Abstract
This study aimed to evaluate the association between age at menarche and cardiovascular (CV) events through a systematic review and meta-analysis of observational studies. A comprehensive literature search covering studies published from January 1, 2000, to October 31, 2023, was conducted in PubMed, MEDLINE, Embase, and Scopus. Twenty-nine observational studies involving 4,931,160 adult women aged 18 years or older were included. The meta-analysis revealed a J-shaped association between age at menarche and CV events. Individuals with menarche at 12–13 years exhibited the lowest risk, while those with younger (≤ 11 years) or older ages (14–15 years and ≥ 16 years) showed an increased risk. Notably, individuals with age at menarche of 16 years and older had the highest risk of CV events. The pooled odds of CV mortality in age at menarche categories 14–15 years and ≥ 16 years were 37% (OR: 1.37, 95% CI 1.14–1.64, I2: 76.9%) and 64% (OR: 1.64, 95% CI 1.20–2.24, I2: 87%) higher than referent age at menarche 12–13 years. No statistically significant difference was found in CV mortality risk between individuals with age at menarche ≤ 11 years and those with age at menarche 12–13 years. The ORs for coronary heart disease were significantly higher for age at menarche ≥ 16 years (35% increase), while no significant difference was found for age at menarche ≤ 11 years or 14–15 years compared to age at menarche 12–13 years. Regarding stroke, the ORs for age at menarche ≤ 11, 14–15, and ≥ 16 years were significantly higher (7%, 24%, and 94% increase, respectively) compared to age at menarche 12–13 years. Dose–response meta-analysis and one-stage random-effect cubic spline models confirmed the J-shaped risk pattern. Meta-regression indicated that age and BMI were not significant sources of heterogeneity. Sensitivity analyses and the absence of publication bias further supported the robustness of the findings. This study concludes that age at menarche is independently associated with CV events, with a J-shaped pattern. The findings underscore the significance of considering menarche age as an independent risk factor for CV events. Further research is warranted to validate these findings and explore potential underlying mechanisms.
Similar content being viewed by others
Introduction
Cardiovascular (CV) events are the leading cause of mortality and major morbidity in both developed and developing countries1. The age-standardized prevalence of CV events in men is higher than in women, however, it is responsible for causing approximately 35% of annual female mortality and is one of the most common reasons for disability-adjusted life-years lost among women2,3.
Despite its importance, CV events in women are inadequately acknowledged, and their risk factors remain understudied4. However, along with well-known cardiovascular risk factors such as smoking and obesity, there are other female-specific risk factors, such as reproductive age characteristics, which could also influence women’s CV disease risk throughout their lifespan5.
Age at menarche, defined as the age at first menstruation in adolescent girl, is one of the most important reproductive characteristics of women and is a milestone of pubertal development6. Emerging evidence suggests that the timing of menarche is associated with a higher risk of some cancers7,8, chronic disorders9,10, cardiometabolic disturbances11,12,13,14, higher adult body mass index (BMI)15,16 and hypertension17.
In addition, some studies specifically investigate the association between age at menarche and CV events. While certain systematic review and meta-analysis studies have indicated that an early onset of menarche is linked to an increased risk of all-cause mortality, the evidence concerning its association with mortality related to CV events or other CV events remains somewhat inconclusive18,19. Besides, in a separate systematic review study, Luijken et al. reviewed the data on the association between age at menarche and different subtypes of CVD20. They observed that among eight studies involving Caucasian populations, a consistent inverse linear relationship was reported between age at menarche (AAM) and cardiovascular disease (CVD) risk. However, a significant U-shaped relationship was observed in a large-scale study (n = 1,200,000)21. However, data from Asian populations yielded inconclusive results regarding the association between AAM and CVD risk. It should be noted that using different criteria for early age at menarche may lead to discrepancies between studies. In a pooled Analysis of Individual Patient Data of 307,855 women, Mishra et al.22 reported the U-shaped association between age at menarche and CVD. However, in a separate systematic review, Luijken et al.20 highlighted heterogeneity among the findings of available studies.
These findings suggest that the association between age at menarche and CV events is not entirely clear and needs to be precisely estimated. Therefore, the aim of this systematic review and meta-analysis of observational study was to assess the associations between age at the menarche and CV events among women.
Material and methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines23. The trial protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42023453056.
The review question was framed using the PICO (population, intervention/index, control, and outcomes) statement as follows: P: all women who experience menarche which was classified into different monarchial age groups; I: age at menarche; C: women who experienced menarche at the normal age24,25,26; O: cardiovascular events including stroke, coronary heart disease and CV mortality.
Eligibility criteria
This review considered all types of analytic observational studies and assessed the association between age at menarche and any cardiovascular events. Eligible studies were required to clearly define the age at menarche. Additionally, eligible studies needed to report an accurate number of CV events. To ensure the applicability of findings to the general population, studies focusing on women with severe diseases or serious conditions were excluded. Also, gray literature and non-original studies including reviews, commentaries, editorials, letters, meeting abstracts, case reports, conference proceedings, governmental or organizational reports, dissertations, theses, unpublished data, and presentations that did not provide accurate and clear data on research variables, were excluded. Articles not published in English were also excluded. Data for eligible articles in the press were requested from the study authors.
Search strategy
A systematic computerized literature search of four electronic databases including PubMed, EMBASE, Scopus and Web of Science, covering the period from January 1, 2000, to October 31, 2023. A set of relevant terms was combined and used to narrow the search. Truncations were applied where appropriate, following the syntax rules of each database. Two filters, selecting only human studies and English publication, were applied. Additionally, a manual search in the references lists of selected studies and other relevant reviews was performed. The specific search strategy is presented in Supplementary Table 1, using PubMed as an example.
Study selection and extraction
EndNote software (version X8, Clarivate Analytics, 2017 Boston, MA) was used to export identified references. After removing duplicates, titles, abstracts, and full texts were screened based on the aforementioned selection criteria. Two researchers (SB-G and RBY) completed all stages of the screening process independently, and discrepancies were resolved through discussion. If necessary, additional reviewers were consulted for further input. Throughout the review of abstracts and full-text articles, a list of references that did not meet the eligibility criteria was maintained, along with notes on the reasons for exclusions. Data on study characteristics, participant descriptions, association details, outcomes, and statistics were independently extracted by two reviewers. For missing relevant data, authors of eligible studies were contacted via email. The characteristics of included studies were summarized in Table 1.
Exposure and outcomes of study
Age at menarche was defined as the age in whole years at the first menstrual period. This variable was initially categorized into four groups (age at menarche: ≤ 11, 12–13, 14–15, and ≥ 16 years, with the reference group being women who were aged 12–13 years at menarche). The outcome of study included the specific subtypes of CV events including coronary heart disease, stroke and CV mortality. The criteria for stroke included subarachnoid hemorrhage, ischemic stroke, intracerebral hemorrhage, and unspecified stroke. Coronary heart disease was defined as myocardial infarction, coronary artery bypass graft surgery, or percutaneous coronary intervention and other coronary disease.
Quality appraisal and statistical analysis
Two authors (CFM and SB-G) independently conducted a thorough critical appraisal of the selected studies. Any disagreements or discrepancies that arose during this process were resolved through discussion. In cases where necessary, other reviewers (IS and RB-Y) were consulted. The Newcastle–Ottawa Scale (NOS) was used for methodological structures and result presentation of the studies27. This scale includes three criteria: (i) participant selection (maximum of four stars); (ii) comparability of study groups (maximum of two stars) and (iii) assessment of outcome or exposure (maximum of three stars) for the outcome/exposure category. Studies with scores above 7 were considered high quality, those with scores between 4 and 7 were categorized as moderate quality, and those with scores less than 4 were judged as low quality. However, we planned to conduct a subgroup analysis by including and excluding results from studies of low quality, if any such studies were identified.
Statistical analysis
All statistical analysis was performed using STATA software (version 14; STATA, INC., College Station, TX, USA). We conducted two types of analyses. First, to estimate pooled Odds Ratio (OR) and 95%CI of the outcomes of interest in menarche age groups versus controls, we employed DerSimonian & Laird and inverse variance methods to run random/fixed effect models. It is important to note that raw data were extracted from different types of studies such as case–control, prospective and cross-sectional. For each one, the OR (SE log OR) were estimated, separately. However, some approximation was considered for relative risks and Hazard ratio with odds ratios28. Heterogeneity was quantified using the I-squared measure and Chi-squared test. In this case, an I-squared value exceeding 50% was considered medium to high heterogeneity. A significant result in the Chi-squared test was also considered as heterogeneity. In the presence of significant heterogeneity, a random effect model was applied.
Forest plots were generated to display the included studies for estimation of pooled OR (95% CI). Publication bias was assessed via Begg’s and Harbord’s tests. Sensitivity analysis was run to investigate the influence of each individual study on the overall meta-analysis summary estimate. Additionally, meta-regression analysis was conducted to explore the potential sources of heterogeneity related to age and BMI. Significant level was set at 0.05.
Second, dose Response meta-analysis was also performed to consider menarche age as continues variable and show the trend of risks according to the exposure dose. dose One-stage random-effect (for both intercept and slope coefficients) dose–response Linear and restricted cubic spline with the three selected knots models were fitted to detect the trend of risks considering menarche age 13 as reference age29.
Linear dose response model consider age at menarche as a continuous variable so the exponential of regression coefficient shows the linear trend. On the contrary, the cubic spline model considers a non-linear association between age at menarche and risk of adverse events. Based on the model represented by Harrell FE Jr.30.
Restricted cubic spline models with three knots (at percentiles 10, 50 and 90) defines as,
We consider menarche age 13 year as referent, so exp (B1) and exp (B2) showed the odds ratios of adverse events at age lower 13 and upper 13 year, respectively.
Results
Identification of literature
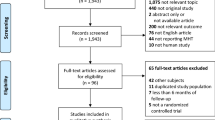
Through electronic searches, we identified 2299 unique articles. No additional studies were identified through manual searches or contact with authors. We excluded duplicates that appeared in multiple databases. Subsequently, we evaluated the titles and abstracts of the remaining articles, resulting in 58 articles for further evaluation. After screening the full texts of these articles to ensure they meet the eligibility criteria, we included 29 articles14,21, 31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 in the current review. The PRISMA diagram is presented in Fig. 1.
Characteristics of included studies
The main characteristics of the included studies are presented in Table 1. Of the 29 included articles, data were derived from two independent case–control studies31,32, one cross-sectional study37 and the remaining studies were prospective. In total, these studies, involving a total of 4,931,160 participants, focused on adult women aged 18 years or older.
Age at menarche was assessed using self-report questionnaire in all studies. The length of recall of age at menarche was heterogeneous and predominantly reported in middle age. The included studies were published between 2006 and 2023, covering a study period of almost 50 years (1976–2023). A total of seven studies were conducted in Europe (including Spain31, Italy32, UK14,21,46, Norway39, Netherlands53), five in the USA34,34,36,40, 47, one in Mexico38) and 16 in Asia, including (China33,37,42, 50,51,52,55, South Korea41,44,45,54,56, Japan43,49,57, Singapore48).
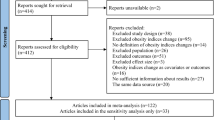
The quality appraisal of the included studies has been presented in Supplementary Tables 2–4. Among them, a total of 23 studies were judged as high quality14,21,33,34,35,36,38,39,40,41,42,43,44,46,47,48,49,50,51,53, 54,56,57; six were rated as moderate quality31,32,37,45,52,55; and none were considered low quality. As no study was assessed as low quality, all studies included in the final analysis. The results of Begg’s and Harbord’s tests suggested no publication bias, however lower number of studies should be considered (Table 2).
Result of meta-analysis
For the meta-analysis, we included studies21,31,32,33,34,35, 37,38,39,40,53,54,57 in which the classification of age at menarche was compatible with the following categories: ≤ 11 years, 12–13 years (reference group), 14–15 years, and ≥ 16 years.
Results of meta-analysis revealed a J-shaped association between age at menarche and the outcomes of CV events (Fig. 2), and women with an age at menarche of 12–13 years had a lower risk than other groups. Results are presented Table 2 and Fig. 3A–C.
Forest plot of pooled OR and 95% confidence interval (CI) of cardiovascular events (A) among women with age at menarche ≤ 11 years compared to those with age at menarche 12–13 years (B) among women with age at menarche 14–15 years compared to those with age at menarche 12–13 years (C) among women with age at menarche ≥ 16 years compared to those with age at menarche 12–13 years.
The pooled odds of CV mortality in age at menarche categories 14–15 years and ≥ 16 years were 37% (OR: 1.37, 95% CI 1.14–1.64, I2: 76.9%) and 64% (OR: 1.64, 95% CI 1.20—2.24, I2: 87%) higher than referent age at menarche 12–13 years. Although it was higher, but no statistically significant difference was found in the risk of CV mortality between individuals with age at menarche ≤ 11 years and those with age at menarche 12–13 years.
As well, the pooled odds of coronary heart disease in age at menarche ≥ 16 years were significantly 35% (OR: 1.35, 95% CI 1.03—1.76, I2: 95.4%) higher than among those with age at menarche 12–13 years. No statistically significant difference was found in the risk of coronary heart disease between individuals with age at menarche ≤ 11 years or 14–15 years and those with age at menarche 12–13 years.
Regarding the stroke, the pooled odds of stroke in age at menarche ≤ 11, 14–15 and ≥ 16 years were significantly 7% (OR: 1.07, 95% CI 1.04–1.11, I2: 22.7%), 24% (OR: 1.24, 95% CI 1.10–1.41, I2: 81.1%), 94% (OR: 1.94, 95% CI 1.39–2.70, I2: 96.9%), higher than among those with age at menarche 12–13 years, respectively.
Secondary, using dose response meta-analysis, we included all studies14,21,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57 used various classification of age at menarche. Results of dose response meta-analysis were also confirmed the obtained results. Considering AIC, one stage random effect Cubic spline model had better fit on data, showing J-shaped risk of CV events according to the menarche age considering 13 years as reference age (Supplementary Table 4 and Supplementary Fig. 3).
Results of restricted cubic spline estimated positive values of regression coefficients (B1 and B2) which interpreted that comparing age at menarche 13, lower and upper menarche age had an increasing trend of risk for a dose of 1 unit (menarche age). Compare to menarche age lower 13, odds ratio of stroke, CHD and CV mortality increase by 5% (OR: 1.05, 95% CI 0.94–1.11), 5% (OR: 1.05, 95% CI 0.97–1.14), and 7% (OR: 1.07, 95% CI 0.95–1.20), respectively. Age at menarche upper 13 year also showed the same increasing trend of risk of stroke, CHD and CV mortality increase by 4% (OR: 1.04, 95% CI 0.99–1.15), 2% (OR: 1.02, 95% CI 0.94–1.12), and 4% (OR: 1.04, 95% CI 0.94–1.16), respectively. The p-value for testing non-linearity (H0: β2 = 0) was significant for stroke, however for the rest of outcomes results showed lower power (Table 5-supplementary).
Results of meta-regression also showed that age and BMI at the time of recruitment of the study were not significant sources of heterogeneity (Supplementary Fig. 1). Results of sensitivity analyses showed that no single study essentially changed the pooled odds ratio of all outcomes (Supplementary Fig. 2). No publication bias was also detected (Table 2).
Discussion
We have provided quantitative estimates for the associations between age at menarche and cardiovascular events after adjusting for confounding factors of age and BMI through a systematic search and comprehensive meta-analysis. The results of meta-analysis revealed a J-shaped association between age at menarche and the outcomes of CV events. Women with an age at menarche of 12–13 years had a lower risk compared to other groups with younger (≤ 11 years) or older (14–15 years and ≥ 16 years) age at menarche. However, individuals with age at menarche of 16 years and older exhibited the highest risk of cardiovascular events. Subgroup analysis revealed similar J-shaped associations for specific CV events including stroke, coronary heart disease and CV mortality. Notably, the magnitude of the risks for CV mortality was stronger than that observed for coronary heart disease and stroke. This finding further supports that age at menarche may be an independent risk factor for CV events later in life.
Cardiovascular events continue to be a significant causes of mortality and morbidity among women58. In addition to some traditional risk factors such as diabetes mellitus, smoking and obesity, a number of clinical conditions exclusive to women have been demonstrated to elevate the risks of CV events. In this respect, there is evidence showing the association between women’s reproductive age including age at menopause and menarche and the subsequent risk of CV events22,47,59,60. Menopause marks the end of the reproductive period and is recognized as one of the important CVD risk factors among women61,62. Menarche, on the other hand, is a marker of puberty signifies the onset of ovarian and other endocrine functions relating to reproduction. Nevertheless, the results of studies focusing on the association between CV events and age at menarche were controversial, and the current meta-analysis study contributes to the clarification of conflicting results reported by previous studies.
Our findings suggest that both an age at menarche of less than 12 years and an age at menarche of later than 13 years may contribute to an increased risk of CV events. This trend appears to be more pronounced in individuals with an age at menarche of 16 years and older. Our findings are consistent with earlier studies that demonstrated the association between age at menarche and CV events. In agreement with this study22, Mishra et al. in a pooled analysis of individual patient data from 12 Studies, showed that short reproductive life span (< 33 years) was associated with an increased risk of CVD events in midlife. Women who had both a short reproductive life span and early menarche (age ≤ 11 years) had the most pronounced risk of CVD events. On the other hand, using the similar criteria for early and late menarche definition as in our study, they reported the U- shaped association between age at menarche and CVD, with a higher risk of CVD for both early menarche (age ≤ 11 years) and late menarche (age ≥ 15 years).
In a separate systematic review study, Luijken et al. (2017) reviewed the data on the association between age at menarche and different subtypes of CVD20. They noted that among eight studies involving Caucasian populations, an inverse linear relationship was consistently reported between age at menarche (AAM) and cardiovascular disease (CVD) risk. However, a significant U-shaped relationship was observed in a large-scale study (n = 1,200,000)21. However, data from Asian populations were characterized by inconclusive results regarding the association between AAM and CVD risk. It should be noted that using different criteria for early age at menarche may lead to discrepancy between studies. In another systematic review and meta-analysis study, Charalampopoulos et al. reported that while no significant association was observed between an earlier age at menarche and CV mortality (HR = 1.05 (95% CI 0.90, 1.21), however, each 1-year increase in age at menarche was associated with a 3% lower relative risk of total CV mortality18.
While the precise mechanisms underlying the association between earlier age at menarche and CV events in the future are not entirely elucidated, there are critically potentially mediating factors indicating that an earlier age at menarche is associated with increased risk of childhood and adulthood obesity, hypertension, and metabolic syndrome15,63,64,65,66. Furthermore, history of low birth weight, and rapid infancy growth67,68,69 are critical mediating factors for the relation of early menarche with risks of coronary and CV events. Besides, emerging evidence indicated that genetic components influencing both puberty timing and BMI could act as a shared genetic connection that might explain the relationship between the age at menarche and the risk of developing cardiovascular disease70. Recently, Ardissino M, et al.60, provided genetic evidence to support that earlier menarche are associated with higher risk of atrial fibrillation, coronary artery disease, heart failure, and stroke in women. Utilizing Mendelian randomization, the authors established a causal relationship between reproductive factors and cardiovascular diseases in the female population. They showed that earlier genetically predicted age at menarche increased risk of coronary artery disease (OR per year, 1.10, 95% CI 1.06–1.14) and heart failure (OR, 1.12, 95% CI 1.07–1.17); both associations were at least partly mediated by BMI.
The results of the study demonstrated that later age at menarche was associated with a higher risk of composite and subtypes of CV events. The higher risk of CV events in these women may be partly explained by shorter reproductive lifespan and subsequently shorter exposure to estrogen. In this respect, it is reported that estrogen, particularly estradiol (E2), acts as a mediator in CVD protection by promoting angiogenesis, vasodilation and decreasing reactive oxygen species, oxidative stress, and fibrosis71,72. In agreement with this finding, Mishra et al. in a systematic review and meta-analysis reported that a shorter reproductive lifespan was associated with a higher risk of CVD events, particularly stroke73. Consistent with our findings, they reported no evidence of an association between early age at menarche and CVD mortality (RR: 1.05, 95% CI 0.95, 1.14; heterogeneity I2 = 0.2%, p = 0.391). However, in contrast to our finding, they reported that early age at menarche was not significantly associated with a moderately higher risk of stroke events (RR: 1.17, 95% CI 0.20, 2.14; heterogeneity I2 = 69.6%, p = 0.070) (5, 28). We, on the other hand, found that the risk of stroke in individuals with age at menarche ≤ 11 years was significantly 8% higher (OR: 1.08, 95% CI 1.04–1.12, I2 = 0) than among those with age at menarche between 12 and 13 years. This discrepancy may be related to the fact that the definition of early age at menarche in these two meta-analyses differs. In our meta-analysis, we used the precise definition, where age at menarche was defined as being less than or equal to 11 years. In the Mishra study, they used the definitions of each individual study, including two studies that used age at menarche ≤ 1349 and ≤ 12 years44, which may have affected the final findings.
In the current meta-analysis, we found no publication bias. Meta-regression showed that the age and BMI of participants were not the source of heterogenicity. However, the subgroup analysis based on subtypes of CV events revealed decreased I2 among them, suggesting that the type of CV events contributed to those heterogeneities. Despite this, concerns remain regarding the lower power of meta-regression analysis and sample size.
In addition, ethnicity appears to play a significant role in determining the age of menarche. Generally, some studies have indicated that the natural mean age at menarche is higher in Asian populations when compared to Caucasian populations20. Furthermore,—African-American girls tend to experience menarche earlier than their Caucasian counterparts74,75. Nevertheless, our search did not yield relevant studies concerning the relationship between age at menarche and cardiovascular (CV) events within African and Middle-East Asian populations, which may exhibit different characteristics of the Western countries or East Asian populations. Hence, due to a lack of data, we could not perform such a sub-analysis based on ethnicity.
Our results may have potential public health implications. In this respect, data on the onset of menstruation as a potential risk factor for CVD may be valuable for intervention strategies targeting modifiable factors, aiming at improved CV health outcomes in the long-term.
Our study had some limitations. Despite conducting an extensive literature search, certain unpublished studies that, those written in languages other than English, or those presenting age at menarche as a continuous variable were not included. All of the original studies included relied on self-reported data for age at menarche, potentially introducing results with recall biases. Furthermore, the length of recall of age at menarche was heterogeneous.
However, to reduce recall bias, various strategies were implemented by separate included studies. These strategies included assessing the correlation with age at puberty, calculating the duration of reproductive years by subtracting the age at menarche from the age at menopause, restricting the analysis to women who consistently provided reports without hesitation or had documented gynecologic histories, and evaluating the accuracy of self-reported age at menarche. These approaches enhanced the validity and reliability of self-reported age at menarche. As such, numerous studies have indicated that self-reported age at menarche is sufficiently accurate for utilization in epidemiological investigations studies76,77. All the studies included had an observational framework, which implies that residual or unmeasured confounding might not have been entirely controlled. Despite the stringent inclusion criteria that led to the inclusion of a limited number of studies in this meta-analysis, the findings of our meta-analysis could give a better, more unbiased and professional impression and offering up-to-date evidence on this crucial topic. Moreover, it's important to highlight that the majority of included studies in the current meta-analysis were conducted with a population-based approach, as a representative of general population characteristics with minimizing the potential for selection bias. As a result, the outcomes of this study are applicable for general population extrapolation. It is worth mentioning that, for most studies with different study design we extracted the raw data and estimated the OR directly. However, there were some concerns regarding the overestimation or of pooled estimates of OR when it comes to cohort studies. Problems may arise, if the odds ratio is misinterpreted as a risk ratio or hazard ratio in cohort studies. For exposures that increase the chances of events, the odds ratio will be larger than the risk ratio, so the misinterpretation will tend to overestimate the exposure effect, especially when events are common (risks of events more than10%) For exposure that reduce the chances of events, the odds ratio will be smaller than the risk ratio, so that, again, misinterpretation overestimates the effect of the exposure78,79. There were 3 cohort studies with prevalence over 10% regarding the CV mortality outcome, although results of sensitivity analysis did not show strong influence of these studies. In this study, we have also explored a J-shape pattern for the risk of cardiovascular events by menarche age using a restricted random-effect cubic spline model with three knots, although based on the figures upper limit of 95% CI for cubic spline model showed the J-shaped pattern much better. This inconsistency might be a matter of sparse data especially in lower age 13 (stroke: 18 records lower 13 year out of 65 upper 13 year, CHD: 23 records out of 78 and CV mortality: 17 records out of 54), which model could not fit well and detect true pattern due to insufficient evidence. The p-value for testing non-linearity (H0: β2 = 0) was not significant for the outcomes except stroke, it might be due to the low power. In addition, Stone and Koo proved cubic spline functions have a drawback that behaved poorly in the tails, that is before the first knot and after the last knot. They cite advantages of constraining the function to be linear in the tails, called natural splines80.
Conclusion
In conclusion, we observed a J-shaped association between age at menarche and CV events. The risk was lowest for menarche at 12–13 years of age, increasing with younger (≤ 11 years) and older (14–15 years and ≥ 16 years) age at menarche. Individuals with age at menarche of 16 years and older exhibited the highest risk of CV events. This finding further supports that age at menarche may be an independent risk factor for CV events later in life. Future studies are warranted to confirm these findings and to explore the potential underlying mechanism linking CV events and onset of age at menarche.
Data availability
All data generated or analyzed during the present study are included in this published article.
References
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010 (2020).
Keteepe-Arachi, T. & Sharma, S. Cardiovascular disease in women: Understanding symptoms and risk factors. Eur. Cardiol. 12, 10–13. https://doi.org/10.15420/ecr.2016:32:1 (2017).
Woodward, M. Cardiovascular disease and the female disadvantage. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph16071165 (2019).
Vogel, B. et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 397, 2385–2438. https://doi.org/10.1016/s0140-6736(21)00684-x (2021).
Elder, P., Sharma, G., Gulati, M. & Michos, E. D. Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am. J. Prev. Cardiol. 2, 100028. https://doi.org/10.1016/j.ajpc.2020.100028 (2020).
Lee, H. S. Why should we be concerned about early menarche?. Clin. Exp. Pediatr. 64, 26–27. https://doi.org/10.3345/cep.2020.00521 (2021).
Gong, T. T., Wang, Y. L. & Ma, X. X. Age at menarche and endometrial cancer risk: A dose-response meta-analysis of prospective studies. Sci. Rep. 5, 14051. https://doi.org/10.1038/srep14051 (2015).
Fuhrman, B. J. et al. Association of the age at menarche with site-specific cancer risks in pooled data from nine cohorts. Cancer Res. 81, 2246–2255. https://doi.org/10.1158/0008-5472.Can-19-3093 (2021).
Janghorbani, M., Mansourian, M. & Hosseini, E. Systematic review and meta-analysis of age at menarche and risk of type 2 diabetes. Acta Diabetol. 51, 519–528. https://doi.org/10.1007/s00592-014-0579-x (2014).
Gaudino, R. et al. Delayed age at menarche in chronic respiratory diseases. Eur. J. Clin. Investig. 51, e13461. https://doi.org/10.1111/eci.13461 (2021).
Bhuiyan, A. R. et al. Timing of menarche related to carotid artery intima-media thickness in black and white young adult women: The Bogalusa Heart Study. Ann. Epidemiol. 25, 414–419. https://doi.org/10.1016/j.annepidem.2015.02.001 (2015).
Bleil, M. E. et al. Early life adversity and pubertal timing: Implications for cardiometabolic health. J. Pediatr. Psychol. 46, 36–48. https://doi.org/10.1093/jpepsy/jsaa082 (2021).
Chen, L. et al. Age at menarche and risk of hypertension in Chinese adult women: Results from a large representative nationwide population. J. Clin. Hypertens. (Greenwich) 23, 1615–1621. https://doi.org/10.1111/jch.14321 (2021).
Day, F. R., Elks, C. E., Murray, A., Ong, K. K. & Perry, J. R. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: The UK Biobank study. Sci. Rep. 5, 11208. https://doi.org/10.1038/srep11208 (2015).
Prentice, P. & Viner, R. M. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. Int. J. Obes. (Lond.) 37, 1036–1043. https://doi.org/10.1038/ijo.2012.177 (2013).
Bubach, S., Horta, B. L., Gonçalves, H. & Assunção, M. C. F. Early age at menarche and metabolic cardiovascular risk factors: Mediation by body composition in adulthood. Sci. Rep. 11, 148. https://doi.org/10.1038/s41598-020-80496-7 (2021).
Guo, L. et al. Age at menarche and prevention of hypertension through lifestyle in young Chinese adult women: Result from project ELEFANT. BMC Womens Health 18, 182. https://doi.org/10.1186/s12905-018-0677-y (2018).
Charalampopoulos, D., McLoughlin, A., Elks, C. E. & Ong, K. K. Age at menarche and risks of all-cause and cardiovascular death: A systematic review and meta-analysis. Am. J. Epidemiol. 180, 29–40. https://doi.org/10.1093/aje/kwu113 (2014).
Muka, T. et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: A systematic review and meta-analysis. JAMA Cardiol. 1, 767–776. https://doi.org/10.1001/jamacardio.2016.2415 (2016).
Luijken, J., van der Schouw, Y. T., Mensink, D. & Onland-Moret, N. C. Association between age at menarche and cardiovascular disease: A systematic review on risk and potential mechanisms. Maturitas 104, 96–116. https://doi.org/10.1016/j.maturitas.2017.07.009 (2017).
Canoy, D. et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation 131, 237–244. https://doi.org/10.1161/circulationaha.114.010070 (2015).
Mishra, S. R. et al. Association between reproductive life span and incident nonfatal cardiovascular disease: A pooled analysis of individual patient data from 12 studies. JAMA Cardiol. 5, 1410–1418. https://doi.org/10.1001/jamacardio.2020.4105 (2020).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Ramraj, B., Subramanian, V. & Vijayakrishnan, G. (2021).
Biro, F. M. et al. Age of menarche in a longitudinal US cohort. J. Pediatr. Adolesc. Gynecol. 31, 339–345. https://doi.org/10.1016/j.jpag.2018.05.002 (2018).
Leone, T. & Brown, L. J. Timing and determinants of age at menarche in low-income and middle-income countries. BMJ Glob. Health https://doi.org/10.1136/bmjgh-2020-003689 (2020).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. https://doi.org/10.1007/s10654-010-9491-z (2010).
Jewell, N. P. Statistics for Epidemiology (CRC Press, 2003).
Crippa, A., Discacciati, A., Bottai, M., Spiegelman, D. & Orsini, N. One-stage dose-response meta-analysis for aggregated data. Stat. Methods Med. Res. 28, 1579–1596. https://doi.org/10.1177/0962280218773122 (2019).
Harrell, F. E. Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. (Springer, New York, 2015).
Alonso de Leciñana, M. et al. Risk of ischemic stroke and lifetime estrogen exposure. Neurology 68, 33–38. https://doi.org/10.1212/01.wnl.0000250238.69938.f5 (2007).
Bertuccio, P., Tavani, A., Gallus, S., Negri, E. & La Vecchia, C. Menstrual and reproductive factors and risk of non-fatal acute myocardial infarction in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 134, 67–72. https://doi.org/10.1016/j.ejogrb.2007.01.005 (2007).
Hu, Z. B., Lu, Z. X. & Zhu, F. Age at menarche, age at menopause, reproductive years and risk of fatal stroke occurrence among Chinese women: The Guangzhou Biobank Cohort Study. BMC Womens Health 21, 433. https://doi.org/10.1186/s12905-021-01579-9 (2021).
Jacobsen, B. K., Oda, K., Knutsen, S. F. & Fraser, G. E. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: The Adventist Health Study, 1976–88. Int. J. Epidemiol. 38, 245–252. https://doi.org/10.1093/ije/dyn251 (2009).
Lee, J. J. et al. Age at menarche and risk of cardiovascular disease outcomes: Findings from the national heart lung and blood institute-sponsored women’s ischemia syndrome evaluation. J. Am. Heart Assoc. 8, e012406. https://doi.org/10.1161/jaha.119.012406 (2019).
Liang, Z. et al. Joint associations of actual age and genetically determined age at menarche with risk of mortality. JAMA Netw. Open 4, e2115297. https://doi.org/10.1001/jamanetworkopen.2021.15297 (2021).
Liu, G. et al. Association of age at menarche with obesity and hypertension among southwestern Chinese women: A new finding. Menopause 25, 546–553. https://doi.org/10.1097/gme.0000000000001027 (2018).
Lozano-Esparza, S. et al. Menarche characteristics in association with total and cause-specific mortality: A prospective cohort study of Mexican teachers. Ann. Epidemiol. 62, 59–65. https://doi.org/10.1016/j.annepidem.2021.06.007 (2021).
Lundblad, M. W. & Jacobsen, B. K. Is age at menarche associated with total mortality? The Tromsø Study. Int. J. Womens Health 10, 203–209. https://doi.org/10.2147/ijwh.S158706 (2018).
Zhang, X., Liu, L., Song, F., Song, Y. & Dai, H. Ages at menarche and menopause, and mortality among postmenopausal women. Maturitas 130, 50–56. https://doi.org/10.1016/j.maturitas.2019.10.009 (2019).
Chang, H. S., Odongua, N., Ohrr, H., Sull, J. W. & Nam, C. M. Reproductive risk factors for cardiovascular disease mortality among postmenopausal women in Korea: The Kangwha Cohort Study, 1985–2005. Menopause 18, 1205–1212. https://doi.org/10.1097/gme.0b013e31821adb43 (2011).
Chen, L. et al. Age at menarche and menopause, reproductive lifespan, and risk of cardiovascular events among chinese postmenopausal women: Results from a large national representative cohort study. Front. Cardiovasc. Med. 9, 870360. https://doi.org/10.3389/fcvm.2022.870360 (2022).
Cui, R. et al. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: The JACC study. J. Epidemiol. 16, 177–184. https://doi.org/10.2188/jea.16.177 (2006).
Jung, K. J., Kim, M. R., Yun, Y. D., Kim, H. C. & Jee, S. H. Duration of ovarian hormone exposure and atherosclerotic cardiovascular disease in Korean women: The Korean Heart Study. Menopause 23, 60–66. https://doi.org/10.1097/gme.0000000000000489 (2016).
Kim, H. L. et al. Reproductive factors predicting angiographic obstructive coronary artery disease: The Korean women’s chest pain registry (Korose). J. Womens Health (Larchmt) 25, 443–448. https://doi.org/10.1089/jwh.2015.5381 (2016).
Lakshman, R. et al. Early age at menarche associated with cardiovascular disease and mortality. J. Clin. Endocrinol. Metab. 94, 4953–4960. https://doi.org/10.1210/jc.2009-1789 (2009).
Ley, S. H. et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.117.006713 (2017).
Mueller, N. T. et al. Age at menarche and cardiovascular disease mortality in Singaporean Chinese women: The Singapore Chinese Health Study. Ann. Epidemiol. 22, 717–722. https://doi.org/10.1016/j.annepidem.2012.08.002 (2012).
Murakami, K. et al. Menstrual factors and stroke incidence in Japanese postmenopausal women: The Ohasama study. Neuroepidemiology 47, 109–116. https://doi.org/10.1159/000452220 (2016).
Wu, X. et al. Age at menarche and natural menopause and number of reproductive years in association with mortality: Results from a median follow-up of 11.2 years among 31,955 naturally menopausal Chinese women. PLoS One 9, e103673. https://doi.org/10.1371/journal.pone.0103673 (2014).
Yang, L. et al. Age at menarche and risk of major cardiovascular diseases: Evidence of birth cohort effects from a prospective study of 300,000 Chinese women. Int. J. Cardiol. 227, 497–502. https://doi.org/10.1016/j.ijcard.2016.10.115 (2017).
Zheng, Y., Zhang, G., Chen, Z. & Zeng, Q. Association between age at menarche and cardiovascular disease risk factors in China: A large population-based investigation. Cardioren. Med. 6, 307–316. https://doi.org/10.1159/000445506 (2016).
Zhu, F., Qi, H., Bos, M., Boersma, E. & Kavousi, M. Female reproductive factors and risk of new-onset heart failure: Findings From UK Biobank. JACC Heart Fail. 11, 1203–1212. https://doi.org/10.1016/j.jchf.2023.02.019 (2023).
Jeong, S. M. et al. Association of reproductive factors with cardiovascular disease risk in pre-menopausal women: Nationwide population-based cohort study. Eur. J. Prev. Cardiol. 30, 264–273. https://doi.org/10.1093/eurjpc/zwac265 (2023).
Sun, Z. et al. Association of age at menarche with valvular heart disease: An analysis based on electronic health record (CREAT2109). Front. Cardiovasc. Med. 10, 1029456. https://doi.org/10.3389/fcvm.2023.1029456 (2023).
Jeong, S. M. et al. Associations of reproductive factors with incidence of myocardial infarction and ischemic stroke in postmenopausal women: A cohort study. BMC Med. 21, 64. https://doi.org/10.1186/s12916-023-02757-2 (2023).
Ota, K. et al. Relationships between age at menarche and risk of cardiovascular disease mortality among Japanese Women: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. J. Atheroscler. Thromb. 30, 247–254. https://doi.org/10.5551/jat.63321 (2023).
Elgendy, I. Y., Mansoor, H. & Pepine, C. J. Reproductive lifespan and incident stroke risk among post-menopausal women: Is it time for sex-specific risk prediction tools?. Int. J. Cardiol. 328, 218–219. https://doi.org/10.1016/j.ijcard.2020.12.032 (2021).
Mansoor, H., Elgendy, I. Y., Segal, R. & Hartzema, A. Duration of reproductive years and the risk of cardiovascular and cerebrovascular events in older women: Insights from the National Health and Nutrition Examination Survey. J. Womens Health (Larchmt) 26, 1047–1052. https://doi.org/10.1089/jwh.2016.6013 (2017).
Ardissino, M. et al. Sex-specific reproductive factors augment cardiovascular disease risk in women: A Mendelian randomization study. J. Am. Heart Assoc. 12, e027933. https://doi.org/10.1161/jaha.122.027933 (2023).
Behboudi-Gandevani, S., Arntzen, E. C., Normann, B., Haugan, T. & Bidhendi-Yarandi, R. Cardiovascular events among women with premature ovarian insufficiency: A systematic review and meta-analysis. Rev. Cardiovasc. Med. 24, 193 (2023).
Yoshida, Y. et al. Early menopause and cardiovascular disease risk in women with or without type 2 diabetes: A pooled analysis of 9374 postmenopausal women. Diabetes Care 44, 2564–2572. https://doi.org/10.2337/dc21-1107 (2021).
Kim, Y. & Je, Y. Early menarche and risk of metabolic syndrome: A systematic review and meta-analysis. J. Womens Health (Larchmt) 28, 77–86. https://doi.org/10.1089/jwh.2018.6998 (2019).
Jung, H. et al. Relationship between age at menarche and metabolic diseases in Korean postmenopausal women: The Korea National Health and Nutrition Examination Survey 2016–2018. PLoS One 18, e0280929. https://doi.org/10.1371/journal.pone.0280929 (2023).
Elks, C. E. et al. Age at menarche and type 2 diabetes risk: The EPIC-InterAct study. Diabetes Care 36, 3526–3534. https://doi.org/10.2337/dc13-0446 (2013).
Bubach, S. et al. Early menarche and blood pressure in adulthood: Systematic review and meta-analysis. J. Public Health (Oxf) 40, 476–484. https://doi.org/10.1093/pubmed/fdx118 (2018).
Sloboda, D. M., Hart, R., Doherty, D. A., Pennell, C. E. & Hickey, M. Age at menarche: Influences of prenatal and postnatal growth. J. Clin. Endocrinol. Metab. 92, 46–50. https://doi.org/10.1210/jc.2006-1378 (2007).
Zhou, X. et al. Overweight/obesity in childhood and the risk of early puberty: A systematic review and meta-analysis. Front. Pediatr. 10, 795596. https://doi.org/10.3389/fped.2022.795596 (2022).
Juul, F., Chang, V. W., Brar, P. & Parekh, N. Birth weight, early life weight gain and age at menarche: A systematic review of longitudinal studies. Obes. Rev. 18, 1272–1288. https://doi.org/10.1111/obr.12587 (2017).
Elks, C. E. et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 42, 1077–1085. https://doi.org/10.1038/ng.714 (2010).
Iorga, A. et al. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 8, 33. https://doi.org/10.1186/s13293-017-0152-8 (2017).
Tokiwa, H., Ueda, K. & Takimoto, E. The emerging role of estrogen’s non-nuclear signaling in the cardiovascular disease. Front. Cardiovasc. Med. 10, 1127340. https://doi.org/10.3389/fcvm.2023.1127340 (2023).
Mishra, S. R., Chung, H. F., Waller, M. & Mishra, G. D. Duration of estrogen exposure during reproductive years, age at menarche and age at menopause, and risk of cardiovascular disease events, all-cause and cardiovascular mortality: A systematic review and meta-analysis. BJOG 128, 809–821. https://doi.org/10.1111/1471-0528.16524 (2021).
Reagan, P. B., Salsberry, P. J., Fang, M. Z., Gardner, W. P. & Pajer, K. African-American/white differences in the age of menarche: Accounting for the difference. Soc. Sci. Med. 75, 1263–1270. https://doi.org/10.1016/j.socscimed.2012.05.018 (2012).
Salsberry, P. J., Reagan, P. B. & Pajer, K. Growth differences by age of menarche in African American and White girls. Nurs. Res. 58, 382–390. https://doi.org/10.1097/NNR.0b013e3181b4b921 (2009).
Cooper, R. et al. Validity of age at menarche self-reported in adulthood. J. Epidemiol. Community Health 60, 993–997. https://doi.org/10.1136/jech.2005.043182 (2006).
Mao, Y. et al. Validity of self-reported age at menarche in computer-assisted interview among Chinese schoolgirls: A cross-sectional study. BMJ Open 8, e016799. https://doi.org/10.1136/bmjopen-2017-016799 (2018).
George, A., Stead, T. S. & Ganti, L. What’s the risk: Differentiating risk ratios, odds ratios, and hazard ratios?. Cureus 12, e10047. https://doi.org/10.7759/cureus.10047 (2020).
Viera, A. J. Odds ratios and risk ratios: What’s the difference and why does it matter?. South Med. J. 101, 730–734. https://doi.org/10.1097/SMJ.0b013e31817a7ee4 (2008).
Stone, C. J. & Koo, C.-Y. Additive splines in statistics. In Proc. of the American Statistical Association Original pagination is p 45, 48 (1985).
Acknowledgements
We are grateful for the contributions of Nord University library for helping us in the process of performing the study.
Funding
Open access funding provided by Nord University. Nord University covered the processing charge to this article.
Author information
Authors and Affiliations
Contributions
S.B.-G., R.B.Y. designed the study, S.B.G. and R.B.Y. gathered data, R.B.Y. performed the statistical analysis, C.F.M. and S.B.-G. performed quality appraisal. S.B.-G., C.F.M., I.S., E.C.A., R.B.Y. interpreted of the results and drafted the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behboudi-Gandevan, S., Moe, C.F., Skjesol, I. et al. The J shaped association of age at menarche and cardiovascular events: systematic review and meta-analysis. Sci Rep 14, 2695 (2024). https://doi.org/10.1038/s41598-024-53011-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53011-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.