Abstract
This study assessed the clinical effectiveness of orange peel polymethoxy-flavonoids rich fraction (OPMF) solid dispersion as a palatal dressing material, compared with Alveogyl, in a randomized clinical trial. After harvesting free gingival grafts for 18 patients in three groups, the donor site in group I received OPMF; group II received Alveogyl; and group III received placebo dough material. The visual analog scale (VAS) pain score in group I showed the lowest value in week one without a significant difference. In week 2, there was a substantial decrease in pain in group I compared to group III. Week 4 showed reduced pain scores in all groups without significant differences. The results of the number of analgesic pills revealed, after 1 week, the lowest number of pills consumed in group I, with a considerable difference compared to group III. Healing process results showed that group I had the highest healing values in each interval, with a significant difference between group I and group III at 1 and 2 weeks. Color matching parameter showed slight differences between the groups’ readings in favor of group I in all intervals without a statistically significant difference. The results suggest OPMF as a palatal dressing material that facilitates hemostasis, pain relief, and palatal wound healing.
Similar content being viewed by others
Introduction
In periodontal surgery, the hard palate is the best place for harvesting a free gingival graft (FGG). Palatal grafts, due to their superior clinical outcomes and autogenous nature, are preferred above other allogenic or synthetic grafts1.
Achieving widened keratinized and attached gingiva, deepening the vestibular depth, covering exposed root surfaces, and changing a thin periodontal phenotype to a thick phenotype are some clinical endpoints associated with the long-standing free gingival graft procedure2,3. These endpoints contribute to efficient primary stability, which is essential for healing4.
After the FGG has been harvested from the donor site, the open wound takes 2 to 4 weeks to heal with primary or secondary intention5. 3 to 5 weeks are typically needed for complete epithelialization6,7.
This healing process progresses through four distinct but overlapping stages: hemostasis, inflammation, granulation, and maturation8. It’s worth noting that wounds in the mouth heal and re-epithelialize more quickly than skin wounds9,10.
The patient morbidity at the palatal donor site is the principal drawback of the FGG treatment11. The most common side effects of FGG harvesting include discomfort, pain, and bleeding at the donor site, potentially affecting a patient’s quality of life, including speech, eating, and drinking problems12.
Assessing the patients’ perception of their treatment is essential to reducing patient discomfort. Various methods have been reported to decrease postoperative pain13.
Therefore, a variety of substances have been investigated for their effects on the palatal donor site, including stents, periodontal packs, growth factors14, absorbable gelatin sponges, absorbable collagen dressings15, hyaluronic acid (HA), Alveogyl, low-level laser therapy16, and medical plant extracts11.
Flavonoids are secondary metabolic plants with a wide variety of potential biological activities. They are polyphenolic compounds that do not contain nitrogen in their chemical structure. Polymethoxy-flavones, a subcategory of flavonoids isolated from citrus peel, are renowned for their potential biological functions, such as analgesics17, anti-inflammatory, antibacterial, and antioxidant activities, which are required for successful wound healing18,19.
This study utilized orange peel extract rich in polymethoxy-flavonoids; despite their beneficial characteristics, their aqueous solubility and oral bioavailability are limited. Additionally, the extract’s consistency resembles a highly viscous exudate, which hinders its pharmaceutical application. Hence, a delivery system that enhances solubility and permeability and offers a localized application for healing palatal wounds after FGG harvesting is deemed necessary20.
Solid dispersion is one of the most attractive techniques for enhancing the dissolution of poorly soluble drugs, where the lipophilic drug is dispersed in a hydrophilic carrier in different ways. The final product is characterized by minimized particle size, enhanced wettability, and solubility20.
Alveogyl, a topical combination of natural substances, is frequently used to effectively treat alveolar osteitis and decrease pain and infection21,22. Alveogyl is a brown fibrous dressing applied topically to prevent dry sockets following extraction. Its active components include iodoform, an iodine-based antibacterial agent23; butamben, an ester local anesthetic; and eugenol, an essential oil with obvious pain-reduction capabilities. Vegetable fibers from the Penghawar djambi plant, which have hemostatic qualities, carry these active components24. Consequently, it can be used as a dressing material25.
However, a few studies have suggested that Alveogyl might extend wound healing in alveolar osteitis treatment. Additionally, three reported cases indicated that Alveogyl caused an unexplained foreign body reaction26,27. Therefore, evaluating novel natural materials and comparing their effectiveness to the widely used Alveogyl is necessary. The present study aimed to clinically compare, for the first time, the effects of orange peel polymethoxy-flavonoids rich fraction (OPMF) versus Alveogyl as a palatal wound dressing or no dressing material on the severity of postoperative pain, amount of analgesic consumed, palatal wound healing, and tissue color matching following free gingival graft harvesting in a randomized controlled clinical trial.
Methods
Ethical approval
The Research Ethics Committee of the Faculty of Medicine, Assiut University, approved this prospective randomized control trial. This trial was conducted following the ethical principles outlined in the Declaration of Helsinki, with IRB permission number 17300948, and it has been registered on clinicaltrial.gov with the ID: NCT05814003 since April 14, 2023. The study was performed and reported according to CONSORT 2010 guidelines.
Eligibility criteria
This study was conducted at the Faculty of Dentistry, Assiut University, Egypt. Informed consent was obtained from each patient after the procedure was explained.
Adult, healthy patients aged 18 years or older were enrolled with keratinized gingiva ≤ 1 mm (evaluated with a UNC periodontal probe) and needed free gingival grafts for various periodontal and peri-implant plastic surgeries. Patients were excluded based on the following criteria: (1) Smoking; (2) Pregnancy or breastfeeding; (3) Systemic diseases that interfere with wound healing, such as diabetes mellitus, immunodeficiency, radiation, metabolic disorders, or immunosuppressive drugs; (4) Use of anti-inflammatory drugs or narcotic analgesics within the past three months; (5) Individuals who have undergone palatal grafting procedures at the same site in the past.
Study design
A three-arm, parallel randomized clinical trial was conducted using simple randomization. Sealed envelopes with numbered cards from 1 to 18 were employed for allocation, with the distribution as follows: cards 1 to 6 for Group I, cards 7 to 12 for Group II, and cards 13 to 18 for Group III, maintaining an allocation ratio of 1:1:1. Two different wound dressing materials were applied to the palatal donor following free gingival graft harvesting, dividing the participants into three groups: Group I received orange peel polymethoxy-flavonoids rich fraction (OPMF) dressing material, Group II received Alveogyl dressing material, and Group III received placebo material.
Sample size and characteristics
Based on a prior study11 and calculation using the G power statistical power analysis tool (version 3.1.9.4)28 for sample size calculation, detection of large effect size (f) = 0.88 requires a total sample size of n = 18, divided into n = 6 in each group. The analysis assumes an actual power (1-error) of 0.8 (80%) and a significance level (error) of 0.05 (5%) for a two-sided hypothesis test (see Fig. 1).
Orange peel polymethoxy-flavonoids rich fraction dough preparation
The orange peel extract was prepared following the method described by Khallaf et al.29. Orange peel was air-dried at room temperature, powdered (10 g), and subjected to extraction by maceration using dichloromethane (50 ml × 3). The extract was concentrated under a vacuum to remove solvent and volatile oil. The resulting solid residue was kept at − 10 °C until the time of the experiments. The solid dispersion of the prepared extract was then prepared using β-cyclodextrin through the co-grinding technique, maintaining a 3:1 ratio of β-cyclodextrin to orange peel extract30.
The placebo material is formed only from β-cyclodextrin, a non-active ingredient used as a carrier or inert additive. Its purpose is to increase the bioavailability of active substances and decrease the concentration of active compounds in the final product without compromising their effectiveness31.
Surgical procedure
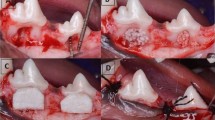
A surgical stent was prepared to protect the donor area by taking an impression of the palatal region before surgery, as shown in Fig. 2a. The stent’s fit was examined before the surgery.
The recipient and palatal donor surgical sites received local anesthesia (4% articaine and 0.001% adrenalin). Additionally, the recipient site underwent the first stage of surgical preparation for the FGG.
A sterile template was utilized to estimate the FGG’s dimensions. A conventional scalpel technique was employed to harvest a 1.0–1.5 mm split-thickness gingival graft (Fig. 2b) from the palatal mucosa adjacent to the premolars and the first molar, positioned 2–3 mm apical to the gingival margin of neighboring teeth.
The graft (Fig. 2c) was placed in the recipient area, firmly adjusted, and stabilized with knotted sutures. Additionally, the recipient site received a gentle compress for 5 min using gauze soaked in saline.
The donor site in the first group received orange peel polymethoxy-flavonoids rich fraction (OPMF) dressing material (Fig. 2d); the donor site in the second group received Alveogyl (Septodont, Niederkassel, Germany) dressing material; and the donor site in the third group received placebo dough material. Subsequently, all groups underwent suturing with resorbable material and the insertion of an acrylic stent.
The participants were not informed about the kind of applied material they would receive, and the tested materials were all the same color.
Following the operation, postoperative instructions were provided to each patient. They were advised to adhere to a soft diet and take one Ibuprofen 600 mg tablet every eight hours on the first postoperative day and then as needed based on the severity of the pain. Additionally, patients were instructed to use a mouthwash containing 0.12 percent chlorhexidine twice daily.
The primary outcome of this trial was the assessment of pain, while the secondary outcomes included the evaluation of the healing process, analgesics consumed, and color matching.
Patient assessment
Subjective assessment
-
a.
Pain assessment The visual analog scale (VAS) measured the patient’s pain level32. During this procedure, patients were asked to rate their pain level on a scale from 0 to 10, where 0 = no pain, 1–3 = mild, 4–6 = moderate, and 7–10 = severe pain.
-
b.
Total analgesics taken The number of Ibuprofen 600 mg pills needed to control postoperative pain during the 14 days following surgery was recorded.
Objective assessment
-
a.
The healing process The Landry, Turnbull, and Howley Healing Index (HI) were used for the evaluation33,34. This index assigns a value from 1 (very poor healing) to 5 (great healing) based on criteria such as redness, hemorrhage, granulation tissue, epithelialization, and suppuration.
-
b.
Color matching The Modified Manchester Scar Scale35 was used to categorize color matching concerning adjacent mucosa into three categories: 1—a perfect match, 2—a minor mismatch, and 3—an evident mismatch.
Follow-up
After surgery, patients were offered follow-up appointments in the first, second, and fourth weeks to collect assessment data.
Statistical analysis
Clinical data were statistically analyzed at 1 week, 2 weeks, and one month using a paired t-test and SPSS (Statistical Package for Social Sciences) software. The Kruskal–Wallis test was employed for comparing all studied groups each week, the Mann–Whitney test for comparisons between two groups in each week, Friedman’s test for comparing all weeks within each group, and the Wilcoxon Signed Ranks test for comparing different weeks within each group. Statistical significance was considered at a p-value of < 0.05.
Results
This study involved 18 participants randomly assigned to three groups, with six participants in each group. Among the participants, 11 (61.1%) were females, and 7 (38.9%) were males.
Regarding the VAS of pain, at 1 week postoperative, the highest pain score was presented in the placebo group (Group III) of 5.5 ± 1.64, indicating moderate pain. Group II, which received Alveogyl, showed a pain score of 4.33 ± 1.21, indicating moderate pain. The orange peel polymethoxy-flavonoids rich fraction (OPMF) group (Group I) had the lowest value of 3.83 ± 0.75, representing moderate pain. However, the different groups had no significant difference in pain scores (p = 0.157).
At 2 weeks postoperative, the pain score was mild in Group I (1.67 ± 0.52), mild in Group II (2 ± 0.89), and moderate in Group III (3.17 ± 0.75). There was a statistically significant difference between Group I and Group III (p < 0.01) and between Group II and Group III (p < 0.05).
At 4 weeks postoperative, the pain score was very mild in Group I and Group II (0.33 ± 0.52), mild in Group III (0.83 ± 0.98), and there was no statistically significant difference in pain scores among different groups (p = 0.558).
Intragroup comparisons showed statistically significant differences within each group across different intervals (p < 0.05), as illustrated in Table 1 and Fig. 3.
The number of analgesic pills results after 1 week postoperative revealed that Group III had the highest values (18.17 ± 2.79), showing a significant difference compared to Group I (13.33 ± 3.01) (p < 0.05). Group II had a value of 16.83 ± 3.13, with no statistically significant difference from both other groups.
At 2 weeks, the highest value was observed in Group III (6 ± 1.41), followed by Group II (5.17 ± 2.14), while the lowest value was found in Group I (3.83 ± 1.17). Intragroup comparisons showed statistically significant differences within each group across different intervals (p < 0.05), as demonstrated in Table 2 and Fig. 4.
The healing process results showed that Group I had the highest healing values in each interval. At 1 week and 2 weeks, there was a significant difference between Group I (3.33 ± 0.52, 4.17 ± 0.75) and Group III (1.83 ± 0.75, 3 ± 0.89) (p < 0.01 and p < 0.05, respectively). At 4 weeks, the highest value was found in Group I (4.67 ± 0.52), followed by Group II (4.50 ± 0.55), while the lowest value was found in Group III (4 ± 0.89). The three groups had no statistically significant difference, as presented in Table 3 and Figs. 5 and 6.
The color matching parameter showed a slight difference in reading between Group I (2.89 ± 0.41, 2.33 ± 0.52, 1.67 ± 0.52) in the first, second, and fourth weeks and both Group II (3, 2.5 ± 0.55, 2) and Group III (3, 2.67 ± 0.52, 2.33 ± 0.52). However, there was no statistically significant difference among the three groups, as shown in Table 4 and Figs. 5 and 7.
Discussion
The most common issues after FGG procedures include postoperative bleeding, pain, and discomfort at the donor site36.
Various dressing materials have been recommended to preserve the resulting partial-thickness wound at the palatal donor site, enhance comfort, support the process of re-epithelialization, and protect the palatal connective tissue from physical and chemical irritation as well as colonization by oral microorganisms already present37.
Applying a topical dressing to the palatal wound allows for the local concentration of healing-promoting, analgesic, and antiseptic substances. At the same time, the risks of side effects or sensitization associated with systemic administration were reduced24.
Orange peel polymethoxy-flavonoids (OPMF) are natural and safe extracts38 from the flavonoid family, playing a significant role in wound healing in several ways. First, they exhibit an antioxidant action by inhibiting reactive oxygen species (ROS), thereby minimizing oxidative stress and accelerating wound healing39. In a previous study performed using a mouse model, it was found that treatment of diabetic foot ulcers with the flavonoid hesperidin resulted in complete healing of the wound within less than 21 days. This effect may be attributed to the enhancement of the expression of the Vascular Endothelial Growth Factor (VEGF-c), Angiopoietin-1 (Ang-1)/Tie-2, and Transforming Growth factor (TGF), leading to accelerated angiogenesis and stimulating new tissue restoration40,41. Flavonoids activated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), reducing oxidative stress and promoting cell proliferation, neovascularization, and wound healing. Additionally, Nrf2 activation inhibits cytoprotective genes, thereby upgrading keratinocyte apoptosis. Flavonoids also exert their analgesic and anti-inflammatory action by inhibiting the expression of nuclear factor kappa B (NF-kB), thereby minimizing the levels of inflammatory mediators such as prostaglandin E2 (PGE2), leukotriene B4 (LTB-4), interleukin 1 (IL-1), tumor necrosis factor (TNF-), interleukin 6 (IL-6), and interferon (IFN-)42. During injury, the commensal bacteria colonize the wound, forming a biofilm that postpones the healing process and makes the wound susceptible to new invasion. The antibacterial action of flavonoids was mediated in different ways, including blocking microbial adhesion and growth through complex action with the microorganism’s cell wall. Additionally, flavonoids mediate bacterial enzyme inhibition, such as tyrosyl‐tRNA synthetase. Baicalein, a flavonoid, when combined with cefotaxime, forms a powerful bactericide that minimizes the Pseudomonas aeruginosa‐induced secretion of the inflammatory cytokines (IL‐1β, IL‐6, IL‐8, and TNFα), which are essential for inflammatory injury after infection with P. aeruginosa42.
The active chemicals in Alveogyl include butamben, iodoform, and eugenol. Additionally, it contains olive oil, spearmint oil, sodium lauryl sulfate, calcium carbonate, penghawar djambi, and purified water23.
Iodoform is an iodine-based antibacterial, while butamben is an ester local anesthetic24. Eugenol, an essential oil extracted from various plants, including cloves, possesses exceptional pain-relieving qualities43. Alveogyl consistency is provided by the penghawar djambi, a byproduct of Cibotium barometz tree fibers44,45, which also offers hemostatic properties and ensures easy adherence to soft tissues in the correct dimensions26.
The current study used orange peel polymethoxy-flavonoids (OPMF) and Alveogyl as palatal wound dressing for a palatal wound following free gingival grafting.
Different objective measurements, including index and scales, wound epithelialization tests, visual clinical healing assessments, photographic healing, bleeding evaluations, cytological analyses46,47, laboratory analyses48, and histological examination49, were employed as methods to evaluate the outcomes of postoperative palatal wound healing50.
Pain perception is one of the most widely discussed techniques for evaluating FGG operations. The patients use the visual analog scale to express their perception51. Sousa et al.15 and others52,53 used the VAS-10 scale, ranging from 0 (no pain) to 10 (the worst suffering ever experienced).
In addition to evaluating the pain, Zucchelli et al.54 and other authors55 used the number of analgesics consumed in hours, days, or weeks to indicate the pain levels.
This study assessed postoperative pain directly via VAS and indirectly via analgesics. Patients received 600 mg of Ibuprofen on the day of surgery for pain control. Patients were instructed to take analgesic medications only when necessary to ensure that reported pain scores were attributed to the intervention adopted54.
The highest pain VAS score was observed in the placebo group (Group III) at 1 week postoperative, representing moderate pain. The Alveogyl group (Group II) showed moderate pain. The hydroxylated polymethoxy flavones group (Group I) had the lowest value, representing moderate pain, with no significant difference among the different groups.
By the second week, pain severity decreased in all the groups, becoming mild in both Group I and Group II and moderate in Group III, with a statistically significant difference between Group III and the other two groups. By the fourth week, as epithelialization increased and pain decreased, the differences between the groups decreased, and the values were no longer statistically different.
Although the number of analgesic tablets used for pain management by the patients in each group noticeably decreased over 14 days, those in Group III used a significantly higher number of analgesic tablets than the intervention Group I and Group II throughout the 14-day healing period.
Our results align with Ferraz et al.56, who approved the analgesic effect of flavonoids, and Ehab et al.25, who reported a significant reduction in VAS pain scores in the Alveogyl group.
All groups experienced more pain over the first week, gradually subsiding over the subsequent days. This trend is consistent with the findings of Del Pizzo57, who noted an increase in pain during the first 2 weeks following surgery.
According to Burkhardt et al.5, pain was more severe in the early postoperative days, subsiding over the next few days.
A Healing Index (HI) was proposed by Landry et al.34 to describe the extent of clinical healing after periodontal surgery, assessing the quality of the healing process. The HI, ranging from 1 (very poor) to 5 (excellent), combines the presence or absence of five clinical criteria (tissue color, response to palpation, granulation tissue, incision margin, and suppuration)58.
The healing process results showed that Group I had the highest healing values at each interval, with a significant difference between Group I and Group III at 1 week and 2 weeks, consistent with Zulkefli et al.19, who reported the wound-healing capacity of flavonoids.
Complete epithelialization of the palatal wound occurred 4 weeks after FGG surgery, according to Del Piezzo et al.57. Consistent with this research, most patients’ palatal lesions were fully healed in our study within 4 weeks.
Silva et al.59 reported that in most patients (92%), the palatal FGG donor site had completely epithelized and closed by 15 days after surgery.
Comparisons of the palatal donor site with adjacent and opposite sides were conducted by Bahammam et al.14 and others53 through visual inspection of clinical images, considering color match (CM) characteristics. Samani et al.35 used the Modified Manchester Scale to compare the color of the neighboring mucosa, in which 0 represented a perfect match, 1 indicated a slight mismatch, and 2 signified an obvious mismatch. The degree of reepithelization and wound healing will be reflected in the visible color changes when the FGG healing occurred by secondary intention and matched the surrounding normal tissue35.
Color Match (CM) was evaluated in the present study on days 7, 14, and 30. In the first, second, and fourth weeks, the color matching parameter showed a slight difference between Group I and Group II and Group III, with no statistically significant difference among the three groups.
The present trial shared common limitations, including subjective methods for quantifying donor healing, the absence of histology evaluation, and the wide variety and drawbacks of scoring systems.
Conclusion
Within its limitations, the study suggests that using orange peel polymethoxy-flavonoids rich fraction (OPMF) as a wound dressing material, comparable to Alveogyl, may represent a suitable option to improve patients’ healing process and reduce postoperative pain.
Data availability
The data used in the current study are not publicly available due to ethical restrictions but are available from the corresponding author on reasonable request.
References
Cortellini, P. & Pini Prato, G. Coronally advanced flap and combination therapy for root coverage. Clinical strategies based on scientific evidence and clinical experience. Periodontology 2000 59, 158–184 (2012).
Zucchelli, G. et al. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J. Periodontol. 91(1), 9–16 (2020).
Zuhr, O., Baumer, D. & Hurzeler, M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: Critical elements in design and execution. J. Clin. Periodontol. 41(15), 123–142 (2014).
Zucchelli, G. & Mounssif, I. Periodontal plastic surgery. Periodontology 2000(68), 333–368 (2015).
Burkhardt, R., Hammerle, C. H. & Lang, N. P. Self-reported pain perception of patients after mucosal graft harvesting in the palatal area. J. Clin. Periodontol. 42, 281–287 (2015).
Tavelli, L. et al. Wound healing dynamics, morbidity, and complications of palatal soft-tissue harvesting. Periodontology 2000 92(1), 90–119 (2023).
Yildiz, M. & Gunpinar, S. Free gingival graft adjunct with low-level laser therapy: A randomized placebo-controlled parallel group study. Clin. Oral Investig. 23, 1845–1854 (2019).
Ehrlich, H. P. & Krummel, T. M. Regulation of wound healing from a connective tissue perspective. Wound Repair Regen. 4, 203–210 (1996).
Sculean, A., Gruber, R. & Bosshardt, D. D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 41, 6–22 (2014).
Harper, D., Young, A. & McNaught, C. E. The physiology of wound healing. Surgery 32, 445–450 (2014).
Keceli, H. G., Aylikci, B. U., Koseoglu, S. & Dolgun, A. Evaluation of palatal donor site hemostasis and wound healing after free gingival graft surgery. J. Clin. Periodontol. 42, 582–589 (2015).
Wyrębek, B., Górski, B. & Górska, R. Patient morbidity at the palatal donor site depending on gingival graft dimension. Dent. Med. Probl. 55, 153–159 (2018).
Meza-Mauricio, J. et al. Is the use of platelet-rich fibrin effective in the healing, control of pain, and postoperative bleeding in the palatal area after free gingival graft harvesting? A systematic review of randomized clinical studies. Clin. Oral Investig. 25, 4239–4249 (2021).
Bahammam, M. A. Effect of platelet-rich fibrin palatal bandage on pain scores and wound healing after free gingival graft: A randomized controlled clinical trial. Clin. Oral Investig. 22, 3179–3188 (2018).
Sousa, F. et al. Effect of A-PRF application on palatal wound healing after free gingival graft harvesting: A prospective randomized study. Eur. J. Dent. 14, 63–69 (2020).
Eltas, A., Eltas, S., Uslu, M. & Ersöz, M. Evaluation of patient discomfort at the palatal donor site following free gingival graft procedures: A randomized controlled clinical trial. J. Periodontol. Implant Dent. 6(2), 47–53 (2014).
Fernández-Rojas, B. & Gutiérrez-Venegas, G. Flavonoids exert multiple periodontic benefits including anti-inflammatory, periodontal ligament-supporting, and alveolar bone-preserving effects. Life Sci. 209, 435–454 (2018).
Nagula, R. L. & Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control Release 296, 190–201 (2019).
Zulkefli, N. et al. Flavonoids as potential wound-healing molecules: Emphasis on pathways perspective. Int. J. Mol. Sci. 24, 4607 (2023).
Dhirendra, K., Lewis, S., Udupa, N. & Atin, K. Solid dispersions: A review. Pak. J. Pharm. Sci. 22(2), 234–246 (2009).
Taberner-Vallverdú, M., Nazir, M., Sánchez-Garcés, M. & Gay-Escoda, C. Efficacy of different methods used for dry socket management: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 20(5), 633–639 (2015).
Pal, U. S., Singh, B. P. & Verma, V. Comparative evaluation of zinc oxide eugenol versus gelatin sponge soaked in plasma rich in growth factor in the treatment of dry socket: An initial study. Contemp. Clin. Dent. 4, 37–41 (2013).
Supe, N. B. et al. Efficacy of Alvogyl (combination of iodoform + butylparaminobenzoate) and zinc oxide eugenol for dry socket. Ann. Maxillofac. Surg. 8(2), 193–199 (2018).
Rani, A., Mohanty, S., Sharma, P. & Dabas, J. Comparative evaluation of Er:Cr:YSGG, diode laser and Alvogyl in the management of alveolar osteitis: A prospective randomized clinical study. J. Maxillofac. Oral Surg. 15(3), 349–354 (2016).
Ehab, K., Abouldahab, O., Hassan, A. & Fawzy El-Sayed, K. Alvogyl and absorbable gelatin sponge as palatal wound dressings following epithelialized free gingival graft harvest: A randomized clinical trial. Clin. Oral Investig. 24(4), 1517–25 (2020).
AbdullGaffar, B. & Awadhi, F. Be aware of a potential pitfall in oral and dental specimens: Alvogyl fibers. Int. J. Surg. Pathol. 28(3), 280–283 (2020).
AbdullGaffar, B., Alawadhi, F. & Gandour, K. Alvogyl dental dressing: A potential cause of complicated postextraction non-healing sockets: A clinicopathologic study of 7 cases. Int. J. Dent. Oral Health 2(4), 170 (2016).
Charan, J. & Biswas, T. How to calculate sample size for different study designs in medical research? Indian J. Psychol. Med. 35(2), 121–126 (2013).
Khallaf, I., Othman, R., Mahmoud, A., Attia, R. & Tahawy, O. In vivo evaluation of orange peel oil and its major component hesperidin against enteral phase of Trichinella spiralis. EJPMR 5(8), 58–65 (2018).
Yang, C., Xu, X., Wang, J. & An, Z. Use of the co-grinding method to enhance the dissolution behavior of a poorly water-soluble drug: Generation of solvent-free drug–polymer solid dispersions. Chem. Pharm. Bull. 60(7), 837–845 (2012).
Braga, S. S. Cyclodextrins as multi-functional ingredients in dentistry. Pharmaceutics 15(9), 2251 (2023).
Delgado, D. A. et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2(3), 088 (2018).
Beausang, E., Floyd, H., Dunn, K. W., Orton, C. & Ferguson, M. W. A new quantitative scale for clinical scar assessment. Plast. Reconstr. Surg. 102, 1954–1961 (1998).
Landry, R. G., Turnbull, R. S. & Howley, T. Effectiveness of benzydamyne HCl in the treatment of periodontal post-surgical patients. Res. Clin. Forums 10, 105–118 (1988).
Samani, M. K., Saberi, B. V., Ali Tabatabaei, S. M. & Moghadam, M. G. The clinical evaluation of platelet-rich plasma on free gingival graft’s donor site wound healing. Eur. J. Dent. 11, 447–454 (2017).
Griffin, T. J., Cheung, W. S., Zavras, A. I. & Damoulis, P. D. Postoperative complications following gingival augmentation procedures. J. Periodontol. 77, 2070–2079 (2006).
Shetty, V. & Schwartz, H. C. Wound healing and perioperative care. Oral Maxillofac. Surg. Clin. N. Am. 18(1), 107–113 (2006).
Burnett, C., Bergfeld, W. & Belsito, D. V. Safety assessment of citrus peel-derived ingredients as used in cosmetics. Int. J. Toxicol. 40(3), 77–99 (2021).
Heinke, J., Patterson, C. & Moser, M. Life is a pattern: Vascular assembly within the embryo. Front. Biosci. 4, 2269 (2012).
Yeh, C. J. et al. The effects of artocarpin on wound healing: In vitro and in vivo studies. Sci. Rep. 7(1), 15599 (2017).
Li, W., Kandhare, A. D., Mukherjee, A. A. & Bodhankar, S. L. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: Role of TGF-ß/Smads and Ang-1/Tie-2 signaling pathways. EXCLI J. 17, 399 (2018).
Sychrová, A., Škovranová, G., Čulenová, M. & Fialová, S. B. Prenylated flavonoids in topical infections and wound healing. Molecules 27(14), 4491 (2022).
Amiri, A., Dugas, R., Pichot, A. L. & Bompeix, G. In vitro and in vivo [corrected] activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int. J. Food Microbiol. 126(1–2), 13–19 (2008).
Syrjänen, S. M. & Syrjänen, K. J. Influence of Alvogyl on the healing of extraction wound in man. Int. J. Oral Surg. 8(1), 22–30 (1979).
Steinmetz, E. F. Penawar Djambe. Q. J. Crude Drug Res. 9(1), 1326–1327 (1969).
Patel, P. V., Kumar, V., Kumar, S., Gd, V. & Patel, A. Therapeutic effect of topical ozonated oil on the epithelial healing of palatal wound sites: A planimetrical and cytological study. J. Investig. Clin. Dent. 2, 248–258 (2011).
Patel, P. V. et al. Cytological assessment of healing palatal donor site wounds and grafted gingival wounds after application of ozonated oil: An 18-month randomized controlled clinical trial. Acta Cytol. 56, 277–284 (2012).
Keskiner, I., Lutfioğlu, M., Aydogdu, A., Saygun, N. I. & Serdar, M. A. Effect of photobiomodulation on transforming growth factor-β1, platelet-derived growth factor-BB, and interleukin-8 release in palatal wounds after free gingival graft harvesting: A randomized clinical study. Photomed. Laser Surg. 34, 263–271 (2016).
Shanmugam, M., Kumar, T. S., Arun, K. V., Arun, R. & Karthik, S. J. Clinical and histological evaluation of two dressing materials in the healing of palatal wounds. J. Indian Soc. Periodontol. 14, 241–244 (2010).
Malpartida-Carrillo, V. et al. Outcome measurements following palatal soft tissue graft harvesting: A review. J. Clin. Exp. Dent. 13(5), 527–535 (2021).
Tavelli, L. et al. Pain perception following epithelialized gingival graft harvesting: A randomized clinical trial. Clin. Oral Investig. 23, 459–468 (2019).
Lektemur Alpan, A. & Torumtay Cin, G. PRF improves wound healing and postoperative discomfort after harvesting subepithelial connective tissue graft from palate: A randomized controlled trial. Clin. Oral Investig. 24, 425–436 (2020).
Yıldırım, S., Özener, H. Ö., Doğan, B. & Kuru, B. Effect of topically applied hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J. Periodontol. 89, 36–45 (2018).
Zucchelli, G. et al. Patient morbidity and root coverage outcome after subepithelial connective tissue and de-epithelialized grafts: A comparative randomized-controlled clinical trial. J. Clin. Periodontol. 37, 728–738 (2010).
Heidari, M. et al. Effect of laser photobiomodulation on wound healing and postoperative pain following free gingival graft: A split-mouth triple-blind randomized controlled clinical trial. J. Photochem. Photobiol. B 172, 109–114 (2017).
Ferraz, C. R. et al. Therapeutic potential of flavonoids in pain and inflammation: Mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules 25(3), 762 (2020).
Del Pizzo, M., Modica, F., Bethaz, N., Priotto, P. & Romagnoli, R. The connective tissue graft: A comparative clinical evaluation of wound healing at the palatal donor site. J. Clin. Periodontol. 29, 848–854 (2002).
Pippi, R. Post-surgical clinical monitoring of soft tissue wound healing in periodontal and implant surgery. Int. J. Med. Sci. 14(8), 721–728 (2017).
Silva, C. O., Ribeiro Edel, P., Sallum, A. W. & Tatakis, D. N. Free gingival grafts: Graft shrinkage and donor-site healing in smokers and non-smokers. J. Periodontol. 81, 692–701 (2010).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.M.F. and A.A.A. conducted the surgical part and collected the clinical data, A.U.A., I.S.A.K., A.S.H., and M.A.S. prepared OPMF material and analyzed the data. All authors shared in the writing and revision of the work, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alghriany, A.A., Ali, A.U., Khallaf, I.S.A. et al. Clinical effectiveness of orange peel polymethoxy-flavonoids rich fraction as a palatal dressing material compared to Alveogyl: randomized clinical trial. Sci Rep 14, 3067 (2024). https://doi.org/10.1038/s41598-024-53511-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53511-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.