudi Arabia (Table 1). Following data merging and classification, 3 prevalence measures on any type of parasomnias disorder were included in the meta-analysis.
The pooled prevalence of parasomnia disorders ranged between 5.6% in Jordan and 17.4% in Saudi Arabia (Table 2). A significant difference in the prevalence of parasomnia disorders was found between Jordan and Saudi Arabia. Only one study reported prevalence data of parasomnias segregated by sex71, and another one67 during the COVID-19 pandemic lockdown in Saudi Arabia.
No significant difference was reported in the prevalence of sleep walking (p-value = 0.090) between males and females71 (Table 3). The reported factor associated with an increased odds of having nightmares was being a female medical student (p value = 0.022)71.
Sleep-related movement disorders
A total of 14 prevalence measures classified under sleep-related movement disorders were retrieved from 6 studies conducted in Jordan, Egypt, Pakistan, and Saudi Arabia (Table 1).
Following data merging and classification, 9 prevalence measures on any type of sleep-related movement disorder were included in the meta-analysis.
The pooled prevalence of sleep-related movement disorders ranged between 5.9% in Egypt and 30.6% in Saudi Arabia (Table 2). Only one study67 reported a prevalence of restless leg syndrome (RLS) during the COVID-19 pandemic lockdown in Saudi Arabia. Significant differences in the pooled prevalence of sleep-related movement disorders were found between Egypt, Jordan, Saudi Arabia, and Pakistan. 5.1% of medical students had a moderate-level of sleep-related movement disorders and 3.0% had a mild-level. No statistically significant difference in sleep-related movement disorders was found between males and females. The reported factor associated with decreased odds of having periodic limb movement disorder/RLS was being a male medical student71 (Table 3).
Undefined sleep disorder
Only one study reported a prevalence measure of any SD (without type specific SD prevalence), which was 9.5% among a male and female medical student during mixed training periods in Saudi Arabia (Table 1).
Heterogeneity
Between-study heterogeneity was relatively high, and differences between prevalence estimates across subgroups were significant between sex groups, academic training periods, and countries for the majority of SDs (Table 2). Meta-regression analyses revealed that prevalence measures retrieved from studies that did not report the SD measurement tool (n = 2) were significantly higher compared with studies that used a validated tool (n = 47). Although not statistically significant, prevalence measures assessed using non-validated tools seem to provide lower prevalence measures as compared to those using validated tools (Table 4). Although not statistically significant, SD prevalence measures based on ‘non-probability sampling’, ‘sampling method not reported’, ‘sample size £ 100’, and ‘response rates3 75%’ were associated with higher SD prevalence measures when compared to ‘probability sampling’, ‘sampling method reported', ‘sample size > 100’, and ‘response rates < 75%’, respectively.
Study-level quality assessment
Overall, most included primary studies properly reported the information required to allow quality assessment and were of good methodological quality (low RoB) (Supplementary Table S4). Most of the included studies (86%, 19/22) had a low likelihood of nonresponse bias, collected data directly from the targeted population (91%, 20/22), used an acceptable case definition (82%, 18/22), and used a validated instrument to measure SD (77%, 17/22). All included studies used the same mode of data collection for comparison groups. However, only 32% (7/22) of the included studies used a random-sampling method. A total of 20 studies out of 22 (91%) had a high RoB related to the representativeness of the national and target populations. Most of the included studies (73%, 16/ 22) used an appropriate tool to identify cases with a high risk of SDs. Only one study had a high RoB with ‘including appropriate numerators and denominators for the studied SDs’. Overall, the included studies had good internal validity and moderate external validity that could limit the generalizability of the results.
Reporting bias and certainty assessment
Our synthesis was likely impacted by the limited number of primary studies retrieved in some specific SDs categories and MENA countries, which may have consequently limited the representativeness of our pooled prevalence estimates. Most of the primary studies had a low risk of non-response bias, however, they had a high risk of selection bias, which could impact their external validity. Additionally, pooled SD prevalence estimates were likely robust because of the good studies’ internal validity (low risk of measurement and analysis biases); however, identified heterogeneity between studies has likely impacted the precision of the pooled prevalence estimates.
The visual inspection of the Doi plots indicates some positive asymmetry (with studies spread out towards the right limb), for all SDs except central disorders of hypersomnolence (Fig. 2). The LFK index was consistent with no asymmetry of the Doi plot for central disorders of hypersomnolence and therefore no evidence of a publication bias. The LFK index was consistent with a minor positive asymmetry of the Doi plots (minor publication bias) for (1) circadian rhythm sleep–wake disorders, (2) parasomnias, and (3) sleep-related movement disorders (Fig. 2). LFK index was consistent with a positive major asymmetry of the Doi plot for insomnias disorders and sleep-related breathing disorders. Hence, there may be a major publication bias related to studies with a higher prevalence more likely to be published. The prediction 95% intervals for the prevalence of on insomnias disorders and sleep-related breathing disorders were [6.67%-87.59%] and [5.3%-40.40%], respectively.
Based on the above, the certainty of available evidence was rated as moderate.
Discussion
The pooled prevalence of SD categories among MENA medical students was the highest for central disorders of hypersomnolence, insomnia, and CRDs. Statistically significant differences in the pooled prevalence were identified between Egypt, Jordan, Morocco, Pakistan, and Saudi Arabia. While insomnia disorders were generally studied in the region, limited data were found for central disorders of hypersomnolence, CRDs, sleep-related breathing disorders, sleep-related movement disorders, and parasomnias disorders preventing conclusions on their extent and positioning. Limited data on the prevalence of SDs among medical students were available globally77, and a wide range of SD prevalence measures was reported among university students globally9,19,25,78. Additionally, only two studies assessed the impact of the COVID-19 pandemic lockdown on the prevalence insomnias disorders.
Insomnia disorder prevalence among the MENA medical students, ranging between 30.4% and 59.1%, was higher than medical students in China (27.8%)79 and lower than medical students in Georgia (70.11%)80. Insomnia prevalence in MENA medical students was comparable to the global insomnia prevalence reported among healthcare workers (37–38%)81,82. Medical students typically cope with large academic loads and clinical duties that include overnight on-call shifts, which could interfere with their sleep6,26. However, medical students may also overreport symptoms compared to non-medical university students or the general population, because of their medical knowledge and their perceived value on health83, which could contribute to the higher self-reported SD prevalence.
Globally, a wide range of insomnia prevalence measures have been reported in university students9,19,36,37,38,84 and the adult general population5,37,38,81,85,86,87,88,89 preventing comparisons. The differences in the diagnostic criteria used to assess insomnia could explain some of the observed differences in the insomnia prevalence between studies.
The variability in insomnia and other SD prevalence measures in the literature could also be explained by the use of sleep disturbance tools, such as Pittsburgh Sleep Quality Index, for assessing the risk of SDs9,19,25,35. Although sleep disturbance tools are good predictors of the risk of SDs90,91, only a small proportion of students with sleep complaints will meet SD clinical interview and diagnostic criteria78. For instance, 30–40% of the population with sleep disturbances will meet the clinical diagnostic scores for insomnia35, as symptoms related to middle-of-night awakenings or daytime impairments are not captured by sleep quality tools and are required to fulfill the criteria for insomnia disorders92,93.
Our meta-analysis demonstrated that insomnia disorders and sleep-related breathing disorders were significantly more prevalent in female medical students as compared to male students. Sex-related differences were not identified for other SDs. The synthesis of reported factors associated with SDs suggested that the female sex was associated with the occurrence of insomnia disorders, nightmares, periodic limb movement disorder, and RLS; and the male sex was associated with the occurrence of OSA. The higher vulnerability of women to insomnia5,94,95,96,97 and RLS5,98,99 as compared to men is consistent with previous findings in the general population, suggesting the need to target female students when planning interventions.
Differences in SD prevalence by academic training period or disorder severity level assessed in our meta-analyses could not be established given the limited data; however, included studies suggested that late academic years (clinical and late clinical years) were associated with insomnia and OSA. Medical education in general and the clinical years specifically have been identified as causative factors for poor sleep quality worldwide6,100. Our findings highlighted the significant negative impact of insomnia disorders, sleep state misperception, narcolepsy, and CRD disorders on academic performance in medical school. Low academic performance among medical and university students has been correlated with poor sleep quality20,101. Additional studies are required to assess the impact of SDs on the academic performance of MENA medical students. Interventions involving sleep-education24,102, monitoring cognitive behavior24,102, and mindfulness relaxation24,102 during clinical training years can help medical students to improve their sleep. Student well-being services can support students in managing disturbed sleep and its consequences. Additionally, incorporating sleep education into the curriculum has been recommended to address medical students’ poor knowledge and misconceptions about sleep practices and disorders6,103,104 and prevent misdiagnosis and maltreatment of SDs105.
Our synthesis of reported factors associated with SDs suggested that excessive daily internet use was associated with the occurrence of insomnia disorders and OSA. A significant increase in sleep problems and a reduction in sleep duration were found among individuals addicted to the internet106, suggesting a potential association with SDs. Additionally, our synthesis suggested that the negative impact of anxiety on the risk of insomnia disorders and OSA depends on the severity level of anxiety. Both insomnia disorders77,94,107,108,109,110,111,112 and OSA113,114,115 are associated with an increased risk of depression and anxiety in adults and adolescents, and it seems that this relationship is bi-directional116. As anxiety and depressive symptoms are relatively common in medical students117,118, they probably contribute to the increased prevalence of insomnia disorders and OSA in this population. Consequently, interventions designed to prevent or address SDs are also likely to positively impact mental health disorders holistically.
To our knowledge, this is the first SR and meta-analysis focusing on SDs rather than sleep disturbances in MENA medical students. The majority of included studies were of good methodological quality, which reinforces the validity of our findings. A minor impact of the two studies not reporting the tool for measuring SD prevalence on the pooled prevalence is expected given that 85% of the included studies used a validated tool for assessing SDs. There was no evidence for the impact of other study characteristics on the SD prevalence (Table 4). All included studies assessed SDs using self-reported questionnaires, which could be subject to recall bias. Also, all included studies have used screening tools for assessing SDs. Therefore, the proportion of medical students meeting the clinical diagnosis criteria is expected to be lower than the estimated proportion of students with SDs considering screening criteria only78. While an objective clinical diagnosis of SDs is generally more reliable, self-reported screening questionnaires are utilized not only in research but also in clinical practice because of their administration efficiency and low cost119. Most studies included in this review were comprised of rather small sample sizes or limited generalizability. Studies with larger samples and geographical coverage are required to confirm our results. Our findings may not be generalizable to all MENA countries given the limited number of countries with available data. Although a publication bias related to the available data on insomnias disorders and sleep-related breathing disorders has been detected, we are confident that the prevalence of these SDs are within the prediction interval. Despite these limitations and the existence of heterogeneity, several subgroup analyses of SD prevalence assessed using validated tools were conducted. Therefore, these limitations do not affect the interpretation of our findings.
As most of the included studies were cross-sectional, temporal sequencing of SD development and potential associated factors cannot be established, which limits conclusions on potential causal associations. However, this synthesis can be used to generate hypotheses and support future study design to assess the risk factors and consequences of SDs in medical students.
Conclusion
SDs with the highest prevalence among medical students in MENA were central disorders of hypersomnolence, insomnia disorders, and CRD disorders. Female sex, latter academic years, anxiety, and excessive internet use were associated with the occurrence of several SDs. SDs negatively impact students’ academic performance. Implementing public health and clinical interventions in medical school settings, particularly targeting high-risk groups (i.e., female students and students in late academic years), should be given serious consideration to help improve students’ overall health, wellbeing, and quality of life.
Data availability
The original contributions presented in the study are included in the article and online supplement. Further inquiries can be directed to the corresponding author.
References
Sateia, M. J. International classification of sleep disorders-third edition: highlights and modifications. Chest 146(5), 1387–1394 (2014).
Hypersomnia Foundation. International classification of sleep disorders (ICSD) 2022 [Available from: https://www.hypersomniafoundation.org/glossary/international-classification-of-sleep-disorders/#:~:text=The%20ICSD%2D3%20groups%20sleep,)%20sleep%2Drelated%20movement%20disorders
American Academy of Sleep Medicine. The International Classification of Sleep Disorders – Third Edition (ICSD-3) 3rd edn. (American Academy of Sleep Medicine, 2014).
American Sleep Association. Sleep disorders – ICD-10 codes and names (2015).
Karna B, Sankari A, Tatikonda G. Sleep Disorder. [Updated 2022 Nov 26]. 2022. In: StatPearls. Treasure Island (FL): Karna B, Sankari A, Tatikonda G. Sleep Disorder. [Updated 2022 Jul 19], in StatPearls [Internet]. Treasure Island (FL) (StatPearls Publishing, 2022). Available from: https://www.ncbi.nlm.nih.gov/books/NBK560720/?report=classic. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560720/?report=classic
Azad, M. C. et al. Sleep disturbances among medical students: A global perspective. J. Clin. Sleep Med. 11(01), 69–74 (2015).
Streatfeild, J., Smith, J., Mansfield, D., Pezzullo, L. & Hillman, D. The social and economic cost of sleep disorders. Sleep 44(11), 132 (2021).
Carney, C. E., Moss, T. G., Lachowski, A. M. & Atwood, M. E. Understanding mental and physical fatigue complaints in those with depression and insomnia. Behav. Sleep Med. 12(4), 272–289 (2014).
Jiang, X. et al. A systematic review of studies on the prevalence of insomnia in university students. Public Health 129(12), 1579–1584 (2015).
Hanin, C. et al. Narcolepsy and psychosis: A systematic review. Acta Psychiatrica Scand. 144(1), 28–41 (2021).
Lo, J. C., Chong, P. L., Ganesan, S., Leong, R. L. & Chee, M. W. Sleep deprivation increases formation of false memory. J. Sleep Res. 25(6), 673–682 (2016).
Wang, C., Tan, J., Miao, Y. & Zhang, Q. Obstructive sleep apnea, prediabetes and progression of type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Investig. 13(8), 1396–1411 (2022).
Qie, R. et al. Obstructive sleep apnea and risk of type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of cohort studies. J. Diabetes 12(6), 455–464 (2020).
Zheng, Z. et al. Meta-analysis of relationship of sleep quality and duration with risk of diabetic retinopathy. Front. Endocrinol. (Lausanne) 13, 922886 (2022).
Partinen, M., Putkonen, P. T., Kaprio, J., Koskenvuo, M. & Hilakivi, I. Sleep disorders in relation to coronary heart disease. Acta Med. Scand. Suppl. 660(S660), 69–83 (1982).
Heilbrunn, E. S., Ssentongo, P., Chinchilli, V. M., Oh, J. & Ssentongo, A. E. Sudden death in individuals with obstructive sleep apnoea: A systematic review and meta-analysis. BMJ Open Respir. Res. 8(1), e000656 (2021).
Chattu, V. K. et al. Insufficient sleep syndrome: Is it time to classify it as a major noncommunicable disease?. Sleep Sci. 11(2), 56–64 (2018).
Liu, Y. et al. Prevalence of healthy sleep duration among adults-United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 65(6), 137–141 (2016).
Li, L. et al. Prevalence of sleep disturbances in Chinese university students: A comprehensive meta-analysis. J. Sleep Res. 27(3), e12648 (2018).
Seoane, H. A. et al. Sleep disruption in medicine students and its relationship with impaired academic performance: A systematic review and meta-analysis. Sleep Med. Rev. 53, 101333 (2020).
Khaksarian, M. et al. Sleep disturbances rate among medical and allied health professions students in Iran: Implications from a systematic review and meta-analysis of the literature. Int. J. Environ. Res. Public Health 17(3), 1011 (2020).
Rao, W. W. et al. Sleep quality in medical students: A comprehensive meta-analysis of observational studies. Sleep Breath 24(3), 1151–1165 (2020).
Russell, K. et al. Sleep problem, suicide and self-harm in university students: A systematic review. Sleep Med. Rev. 44, 58–69 (2019).
Gardani, M. et al. A systematic review and meta-analysis of poor sleep, insomnia symptoms and stress in undergraduate students. Sleep Med. Rev. 61, 101565 (2022).
Chowdhury, A. I., Ghosh, S., Hasan, M. F., Khandakar, K. A. S. & Azad, F. Prevalence of insomnia among university students in South Asian Region: A systematic review of studies. J. Prev. Med. Hyg. 61(4), E525–E529 (2021).
Shad, R., Thawani, R. & Goel, A. Burnout and sleep quality: A cross-sectional questionnaire-based study of medical and non-medical students in India. Cureus 7(10), e361 (2015).
Binjabr MA, Alalawi IS, Alzahrani RA, Albalawi OS, Hamzah RH, Ibrahim YS, et al. The worldwide prevalence of sleep problems among medical students by problem, country, and COVID-19 status: A systematic review, meta-analysis, and meta-regression of 109 studies involving 59427 participants. Curr. Sleep Med. Rep 1–19 (2023).
Jannathul, F. et al. Sleep disruption and its impact on academic performance in medical students: A systematic review. Univers. J. Public Health 11(1), 1–7 (2023).
Jahrami, H. et al. Prevalence of sleep problems among medical students: A systematic review and meta-analysis. J. Public Health 28(5), 605–622 (2020).
Jahrami, H. et al. Predictors of excessive daytime sleepiness in medical students: a meta-regression. Clocks Sleep 1(2), 209–219 (2019).
Al-Ajlouni, Y. A. et al. Effects of the COVID-19 pandemic on sleep health among Middle Eastern and North African (MENA) populations: A systematic review of the literature. BMJ Open 12(12), e066964 (2022).
Salehinejad, M. A., Azarkolah, A., Ghanavati, E. & Nitsche, M. A. Circadian disturbances, sleep difficulties and the COVID-19 pandemic. Sleep Med. 91, 246–252 (2022).
Chandler, L. et al. Improving university students’ mental health using multi-component and single-component sleep interventions: A systematic review and meta-analysis. Sleep Med. 100, 354–363 (2022).
Mulyadi, M., Tonapa, S. I., Luneto, S., Lin, W. T. & Lee, B. O. Prevalence of mental health problems and sleep disturbances in nursing students during the COVID-19 pandemic: A systematic review and meta-analysis. Nurse Educ. Pract. 57, 103228 (2021).
Jahrami, H. A. et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med. Rev. 62, 101591 (2022).
Deng, J. et al. The prevalence of depressive symptoms, anxiety symptoms and sleep disturbance in higher education students during the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatry Res. 301, 113863 (2021).
Zhang, S. X. et al. A Systematic review and meta-analysis of symptoms of anxiety, depression, and insomnia in Spain in the COVID-19 crisis. Int. J. Environ. Res. Public Health 19(2), 1018 (2022).
Zou, Q. et al. Prevalence of anxiety, depressive and insomnia symptoms among the different groups of people during COVID-19 pandemic: An overview of systematic reviews and meta-analyses. Front. Psychol. 13, 1024668 (2022).
Duvivier, R. J., Boulet, J. R., Opalek, A., van Zanten, M. & Norcini, J. Overview of the world’s medical schools: An update. Med. Educ. 48(9), 860–869 (2014).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 62(10), 1006–1012 (2009).
Rethlefsen, M. L. et al. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 10(1), 39 (2021).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15), 2008–2012 (2000).
Chaabna, K. et al. Gray literature in systematic reviews on population health in the Middle East and North Africa: Protocol of an overview of systematic reviews and evidence mapping. Syst. Rev. 7(1), 94 (2018).
Chaabane, S., Chaabna, K., Abraham, A., Mamtani, R. & Cheema, S. Physical activity and sedentary behaviour in the Middle East and North Africa: An overview of systematic reviews and meta-analysis. Sci. Rep. 10(1), 9363 (2020).
Chaabane, S., Chaabna, K., Doraiswamy, S., Mamtani, R. & Cheema, S. Barriers and facilitators associated with physical activity in the Middle East and North Africa region: A systematic overview. Int. J. Environ. Res. Public Health 18(4), 1647 (2021).
Doraiswamy, S. et al. Perinatal mental illness in the Middle East and North Africa region-a systematic overview. Int. J. Environ. Res. Public Health 17(15), 5487 (2020).
Chaabna, K. et al. The state of population health research performance in the Middle East and North Africa: A meta-research study. Syst. Rev. 10(1), 1 (2021).
Chaabna, K., Cheema, S., Abraham, A. & Mamtani, R. Strengthening literature search strategies for systematic reviews reporting population health in the Middle East and North Africa: A meta-research study. J. Evid. Based Med. 13(3), 192–198 (2020).
Chaabna, K. et al. Systematic overview of hepatitis C infection in the Middle East and North Africa. World J. Gastroenterol. 24(27), 3038–3054 (2018).
Chaabane, S., Doraiswamy, S., Chaabna, K., Mamtani, R. & Cheema, S. The impact of COVID-19 school closure on child and adolescent health: A rapid systematic review. Children (Basel) 8(5), 415 (2021).
Chaabane, S. et al. Perceived stress, stressors, and coping strategies among nursing students in the Middle East and North Africa: An overview of systematic reviews. Syst. Rev. 10(1), 136 (2021).
Singh, S. C. S., Matchar, D. B. & Bass, E. B. Grading a body of evidence on diagnostic tests. In Methods Guide for Medical Test Reviews [Internet] (eds Chang, S. M., Matchar, D. B., Smetana, G. W. et al.) (Agency for Healthcare Research and Quality, 2012).
Hoy, D. et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 65(9), 934–939 (2012).
Dekkers, O. M. et al. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLOS Med. 16(2), e1002742 (2019).
Terracciano, L., Brozek, J., Compalati, E. & Schünemann, H. GRADE system: New paradigm. Curr. Opin. Allergy Clin. Immunol. 10(4), 377–383 (2010).
Michael, B., Larry, V. H., Julian, P. T. H. & Hannah, R. R. Random-Effects Model. Introduction to Meta-Analysis 69–75 (Wiley, 2009).
Freeman, M. F. & Tukey, J. W. Transformations related to the angular and the square root. Ann. Math. Stat. 21(4), 607–611 (1950).
Turner, R. M., Bird, S. M. & Higgins, J. P. The impact of study size on meta-analyses: Examination of underpowered studies in Cochrane reviews. PLoS One. 8(3), e59202 (2013).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327(7414), 557–560 (2003).
Schwarzer G. General package for meta-analysis.: CRAN; 2018. Available from: https://github.com/guido-s/meta, http://meta-analysis-with-r.org
Cochrane Handbook for Systematic Reviews of Interventions. Version 6.22021.
Higgins, J. P. T., Green, S. (eds) 9 analysing data and undertaking meta-analyses > 9.6 investigating heterogeneity > 9.6.4 meta-regression. 2011, in Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011] [Internet]. The Cochrane Collaboration. Available from: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Furuya-Kanamori, L., Barendregt, J. J. & Doi, S. A. R. A new improved graphical and quantitative method for detecting bias in meta-analysis. JBI Evid. Implement. 16(4), 195–203 (2018).
Hunter, J. P. et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 67(8), 897–903 (2014).
Spineli, L. M. & Pandis, N. Prediction interval in random-effects meta-analysis. Am. J. Orthod. Dentofac. Orthop. 157(4), 586–588 (2020).
Shamim, M. A. Real-life implications of prevalence meta-analyses? Doi plots and prediction intervals are the answer. Lancet Microbe 4(7), e490 (2023).
Abdelmoaty Goweda, R. et al. Prevalence of sleep disorders among medical students of Umm Al-Qura University, Makkah, Kingdom of Saudi Arabia. J. Public Health Res. 9(s1), 2020 (2021).
Alrashed, F. A. et al. Prevalence of insomnia and related psychological factors with coping strategies among medical students in clinical years during the COVID-19 pandemic. Saudi J. Bio. Sci. 28(11), 6508–6514 (2021).
Essangri, H. et al. Predictive factors for impaired mental health among medical students during the early stage of the COVID-19 pandemic in Morocco. Am. J. Trop. Med. Hyg. 104(1), 95–102 (2021).
Zainab, S. et al. Frequency and predictors of sleep disorders in undergraduate medical students. J Liaquat Univ. Med. Health Sci. 19(2), 109–115 (2020).
Yassin, A. et al. Prevalence of sleep disorders among medical students and their association with poor academic performance: A cross-sectional study. Ann. Med. Surg. (Lond.) 58(58), 124–129 (2020).
Al-mistarehi, A. et al. The impact of sleep disorders on academic performance among medical students. Am. J. Respir. Crit. Care Med. 199, A4296 (2019).
Alshaaer, N. E. F., Marashli, E., Mahgoub, M., & Alashqae, A. A. The prevalence of Insomnia in medical students: Impact of academic performance (2012).
Sleep Foundation. Paradoxical Insomnia: The misperception of your sleep state 2022 Available from: https://www.sleepfoundation.org/insomnia/paradoxical-insomnia
Mohamed, E. et al. Insomnia and related anxiety among medical students. J. Res. Med. Dent. Sci. 8(3), 198–202 (2020).
Burhan, N. M. Prevalence of sleep disorders among medical students at King Abdulaziz University: A cross-sectional study (2019).
Mokarrar, M., Afsharmanesh, A., Afshari, M. & Mohammadi, F. Prevalence of sleep disorder among medical students in an Eastern University in Iran. Iran. J. Health Sci. 5(1), 49–54 (2017).
Thomas, S. J. A survey of sleep disorders in college students: A study of prevalence and outcomes: University of Alabama Libraries (2014).
Zhang, M. et al. Prevalence and factors associated with insomnia among medical students in China during the COVID-19 pandemic: Characterization and associated factors. BMC Psychiatry 23(1), 140 (2023).
Solanki, S., Venkiteswaran, A. & Saravanabawan, P. Prevalence of insomnia and factors influencing its incidence in students of tbilisi state medical university: A cross-sectional study. Cureus 15(9), e46084 (2023).
Cénat, J. M. et al. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 295, 113599 (2021).
Pappa, S. et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav. Immun. 88, 901–907 (2020).
Moss-Morris, R. & Petrie, K. J. Redefining medical students’ disease to reduce morbidity. Med. Educ. 35(8), 724–728 (2001).
Pavlinac Dodig, I. et al. Sleep and lifestyle habits of medical and non-medical students during the COVID-19 lockdown. Behav. Sci. 13(5), 407 (2023).
Roth, T. et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, second edition criteria: Results from the America Insomnia survey. Biol. Psychiatry 69(6), 592–600 (2010).
Buysse, D. J. Insomnia. JAMA. 309(7), 706–716 (2013).
Morin, C. M. et al. Prevalence of insomnia and its treatment in Canada. Can. J. Psychiatry 56(9), 540–548 (2011).
Ohayon, M. M. & Smirne, S. Prevalence and consequences of insomnia disorders in the general population of Italy. Sleep Med. 3(2), 115–120 (2002).
Léger, D., Partinen, M., Hirshkowitz, M., Chokroverty, S. & Hedner, J. EQUINOX (Evaluation of daytime QUality impairment by nocturnal awakenings in outpatient’s eXperience) survey investigators. Characteristics of insomnia in a primary care setting: EQUINOX survey of 5293 insomniacs from 10 countries. Sleep Med. 11(10), 987–998 (2010).
Buysse, D. J. et al. Relationships between the pittsburgh sleep quality index (PSQI), epworth sleepiness scale (ESS), and clinical/polysomnographic measures in a community sample. J. Clin. Sleep Med. 4(6), 563–571 (2008).
Backhaus, J., Junghanns, K., Broocks, A., Riemann, D. & Hohagen, F. Test-retest reliability and validity of the pittsburgh sleep quality index in primary insomnia. J. Psychosom. Res. 53(3), 737–740 (2002).
Edinger, J. D. et al. American academy of sleep medicine work group. Derivation of research diagnostic criteria for insomnia: Report of an American academy of sleep medicine work group. Sleep 27(8), 1567–1596 (2004).
Lichstein, K. L., Durrence, H. H., Taylor, D. J., Bush, A. J. & Riedel, B. W. Quantitative criteria for insomnia. Behav. Res. Ther. 41(4), 427–445 (2003).
Sivertsen, B., Krokstad, S., Øverland, S. & Mykletun, A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J. Psychosom. Res. 67(2), 109–116 (2009).
Phillips, B. A. et al. Sleep disorders and medical conditions in women. J. Womens Health (Larchmt). 17(7), 1191–1199 (2008).
Tamanna, S. & Geraci, S. A. Major sleep disorders among women: (women’s health series). South Med. J. 106(8), 470–478 (2013).
Rodriguez, J. C., Dzierzewski, J. M. & Alessi, C. A. Sleep problems in the elderly. Med. Clin. N. Am. 99(2), 431–439 (2015).
Allen, R. P. et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern. Med. 165(11), 1286–1292 (2005).
Alsafadi, S. et al. Risk factors of primary and secondary restless legs syndrome among a middle-aged population in Saudi Arabia: A community-based study. Ann. Thorac. Med. 13(3), 175–181 (2018).
Mahajan, A. S. Stress in medical education: A global issue or much ado about nothing specific?. South-East Asian J. Med. Educ. 4(2), 9–13 (2010).
Suardiaz-Muro, M. et al. Sleep and academic performance in university students: A systematic review. Rev. Neurol. 71(2), 43–53 (2020).
Friedrich, A. & Schlarb, A. A. Let’s talk about sleep: A systematic review of psychological interventions to improve sleep in college students. J. Sleep Res. 27(1), 4–22 (2018).
Jain, A., Wadhwa, R., Kundu, K., Nebhinani, N. & Gupta, R. Assessment of knowledge about RLS among medical teachers and undergraduate students using newly developed questionnaire: K-RLS. Sleep Vigil. 6(1), 131–137 (2022).
Ozoh, O. B., Iwuala, S. O., Desalu, O. O., Ojo, O. O. & Okubadejo, N. U. An assessment of the knowledge and attitudes of graduating medical students in lagos, nigeria, regarding obstructive sleep apnea. Ann. Am. Thorac. Soc. 12(9), 1358–1363 (2015).
Rosen, R. C., Rosekind, M., Rosevear, C., Cole, W. E. & Dement, W. C. Physician education in sleep and sleep disorders: A national survey of U.S. medical schools. Sleep 16(3), 249–254 (1993).
Alimoradi, Z. et al. Internet addiction and sleep problems: A systematic review and meta-analysis. Sleep Med. Rev. 47, 51–61 (2019).
Khurshid, K. A. Comorbid Insomnia and psychiatric disorders: An update. Innov. Clin. Neurosci. 15(3–4), 28–32 (2018).
Pigeon, W. R., Bishop, T. M. & Krueger, K. M. Insomnia as a precipitating factor in new onset mental illness: A systematic review of recent findings. Curr. Psychiatry Rep. 19(8), 44 (2017).
Sarsour, K., Morin, C. M., Foley, K., Kalsekar, A. & Walsh, J. K. Association of insomnia severity and comorbid medical and psychiatric disorders in a health plan-based sample: Insomnia severity and comorbidities. Sleep Med. 11(1), 69–74 (2010).
Ohayon, M. M. & Roth, T. Place of chronic insomnia in the course of depressive and anxiety disorders. J. Psychiatr. Res. 37(1), 9–15 (2003).
Roane, B. M. & Taylor, D. J. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep 31(10), 1351–1356 (2008).
Neckelmann, D., Mykletun, A. & Dahl, A. A. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 30(7), 873–880 (2007).
Björnsdóttir, E. et al. The prevalence of depression among untreated obstructive sleep apnea patients using a standardized psychiatric interview. J. Clin. Sleep Med. 12(1), 105–112 (2016).
McCall, W. V., Harding, D. & O’Donovan, C. Correlates of depressive symptoms in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2(4), 424–426 (2006).
Macey, P. M., Woo, M. A., Kumar, R., Cross, R. L. & Harper, R. M. Relationship between obstructive sleep apnea severity and sleep, depression and anxiety symptoms in newly-diagnosed patients. PLoS One 5(4), e10211 (2010).
Jansson-Fröjmark, M. & Lindblom, K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J. Psychosom. Res. 64(4), 443–449 (2008).
Zeng, W., Chen, R., Wang, X., Zhang, Q. & Deng, W. Prevalence of mental health problems among medical students in China: A meta-analysis. Med. (Baltimore) 98(18), e15337 (2019).
Mirza, A. A., Baig, M., Beyari, G. M., Halawani, M. A. & Mirza, A. A. Depression and anxiety among medical students: A brief overview. Adv. Med. Educ. Pract. 12, 393–398 (2021).
Luyster, F. S. et al. Screening and evaluation tools for sleep disorders in older adults. Appl. Nurs. Res. 28(4), 334–340 (2015).
Pervez, S. et al. Prevalence of Insomnia among medical students. Pak. J. Med. Health Sci. 15(4), 1228–1230 (2021).
Khan, K. et al. Effects of insomnia on daily performance of medical students: A cross sectional study conducted in university of Lahore, Pakistan. Rawal Med. J. 44(3), 622–625 (2019).
Khurshid, R. et al. Attitudes and reactions of medical students to the dissection room. Pak. J. Med. Health Sci. 15(5), 917–919 (2021).
Ali, A. et al. Influence of excessive mobile phone use on anxiety and academic performance among medical college students. J. Pharm. Res. Int. 31(6), 1–7 (2019).
Shakeel, H. A. et al. Insomnia among medical students: A crosssectional study. Int. J. Res. Med. Sci. 7(3), 893 (2019).
Ram, D. Frequency of insomnia amongst medical students and its correlation with demographic variables. J. Pak. Pyschiatr. Soc. 14(2), 26–29 (2016).
Alfadeel, M. et al. The prevalence of insomnia among female medical students of almaarefa colleges in Riyadh city -Kingdom of Saudi Arabia 2015–2016. Indo Am. J. Pharm. Sci. 6(2), 3377–3391 (2020).
Mansour, T., Yousef, M., Mansour, T. M. A. & Yousef, M. Nightmares among young medical students. Biomed. Res.-India 27(2), 437–441 (2016).
Al-Zahrani, J. M., Aldossari, K. K., Abdulmajeed, I., Al-Ghamdi, S. H., Al-Shamrani, A. M., & Al-Qahtani, N. S., et al. Daytime sleepiness and academic performance among arab medical students. Am. J. Respir. Crit. Care Med. 193 (2016).
Goweda, R. et al. Prevalence and associated risk factor of low back pain among medical student of Umm Al-Qura University, Makkah, Saudi Arabia: Cross-sectional study. Med. Sci. 24(106), 4359–4367 (2020).
Alqudah, M. et al. Insomnia among medical and paramedical students in Jordan: Impact on academic performance. BioMed Res. Int. 2019, 7136906 (2019).
Ishaq, M. et al. Prevalence of restless legs syndrome among medical students of karachi: An experience from a developing country. Sleep Disord. 2020, 7302828 (2020).
Shalash, A. S. et al. Restless legs syndrome in Egyptian medical students using a validated Arabic version of the restless legs syndrome rating scale. Sleep Med. 16(12), 1528–1531 (2015).
Almansour, A. et al. the prevalence of sleep deprivation and its influence on student’’ life attending medical School at King Saud University. Int. J. Pharm. Phytopharmacol. Res. 10(5), 149–156 (2020).
Acknowledgements
We would like to thank Dr. Ross MacDonald, Librarian, Scholarly Communications, Weill Cornell Medicine-Qatar, for his help in developing the search strategy. We would like also to thank the English editing service provided by Samantha Cayo, Library of Weill Cornell Medicine-Qatar for editing our manuscript.
Author information
Authors and Affiliations
Contributions
S.C.1, K.C., D.M, R.M. and S.C.2 collectively contributed to the conception of the study. S.C.1, K.C., J.A., D.M., R.M. and S.C.2 were involved in the literature search. S.C.1, K.C., DM, S.K., J.A., R.M., and S.C.2 were involved in the screening step. S.C.1, K.C., S.K., J.A., R.M., and S.C.2 were involved in the data extraction step. S.C.1, K.C., S.K., and J.A., were involved in table preparation. Statistical analysis and manuscript drafting were implemented by S.C.1. All authors read and reviewed the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaabane, S., Chaabna, K., Khawaja, S. et al. Sleep disorders and associated factors among medical students in the Middle East and North Africa: a systematic review and meta-analysis. Sci Rep 14, 4656 (2024). https://doi.org/10.1038/s41598-024-53818-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53818-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.