Abstract
Tamoxifen (TAM) is one of the most successful treatments for breast cancer; however, TAM resistance continues to be a significant barrier. TAM resistance has been reported to be associated with increased expression of human telomerase reverse transcriptase (hTERT). This enzyme shares structural similarity with RNA-dependent RNA polymerase (RdRp) enzyme of RNA viruses, suggesting that RdRp inhibitors may also inhibit hTERT. Favipiravir (FAV) is an antiviral drug that inhibits RdRp of RNA viruses. Thus, we propose that FAV may also elicit an antitumor effect by suppressing hTERT. This study aimed to investigate the effect of FAV and TAM on TAM-resistant breast cancer (TAMR-1). The cell viabilities were determined. The levels of CDK1/ hTERT, in addition to regulators of hTERT-targeted signaling pathways were measured. Apoptosis, migration, and cell cycle distribution were also determined. Our data revealed that the combination of TAM and FAV suppressed cell proliferation synergistically (CI < 1) and resulted in a significant change in cell migration and apoptosis. Indeed, this was associated with reduced levels of hTERT and CDK1 and shift in the cell cycle distribution. Our findings suggest that the TAM/FAV combination exhibits synergistic effects against TAMR-1 human breast cancer cells by targeting hTERT.
Similar content being viewed by others
Introduction
Cancer is a complex disease marked by uncontrolled cell proliferation and the capacity to expand to other tissues. It is considered the second most likely cause of death and accounted for around 10 million deaths worldwide in 20191. Among all cancer types, breast cancer is the most common cancer in females, with an estimated 2.3 million new cases worldwide in 2020. Breast cancer is the fifth most prevalent cause of death due to cancer, with around 600,000 deaths worldwide in 20202. Patients being treated for cancer have a four-fold increased risk of viral infection and a ten-fold increased incidence of death3.
Endocrine therapy is crucial in breast cancer treatment. Appropriate treatment is implemented according to the type of hormone receptor expressed on the cell surface4. Tamoxifen (TAM) is considered the drug of choice for estrogen receptor-positive patients. TAM works by competing with estrogen for estrogen receptor binding in the breast tissue; thus, abolishing its consequent effects on tumor tissues5. Moreover, TAM is an inexpensive drug, which increased its prominent role. TAM has played a central role in saving many lives in addition to increasing the survival rate of patients with breast cancer around the globe6. Nevertheless, this therapy is limited by the development of TAM resistance and progression to metastasis. Around 20–30% of breast cancers are resistant to TAM after 3–5 years of treatment7; therefore, effective strategies are required to decrease TAM resistance. TAM resistance involves a number of signaling pathways, cell cycle regulators, growth factors, autophagy, and transcription factors that control estrogen receptor expression8,9,10. Interestingly, it has been reported that the suppression of telomerase rendered cells more sensitive to anticancer drugs regardless of their mode of action11,12,13. Telomerase is a ribonucleoprotein enzyme complex that prevents telomere shortening during cell division cycles. Human telomerase reverse transcriptase (hTERT) is a crucial catalytic member of the enzyme complex that adds DNA telomeric repeats to the ends of chromosomes to prevent telomere shortening14. Elevated levels of telomerase have been reported in 85% of human carcinomas and 90% of breast cancers13,14,15. hTERT has been found to be overexpressed in resistant cancer cells16,17. Cell immortality correlates with the maintenance of telomere length and the restoration of hTERT activity. Therefore, inhibiting the catalytic activity of hTERT in breast cancer cells may induce telomere shortening and thus block tumor cell proliferation18,19. Indeed, the combination of anticancer drugs with telomerase inhibitors has been found to effectively kill breast cancer cells and resensitize cells to anticancer agents13. Several telomerase inhibitors have been studied, including immunotherapeutic agents, antisense oligonucleotides, and hTERT inhibitors21. Although hTERT is an RNA-dependent DNA polymerase, recent 3-D structural analyses have revealed that the hTERT protein shares structural similarity with viral RNA-dependent RNA polymerases (RdRps), including the typical right-handed architecture21,22. Therefore, we propose that RdRp inhibitors may elicit a potent antitumor effect.
Recently, favipiravir (FAV), an RdRp inhibitor, has gained increased attention due to its use in several countries, including Japan, Italy, Russia, Turkey, and Egypt for the treatment of COVID-19 infection with emergency approval23,24. In addition, FAV is effective against a variety of RNA viruses, including Ebola, norovirus, respiratory syncytial virus, rhinovirus, and influenza26. In 2014, Japan licensed the use of FAV (brand-name, Avigan) for the treatment of avian flu and neuraminidase inhibitor-resistant influenza26,27. It is worth noting that various antiviral drugs have shown promise in the prevention and treatment of cancer29. Repurposing of antivirals for cancer treatment is based upon the fact that cancer patients, due to their immunosuppressant state, often receive antimicrobials for prophylaxis or treatment against different infectious agents in addition to their chemotherapeutics. Interestingly, cancer patients receiving these combined treatment regimens, have shown a higher survival rate and better outcome than patients receiving chemotherapy alone30. Hence, combining antiviral agents with anticancer drugs for combating tumor progression is becoming more attractive due to synergistic anticancer effects, decreasing the probability of resistance development and side effects.
Furthermore, cyclin-dependent kinase 1 (CDK1), a serine/threonine kinase, is responsible for the phosphorylation of hTERT which is substantial for hTERT-mediated RdRp activity and has been reported to be associated with tumor aggressiveness31.
Additionally, telomerase activity is also affected by tumor necrosis factor-α (TNF-α), a major inflammatory cytokine that is significantly expressed in resistant breast cancer32 by causing hTERT to bind to NF-B p65 to translocate from the cytoplasm to the nucleus, a crucial step in telomere elongation induction31,32. TNF-α regulates the mTOR pathway as assessed by the phosphorylation status of the mTOR substrate, 4E-BP133,34. mTOR mediates the phosphorylation of 4E-BP1, leading to the dissociation and release of eIF4E from 4E-BP1, enabling eIF4E to promote cap-dependent translation, resulting in increased protein synthesis of oncogenic mRNA36. Most cancer cells, including breast cancer cells, have increased eukaryotic translation initiation factor 4E (eIF4E) expression, which leads to the upregulation of cancer-promoting genes37 and overexpression of eukaryotic initiation factor 4E-binding protein (4E-BP1), which is linked to endocrine resistance and poor prognoses29.
The role of some antivirals such as zidovudine, abacavir and lamivudine in sensitization of cancer cell lines to radiation was previously reported38, yet the effect of FAV on cancer cells and its mechanism of action was not yet investigated. Therefore, the present study focuses on investigating for the first time the proposed role of FAV alone and its combination with TAM in inducing breast cancer cell death in TAM-resistant MCF-7 breast cancer cell lines (TAMR-1). The effect of TAM or FAV, and the combination of both drugs on the migratory potential, proliferative capacity, cell cycle, and apoptotic activities of MCF-7 and TAMR-1 cells was investigated. The levels of hTERT, in addition to hTERT-targeted pathways regulators; CDK-1, 4E-BP1, eIF4E, and TNF-α were measured in resistant and sensitive MCF-7 cells.
Results
Tamoxifen and favipiravir monotherapy decreased the viability of MCF-7 and TAMR-1 cancer cells while cotreatment potentiated the effects
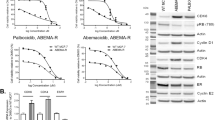
As a first step, the viability of the carcinoma cell lines in the presence of TAM or FAV or their combination was determined using sulforhodamine B (SRB) assay. It was observed that FAV and TAM had an inhibitory effect on MCF-7 and TAMR-1 breast cancer cells in a dose-dependent manner following 48 h of drug incubation. The average IC50 values of TAM and FAV in MCF-7 cells were 4.5 μM and 40 μM, respectively (Fig. 1a,b), whereas the average IC50 values of TAM and FAV in TAMR-1 cells were 51 μM and 40 μM, respectively (Fig. 1c,d). Although MCF-7 cells are more sensitive to TAM monotherapy than TAMR-1 cells, both cell lines showed the same sensitivity toward FAV with equal IC50 values of 40 μM.
The surviving fractions of MCF-7 and TAMR-1 cells with different concentrations of TAM (a,b) or FAV (c,d), or their combination (e,f), respectively, for 48 h. The values shown are the mean ± SD of two independent experiments performed in triplicate. The surviving fraction is the percentage of viable cells in comparison to the control. Surviving fraction = optical density (treated cells)/optical density (untreated cells).
In both cell lines, there was a significant decrease in viability in all combination-treated samples as compared to monotherapy (Fig. 1e,f). When TAM (50 μM) and FAV (50 μM) were combined to treat TAMR-1 cells, the inhibitory rate reached 78%, whereas treatment with TAM alone achieved an inhibitory rate of 48%. In MCF-7 cells, treatment with TAM (2.25 μM) in combination with FAV (100 μM) inhibited cell growth by approximately 16% when compared to TAM alone.
CompuSyn software was used to analyze the effect of the combination’s interaction to see whether combinations would have a synergistic effect and result in a greater reduction in cell growth than the single drugs. For both drugs, synergism equivalent to a combination index (CI) of < 1 invariably resulted in a favorable dose-reduction index of > 1 (see Supplementary Fig.*** S1 online). As a result, for the subsequent studies using TAMR-1 cells, a 50 μM FAV and 50 μM TAM combination regimen was chosen since it had the lowest CI (0.75) when compared to other combinations.
The effect of TAM (50 μM), FAV (50 μM), or their combination on the gene expression of hTERT and CDK1 in TAMR-1 cells
To investigate the putative suppressive role of FAV on hTERT expression and consequent synergistic effect in TAMR-1 cells, the gene expression levels of hTERT and CDK1 were assessed after treating cells with TAM or FAV or their combination. The gene expression levels of hTERT and CDK1 were found to be significantly higher in TAMR-1 cells than in MCF-7 cells (P < 0.0001) (Fig. 2), indicating that the upregulation of these proteins correlates with TAM resistance. Each drug and their combination significantly decreased hTERT and CDK1 expression when compared to the TAMR-1 control (P < 0.0001). Moreover, the combination significantly decreased the hTERT and CDK1 gene expression levels when compared to TAM monotherapy.
qPCR analysis for the expression levels of CDK1 (a) and hTERT (b) in MCF-7 and TAMR-1 cells. Data represented in the bar graphs are from three independent experiments. The statistical differences were investigated using a one-way ANOVA test followed by a Games Howell posthoc test. ***Significant at P < 0.0001. ****Significant at P < 0.00001.
The effect of TAM (50 μM), FAV (50 μM), or their combination on TNF-α, elF4E, and 4E-BP1 levels in TAMR-1 cells
Figure 3 shows that TAMR-1 cells expressed significantly higher levels of TNF-α, eIF4E, and 4E-BP1 compared to MCF-7 cells (P < 0.0001). In TAMR-1 cells, the levels of TNF-α, eIF4E, and 4E-BP1 significantly decreased after TAM/FAV monotherapy and the combined therapy compared to the untreated TAMR-1 cells. In addition, FAV treatment induced lower levels of TNF-α, eIF4E, and 4E-BP1 expression when compared to TAM. The combination significantly lowered the levels of TNF-α, eIF4E, and 4E-BP1 when compared to treatment with each drug alone (P < 0.0001). Interestingly, their levels in the combination-treated TAMR-1 cells did not differ when compared to the untreated MCF-7 cells.
TNF-α (a), eIF4E (b), and 4E-BP1 (c) levels in MCF-7 and TAMR-1 cells. Data represented in the bar graphs are from three independent experiments. One-way ANOVA test followed by Games Howell post hoc test was used to examine statistical differences. **Significant at P < 0.001. ***Significant at P < 0.0001.
The effect of TAM (50 μM), FAV (50 μM), or their combination on wound healing in TAMR-1 cells
The ability of TAMR-1 cells to migrate was examined using a wound healing test to see if FAV may improve TAM treatment and prevent TAMR-1 cell migration. Figure 4a illustrates TAMR-1 control cells at 0 h. When compared to the control at 48 h (Fig. 4b), TAM monotherapy significantly reduced migratory capability (Fig. 4c), while FAV monotherapy (Fig. 4d) had no significant effect on the wound healing ability of TAMR-1 cells (P > 0.05). Interestingly, the combination of FAV (50 μM) and TAM (50 μM) resulted in a substantial delay in wound healing when compared to FAV and TAM monotherapies (P < 0.0001) (Fig. 4e).
Wound healing assay of the treated TAMR-1 cells. Panel (a) represents the control at 0 h, (b) represents the control at 48 h. (c–e) represent TAM, FAV, and their combination at 48 h, respectively, at concentrations of 50 μM each. Panel (f) shows the bar graph quantifying the migration of the cells for each drug. Plotted points are the mean representation of the data ± SD. The statistical differences were investigated using a one-way ANOVA test followed by a Games Howell post hoc test. ***Significant at P < 0.0001.
The effect of TAM (50 μM), FAV (50 μM), or their combination on apoptosis induction in TAMR-1 cells
To investigate whether TAMR-1 cells undergo apoptosis upon treatment with tamoxifen, favipiravir, or their combination, cell staining with annexin V and propidium iodide (PI) was performed and cells were analyzed using flow cytometry. Induction of cell apoptosis of TAMR-1 cells treated with TAM (50 μM) or FAV (50 μM) monotherapy or their combination was observed at 48 h (Fig. 5). In comparison to the single-drug treatment groups, the number of late apoptotic and necrotic cells in the combination-treatment group was increased. The percentage of TAMR-1 cells that were found to be necrotic or apoptotic is shown in Fig. 5b. To confirm that combination therapy induces apoptosis in TAMR-1 cells, the levels of the proapoptotic protein, BAX, and the antiapoptotic protein, BCL-2, were measured (Fig. 5c). The Western blot results revealed that the expression level of BAX was significantly increased, while the expression of BCl-2 was decreased after treatment with the drug combination (P < 0.05). These outcomes matched the outcomes of the SRB test, suggesting that the addition of FAV to TAM has a synergistic effect on TAMR-1 cells.
The effect of TAM or FAV or their combination on TAMR-1 cell apoptosis. (a) TAMR-1 early apoptotic cells, late apoptotic cells, and necrotic cells were detected by flow cytometry at 48 h using annexin V/propidium iodide (PI). (b) The bar chart shows a higher proportion of late apoptotic and necrotic cells after combination therapy compared to monotherapy with either drug. Cell numbers (percentages) from four different quadrants are represented in the representative dot plots (UL; necrosis, UR; late apoptosis, LL; live, LR; early apoptosis). (c) The expression levels of BAX and BCL-2 in TAMR-1 cells using Western blot analysis. Results are expressed as mean ± standard deviation for three independent experiments. **Significant at P < 0.001. ***Significant at P < 0.0001. The original blots are presented in Supplementary Fig. S2.
The effect of TAM (50 μM), FAV (50 μM), or their combination on cell cycle distribution of TAMR-1 cells
The cell cycle distribution was assessed by flow cytometry after 48 h to further determine if the antiproliferative impact caused by TAM, FAV, or their combination is linked to cell cycle arrest. Figure 6 shows that there was a clear alteration in the distribution of different phases when cells were treated with TAM, FAV, or their combination. Remarkably, TAM arrested the cell cycle at the S (58.29%) and G2/M (4.40%) phases, while FAV arrested the cell cycle at the G0/G1 phase (97.06%). The combination of TAM and FAV significantly increased the percentage of cells accumulated at the G2/M phase (9.36%) compared to the control (0.28%) and each drug alone (TAM, 4.4%; FAV, 0.07%). When compared to the control group, TAM-treated cells showed a significant increase in cyclin D1 (CCND1) levels, while FAV treatment significantly decreased the CCND1 level. Interestingly, combining the drugs significantly increased the CCND1 level compared to the control but CCND1 showed a significant decrease compared to its corresponding level in TAM-treated cells. Moreover, the combination significantly decreased cyclin B1 (CCNB1) levels compared to the control and to each drug alone.
Flow cytometry and Western blot analysis of the effect of tamoxifen, favipiravir, or their combination on cell cycle regulation in TAMR-1 cells. (b) Flow cytometry results of PI-stained TAMR-1 cells showing the percentage of the cells in G0/G1 (purple), S (green), and G2/M (orange) phases (b) bar graphs representing cell cycle distribution percentages between the different drugs. (c) Expression levels of CCNB1 and CCND1 in TAMR-1 cells using Western blot analysis. Results are expressed as mean ± standard deviation for three independent experiments. **Significant at P < 0.001. ***Significant at P < 0.0001. The original blots are presented in Supplementary Fig. S3.
Discussion
Breast cancer is the most frequent cancer in females and the main cause of death from cancer2. TAM is one of the most chemotherapeutic medications used to treat breast cancer, however with time, cancer cells become resistant to TAM8,9,10,11. Some genes have been found to be associated with mechanisms of tamoxifen resistance39. Thus, exploration of more effective strategies is demanded to overcome resistance in breast cancer cells, improve TAM efficacy, and decrease undesirable side effects9,38,39,40. Diverse attempts are being investigated to prevail this impediment including novel therapeutic agents, immunotherapy, gene therapy, and combination therapy10,40,41,45. Nonetheless, clinical outcomes are still mediocre urging a thorough understanding of the underlying chemoresistance mechanisms, and improvement of treatment approaches.
A known resistance mechanism focuses on hTERT overexpression16,17. hTERT promotes cancer cell proliferation, angiogenesis, migration, and metastasis39,40. Elevated hTERT levels have been related to poor prognoses in solid tumors, including gastric, cervical, lung and breast cancer48. hTERT has previously been shown to have RdRp activity, which is required for tumor development42,43.
FAV, an RdRp inhibitor, is used against various RNA virus infections. In this study, FAV was combined with TAM due to its RdRp inhibitory effects and the structural similarity between hTERT and RdRp44,45. In addition, infection is more common in patients with cancer than in the normal population46,47. It has been reported that knockdown of hTERT inhibits cancer proliferation and attenuates resistance to radio- and chemotherapy16,48. Moreover, the phosphorylation of hTERT by CDK1 participates in cancer development31. CDK1 is crucial in cell cycle control and is considered as a key element of mitosis56. CDK1 inhibitors is an important target for cancer treatment and are already involved in some clinical trials51,52. Also inhibiting CDK1 has been reported to induce apoptosis selectively in MYC-dependent breast cancer cells59. According to our gene expression analysis, TAMR-1 cells had higher levels of hTERT and CDK1 than MCF-7 cells. Meanwhile, treatment with TAM, FAV, and their combination decreased the expression levels of hTERT and CDK1 in TAMR-1 cells significantly when compared to the TAMR-1 control. In addition, compared to the single agents, the medication combination significantly reduced the expression of CDK1 and hTERT. Interestingly, the combination restored hTERT and CDK-1 levels in TAMR-1 cells to those in MCF-7 cells. These findings are in agreement with a prior report stating that the downregulation of hTERT in MCF-7 cells rendered cells more sensitive to chemotherapeutic drugs17. Combining metformin and silibinin inhibited T47D breast cancer cells’ proliferation synergistically via inhibition of hTERT60. Moreover, a combination therapy of roscovitine, a pan-CDK-1 inhibitor, followed by doxorubicin enhanced doxorubicin efficiency and reduced toxicity in triple-negative breast cancer61.
The possible FAV-induced mechanisms underlying the downregulation of hTERT expression and synergistic potentiation of breast cancer cell death was also investigated in this study. hTERT was found to be regulated by wingless (Wnt), mammalian target of the rapamycin (mTOR), and mitogen-activated protein kinase (MAPK) signalling pathways54,55. TNF-α controls protein synthesis by activating MAPK and regulates telomerase activity by translocating hTERT protein coupled to NF-B p65 from the cytoplasm to the nucleus, a substantial step for inducing telomere elongation33. MAPK then causes the eukaryotic initiation factor, eIF4E, to be phosphorylated. Moreover, TNF-α regulates the mTOR pathway as assessed by the phosphorylation status of the mTOR substrate, 4E-BP133,34. mTOR mediates the phosphorylation of 4E-BP1, leading to the dissociation and release of eIF4E from 4E-BP1, enabling eIF4E to promote cap-dependent translation, resulting in increased protein synthesis of oncogenic mRNA36. Dependent on the tumor microenvironment, 4E-BP1 plays a regulatory role by selectively modulating the translation of particular transcripts that function as drivers of cancer cell proliferation and progression by adapting the tumor to metabolic and genotoxic stress64.
Moreover, Zhang et al.65 reported that high levels of TNF-α promote chemoresistance in breast cancer cells. 4E-BP1 has also been linked with poor prognoses and drug resistance in patients with cancer. Knockdown of 4E-BP1 in breast cancer cells has been found to result in substantial decreases in cell proliferation66. eIF4E is a known protumorigenic factor, with elevated expression or activity in a variety of malignancies. High expression of eIF4E indicates poorer recurrence-free survival levels67. Moreover, CDK1 was recently reported to phosphorylate 4E-BPI in the absence (or low levels) of mTOR kinase as an alternative method to maintain phosphorylation during mitosis68. Our data revealed that TAMR-1 cells showed significantly higher levels of TNF-α, eIF4E, and 4E-BP1 in comparison to MCF-7 cells. Moreover, the combination of TAM and FAV significantly lowered the levels of TNF-α, eIF4E, and 4E-BP1 when compared to each drug alone.
Our study demonstrates that a combination of TAM and FAV can synergistically inhibit cell proliferation and increase apoptosis in MCF-7 and TAMR-1 cells. TAM-treated TAMR-1 cells showed a higher IC50 than TAM-treated MCF-7 cells, whereas FAV showed a consistent IC50 across both cell lines. These results are in agreement with prior reports that demonstrated that TAM treatment inhibited cell growth and apoptosis in MCF-7 cells, and that breast tumors developed resistance to TAM8,60. Furthermore, it has been reported that FAV has an inhibitory effect on A549 lung cancer cells72. Our results showed that the percentage of apoptotic and necrotic cells in the combination-treated group were increased compared with the single-drug treatment group. TAM has been reported to induce apoptosis in MCF-7 cells3. Moreover, downregulating hTERT results in the induction of apoptosis and the suppression of cell viability in MCF-7 cells73. Two apoptotic pathways exist: the extrinsic and intrinsic pathways. The intrinsic pathway, also known as the mitochondrial pathway, is controlled by BCL-2 family proteins, including the proapoptotic protein, BAX, and the antiapoptotic protein, BCL-2. In cancer cells, BCL-2 is usually upregulated, inhibiting the proapoptotic BAX; therefore, inhibiting apoptosis63,64. Our data demonstrate that using a combined therapy of TAM and FAV promotes downregulation of BCL-2 antiapoptotic proteins and the upregulation of BAX proapoptotic proteins in TAMR-1 cells. The increased expression of BAX protein, associated with BCL-2 decrease, in cells incubated with combined doses of FAV and TAM illustrates that combination therapy ameliorates the BAX effect, leading to the induction of the intrinsic apoptosis pathway. Similarly, a previous study showed that combining TAM with histone deacetylase inhibitors in TAMR-1 cells induced apoptosis by downregulating BCL-2, suggesting that a high level of Bcl-2 is a key driver of TAM resistance62.
The distribution of the TAMR-1 cells in the cell cycle was determined for a better understanding of the mechanisms of TAM and FAV underlying cell growth inhibition. The analysis of flow cytometry data revealed that TAM and its combination with FAV caused cells to assemble at the S and G2/M phases, while simultaneously depleting G0–G1-phase cells. Conversely, FAV triggered cells to aggregate at the G0–G1 phase. The drugs inhibited the growth of MCF-7 breast cancer cells by delaying the cell cycle transition. However, different studies have found that TAM causes MCF-7 cells' cell cycle to stop at the G0–G1 stage65,66. Western blot analysis of CCND1 and CCNB1 showed that the drug combination led to a significant increase in CCND1 when compared to the control group, while its level was lower than in TAM-treated cells. It was previously reported that CCND1 overexpression is associated with better outcomes for patients with breast cancer but its overexpression is linked to TAM resistance78,79. CCND1 phosphorylates and inactivates retinoblastoma protein, allowing cells to progress from G1 phase to S phase79. High CCNB1 cyclin B1 expression is associated with poor survival in breast cancer80. This agrees with our results, which showed that the combination significantly decreased its levels.
Our data demonstrated a significant reduction in cell migration of TAMR-1 cells when combination treatment of TAM and FAV was implemented compared to each drug alone. This result was in accordance with a previous study that showed a slowdown in wound healing by TAM in MCF-7 cells in a dose-dependent manner81. Moreover, it was previously reported that hTERT increases cell migration and its regulation with miR-138-5p suppresses cell growth and migration82,83,84.
In conclusion, hTERT overexpression is a possible mechanism contributing to TAM therapy resistance. Our findings confirmed that FAV synergistically enhances the anticancer effect of TAM in TAMR-1 cells by downregulating hTERT, TNF-α, eIF4E, and 4E-BP1 expression. The combination of TAM and FAV elicits inhibition of proliferation and invasiveness as well as inducing apoptosis and a shift in the cell cycle distribution (Fig. 7). Thus, our results identified a new mechanism of action for FAV in TAM-resistant breast cancer cells, suggesting that combining FAV with TAM might be an effective therapeutic option to overcome endocrine resistance in breast cancer. Moreover, our data suggests that hTERT could be a potential therapeutic target for the treatment of TAM resistance in breast cancer.
Materials and methods
Drugs and chemicals
TAM was purchased from Amriya Pharm. Ind. (Alexandria, Egypt), and FAV was acquired from Liptis Pharmaceuticals (Cairo, Egypt). Both drugs were stored at room temperature and before use they were dissolved in RPMI-1640 medium.
Cancer cell lines
The American Type Culture Collection provided the MCF-7 human breast cancer cell line (Minnesota, USA.). The National Cancer Institute in Cairo, Egypt, used serial subculturing to keep cell lines alive. The human tumor cell lines have been grown and maintained in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The TAMR-1 breast cancer cell line was generated by subjecting the MCF-7 cell line to mounting concentrations of TAM over a one year period to induce resistance.
Cell culture and drug assays
In 75-cm2 flasks, cells were cultured in RPMI-1640 medium enriched with 10% FBS and antibiotics. Cells were subcultured to 70% confluence at 37 °C and 5% CO2. After aspirating the media, cells were incubated with fresh medium containing diverse concentrations of TAM or FAV or a mixture of the two, for 48 h at 37 °C and 5% CO2. Supernatants and cells were collected for different concentrations and the control. Cells were harvested with 0.25% trypsin–EDTA, lysed with lysis buffer, and kept at − 80 °C.
Sulforhodamine-B (SRB) (cytotoxicity) assay
MCF-7 and TAMR-1 cells were seeded at a concentration of 2 × 103 cells/well in 96-well plates with RPMI-1640 media, 10% FBS, and antibiotics. Plates were maintained at 37 °C in a 5% CO2 atmosphere overnight. Cells were then treated with different concentrations of TAM or FAV (0–200 μM) and incubated for 48 h. IC50 values were generated using GraphPad Prism 8.4.2. The combination regimen was designed using the IC50 and half IC50 of TAM and increasing doses of FAV (0–200 μM). The SRB assay was used to perform the cytotoxicity test. The optical density for each well was determined using ELISA microplate reader (Sunrise, Tecan, Germany). Control cells were incubated without the drug. Using CompuSyn software, we employed the CI approach85 to examine if the cytotoxic interactions of TAM and FAV were synergistic, additive, or antagonistic.
hTERT and CDK-1 gene expression
Total RNA was extracted from treatment and control samples using a total RNA purification kit (Jena Bioscience, Munich, Germany). A cDNA archive kit (Applied Biosystems, Foster City, California, USA) was used to transform RNA to complementary DNA. GoTaq PCR master mix (Promega Co., Madison, USA) was used for qPCR, which included 25 µL of master mix, 1 µL of forward and reverse primers, 1 µL of cDNA, and 0.25 µL of CXR reference dye then completed to a final volume of 50 µL. All analyses were conducted in triplicates on a 7500 qPCR system (Applied Biosystems, Foster City, CA, USA). The 2−ΔΔCt method was employed for data analysis. Outcome results were recorded as relative expression levels after being adjusted to GAPDH86. The primer sequences used were recorded in Table 1.
TNF-α, elF4E, and 4E-BP1 levels
The TNF-α level was measured in cell culture supernatants, while the 4E-BP1 and eIF4E protein levels were measured in the cell lysate. The TNF-α, 4E-BP1, and eIF4E levels in MCF-7 and TAMR-1 cells were estimated using human TNF-α ELISA kits (ab285312, Abcam, UK), human eIF4eBP1 ELISA kits (ab289651, Abcam), and human eIF4E ELISA kits (ab214564, Abcam), respectively. An ELISA reader (TP-Thermoplate Reader) was used to measure each parameter three times, following the manufacturer’s directions, at 450 nm. Total protein was measured, and the results were expressed as mean concentration per 1 mg of total protein ± standard deviation.
Cell migration
TAMR-1 cells were seeded onto a 6-well plate. Using a sterile pipette, a single scratch was made creating a cell-free area, then the plate was rinsed to remove excess cells. Afterwards, the medium in the wells was aspirated and replaced with medium containing TAM or FAV or their combination. Controls comprised untreated wells at 0 and 48 h. An inverted microscope (DFC290, Leica, Wetzlar, Germany) was used to capture the images to analyze the size of the formed gap for each well.
Annexin V-FITC/PI for detection of apoptosis by flow cytometry
Flow cytometry was performed to examine apoptosis and necrosis of human cancer cells. A FITC Annexin V kit was used to further investigate apoptosis. Before being washed with cold PBS, all aspirates were centrifuged for 5 min at 13,000 rpm at 4 °C. The cells were extracted and centrifuged for 5 min at 2500 rpm at 10 °C. The supernatant was discarded, and the pellet was rinsed with 1 mL PBS before centrifugation at 2500 rpm for 5 min at 10 °C. Pellets were incubated for 30 min in PBS (50 μL) with Annexin V-FITC and PI. After incubation, flow cytometry analysis was carried out in 300 μL of PBS containing incubated cells in a flow cytometer (BD Biosciences LSR II FACS, New Jersey, USA). Apoptotic cells release phosphatidylserine extracellularly, which is detectable by using annexin V-labeled fluorescence. TAM or FAV were added to TAMR-1 cells alone and in combination, with PI antibodies and annexin V. Early apoptotic cells express annexin + PI−, late apoptotic cells expressed annexin + PI+ and necrotic cells expressed annexin–PI+.
Cell cycle analysis
Flow cytometry was utilized to assess the cell cycle in TAMR-1 cells, which were treated with FAV or TAM or their combination. After treatment, cells were trypsinized and fixed using 70% (v/v) ethanol and left overnight at 4 °C. After being rinsed twice with PBS, fixed cells were resuspended in PBS (Beyotime, Jiangsu, China) containing 50 g/mL PI and 0.1 mg/mL RNaseA. Following 30 min of incubation at 37 °C in the dark, the cells were analyzed using a flow cytometer (BD Biosciences LSR II FACS, New Jersey, USA). The cell count was estimated in each stage of the cell cycle (G0/G1, S, and G2/M).
Western blot assay
Ice-cold lysis buffer (1% NP-40, 0.1% SDS, 0.1% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 10 mM Tris–HCl; pH 7.4) was added to the cells to obtain the cell lysate required. To separate the protein from the cell lysate, the proteins were subjected to electrophoresis on 12% SDS–polyacrylamide gels and electrophoretically blotted to Amersham Hybond P Western blotting membranes (GE10600021 Sigma, Sigma-Aldrich, MO, USA). After blocking with 5% skim milk, the membranes were probed with primary antibodies mouse anti-β-actin and CCND1 or CCNB1 or BAX or BCL-2 monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, California, USA) for 1 h followed by HRP-conjugated rabbit antimouse IgG or HRP-conjugated goat antirabbit IgG. Quantification of the protein-bound bands was performed by image analysis using the ChemiDoc MP imaging system (v3, Hercules, California, USA).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author.
References
Bray, F., Laversanne, M., Weiderpass, E. & Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127, 3029–3030 (2021).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries | Enhanced Reader, vol. 71 209–249 https://pubmed.ncbi.nlm.nih.gov/33538338/ (2021).
Rouhimoghadam, M., Safarian, S., Carroll, J. S., Sheibani, N. & Bidkhori, G. Tamoxifen-induced apoptosis of MCF-7 Cells via GPR30/PI3K/MAPKs interactions: Verification by ODE modeling and RNA sequencing. Front. Physiol. 9, 907 (2018).
Rugo, H. S. et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J. Clin. Oncol. 34, 3069–3103 (2016).
Jensen, E. V. & Jordan, V. C. The estrogen receptor: A model for molecular medicine. Clin. Cancer Res. 9, 1980–1989 (2003).
Hammond, M. E. H. et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer (Unabridged Version). Arch. Pathol. Lab. Med. 134, e48–e72 (2010).
Fan, W., Chang, J. & Fu, P. Endocrine therapy resistance in breast cancer: Current status, possible mechanisms and overcoming strategies. Future Med. Chem. 7, 1511 (2015).
Hartkopf, A. D., Grischke, E. M. & Brucker, S. Y. Endocrine-resistant breast cancer: Mechanisms and treatment. Breast Care 15, 347–354 (2020).
Yao, J., Deng, K., Huang, J., Zeng, R. & Zuo, J. Progress in the understanding of the mechanism of tamoxifen resistance in breast cancer. Front. Pharmacol. 11, 1848 (2020).
To, N. B., Nguyen, V., Truong, P., Keshawa Ediriweera, M. & Cho, S. K. Effects of combined pentadecanoic acid and tamoxifen treatment on tamoxifen resistance in MCF-7/SC breast cancer cells. Int. J. Mol. Sci. 23, 11340 (2022).
Jafri, M. A., Ansari, S. A., Alqahtani, M. H. & Shay, J. W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 8, 1–18 (2016).
Ward, R. J. & Autexier, C. Pharmacological telomerase inhibition can sensitize drug-resistant and drug-sensitive cells to chemotherapeutic treatment. Mol. Pharmacol. 68, 779–786 (2005).
Cerone, M. A., Londoño-Vallejo, J. A. & Autexier, C. Telomerase inhibition enhances the response to anticancer drug treatment in human breast cancer cells. Mol. Cancer Ther. 5, 1669–1675 (2006).
Shay, J. W. & Bacchetti, S. A survey of telomerase activity in human cancer. Eur. J. Cancer 33, 787–791 (1997).
Collins, K. & Mitchell, J. R. Telomerase in the human organism. Oncogene 21, 564–579 (2002).
Masutomi, K. et al. Telomerase maintains telomere structure in normal human cells. Cells 114, 241–253 (2003).
Romaniuk-Drapała, A. et al. Htert downregulation attenuates resistance to dox, impairs fak-mediated adhesion, and leads to autophagy induction in breast cancer cells. Cells 10, 867 (2021).
Sun, W., Han, W., Li, A. & Shi, Y. miR10a mediate tamoxifen resistance in ER-positive breast cancer cells through down-regulation of RFPL-3/hTERT. Res. Sq. https://doi.org/10.21203/rs.3.rs-819836/v1 (2021).
Jaiswal, R. K. & Yadava, P. K. Assessment of telomerase as drug target in breast cancer. J. Biosci. 45, 72 (2020).
Sugarman, E. T., Zhang, G. & Shay, J. W. In perspective: An update on telomere targeting in cancer. Mol. Carcinog. 58, 1581–1588 (2019).
White, L. K., Wright, W. E. & Shay, J. W. Telomerase inhibitors. Trends Biotechnol. 19, 114–120 (2001).
Nguyen, T. H. D. et al. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature 557, 190–195 (2018).
Jiang, J. et al. Structure of telomerase with telomeric DNA. Cell 173, 1179-1190.e13 (2018).
Peng, Q. et al. Structural basis of SARS-CoV-2 polymerase inhibition by favipiravir. Innovation 2, 100080 (2021).
Shannon, A. et al. Favipiravir strikes the SARS-CoV-2 at its Achilles heel, the RNA polymerase. BioRxiv https://doi.org/10.1101/2020.05.15.098731 (2020).
De Clercq, E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem. Asian J. 14, 3962–3968 (2019).
Wu, R. et al. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 6, 56 (2020).
Zaraket, H. & Saito, R. Japanese surveillance systems and treatment for influenza. Curr. Treat. Options Infect. Dis. 8, 311 (2016).
https://www.cancer.org/cancer/acs-medical-content-and-news-staff.html. Why People with Cancer Are More Likely to Get Infections. American Cancer Society https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/low-blood-counts/infections/why-people-with-cancer-are-at-risk.html (2020).
Pfab, C., Schnobrich, L., Eldnasoury, S., Gessner, A. & El-Najjar, N. Repurposing of antimicrobial agents for cancer therapy: What do we know?. Cancers (Basel) 13, 3193 (2021).
Yasukawa, M. et al. CDK1 dependent phosphorylation of hTERT contributes to cancer progression. Nat. Commun. 11, 1–18 (2020).
Cruceriu, D., Baldasici, O., Balacescu, O. & Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell Oncol. 43, 1–18 (2020).
Akiyama, M. et al. Nuclear factor-kappaB p65 mediates tumor necrosis factor alpha-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res. 63, 18–21 (2003).
Lang, C. H. & Frost, R. A. Glucocorticoids and TNFα interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol. Med. 12, 291–299 (2006).
Chakravarthy, R. et al. Role of the eIF4E binding protein 4E-BP1 in regulation of the sensitivity of human pancreatic cancer cells to TRAIL and celastrol-induced apoptosis. Biol. Cell 105, 414–429 (2013).
Williamson, D. L., Kimball, S. R. & Jefferson, L. S. Acute treatment with TNF-α attenuates insulin-stimulated protein synthesis in cultures of C2C12 myotubes through a MEK1-sensitive mechanism. Am. J. Physiol. Endocrinol. Metab. 289, 95–104 (2005).
Maracci, C. et al. The mTOR/4E-BP1/eIF4E signalling pathway as source of cancer drug targets. Curr. Med. Chem. 29, 1 (2022).
Chen, X. et al. Zidovudine, abacavir and lamivudine increase the radiosensitivity of human esophageal squamous cancer cell lines. Oncol. Rep. 36, 239–246 (2016).
Wang, X. & Wang, S. Identification of key genes involved in tamoxifen-resistant breast cancer using bioinformatics analysis. Transl. Cancer Res. 10, 5246 (2021).
Vaziri-Gohar, A., Zheng, Y. & Houston, K. D. IGF-1 receptor modulates FoxO1-mediated tamoxifen response in breast cancer cells. Mol. Cancer Res. 15, 489–497 (2017).
Liu, C., Mu, C., Li, Z. & Xu, L. Imazamethabenz inhibits human breast cancer cell proliferation, migration and invasion via combination with Pin1. Mol. Med. Rep. 15, 3210–3214 (2017).
Lü, M. et al. MicroRNA-320a sensitizes tamoxifen-resistant breast cancer cells to tamoxifen by targeting ARPP-19 and ERRγ*. Sci. Rep. 5, 1–10 (2015).
Kim, Y. J. et al. Knockdown of YAP/TAZ sensitizes tamoxifen-resistant MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 601, 73–78 (2022).
Mortensen, A. C. L., Mohajershojai, T., Hariri, M., Pettersson, M. & Spiegelberg, D. overcoming limitations of cisplatin therapy by additional treatment with the HSP90 inhibitor onalespib. Front. Oncol. 10, 532285 (2020).
Maqbool, S. N. et al. Overcoming tamoxifen resistance in oestrogen receptor-positive breast cancer using the novel thiosemicarbazone anti-cancer agent, DpC. Br. J. Pharmacol. 177, 2365–2380 (2020).
Liu, N. et al. hTERT promotes tumor angiogenesis by activating VEGF via interactions with the Sp1 transcription factor. Nucleic Acids Res. 44, 8693–8703 (2016).
Zhang, C. et al. Kmt2a promotes melanoma cell growth by targeting htert signaling pathway. Cell Death Dis. 8, e2940 (2017).
Wang, K. et al. The prognostic significance of hTERT overexpression in cancers. A systematic review and meta-analysis. Medicine 97, 11794. https://doi.org/10.1097/MD.0000000000011794 (2018).
Machitani, M. et al. RNA-dependent RNA polymerase, RdRP, a promising therapeutic target for cancer and potentially COVID-19. Cancer Sci. 111, 3976–3984 (2020).
Sasaki-Tanaka, R., Shibata, T., Okamoto, H., Moriyama, M. & Kanda, T. Favipiravir inhibits hepatitis A virus infection in human hepatocytes. Int. J. Mol. Sci. 23, 2631 (2022).
Shih, W. L., Fang, C. T. & Chen, P. J. Anti-viral treatment and cancer control. Recent Results Cancer Res. 193, 269–290 (2014).
Pathania, A. S. et al. COVID-19 and cancer comorbidity: Therapeutic opportunities and challenges. Theranostics 11, 731–753 (2020).
Lawson, J. S. & Glenn, W. K. Catching viral breast cancer. Infect. Agent Cancer 16, 1–11 (2021).
Jafari, A., Dadkhahfar, S. & Perseh, S. Considerations for interactions of drugs used for the treatment of COVID-19 with anti-cancer treatments. Crit. Rev. Oncol. Hematol. 151, 102982 (2020).
Barczak, W. et al. HTERT gene knockdown enhances response to radio- and chemotherapy in head and neck cancer cell lines through a DNA damage pathway modification***. Sci. Rep. 8, 1–16 (2018).
Malumbres, M. & Barbacid, M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 30, 630–641. https://doi.org/10.1016/j.tibs.2005.09.005 (2005).
Wu, C. X. et al. Blocking CDK1/PDK1/β-Catenin signaling by CDK1 inhibitor RO3306 increased the efficacy of sorafenib treatment by targeting cancer stem cells in a preclinical model of hepatocellular carcinoma. Theranostics 8, 3737–3750 (2018).
Ettl, T., Schulz, D. & Bauer, R. J. The renaissance of cyclin dependent kinase inhibitors. Cancers (Basel) 14, 293 (2022).
Kang, J., Sergio, C. M., Sutherland, R. L. & Musgrove, E. A. Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells. BMC Cancer 14, 32 (2014).
Chatran, M. et al. Synergistic anti-proliferative effects of metformin and silibinin combination on T47D breast cancer cells via hTERT and cyclin D1 inhibition. Drug Res. (Stuttg) 68, 710–716 (2018).
Jabbour-Leung, N. A. et al. Sequential combination therapy of CDK inhibition and doxorubicin is synthetically lethal in p53-mutant triple-negative breast cancer. Mol. Cancer Ther. 15, 593–607 (2016).
Raha, P., Thomas, S., Thurn, K. T., Park, J. & Munster, P. N. Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression. Breast Cancer Res. 17, 26 (2015).
Zhang, Y., Toh, L. L., Lau, P. & Wang, X. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/β-catenin pathway in human cancer. J. Biol. Chem. 287, 32494–32511 (2012).
Musa, J. et al. Eukaryotic initiation factor 4E-binding protein 1 (4E-BP1): A master regulator of mRNA translation involved in tumorigenesis. Oncogene 35, 4675–4688 (2016).
Zhang, Z. et al. Transmembrane TNF-alpha promotes chemoresistance in breast cancer cells. Oncogene 37, 3456–3470 (2018).
Rutkovsky, A. C. et al. Eukaryotic initiation factor 4E-binding protein as an oncogene in breast cancer. BMC Cancer 19, 1–15 (2019).
Huang, C. I. et al. eIF4E and 4EBP1 are prognostic markers of head and neck squamous cell carcinoma recurrence after definitive surgery and adjuvant radiotherapy. PLoS One 14, e0225537 (2019).
Shuda, M. et al. CDK1 substitutes for mTOR kinase to activate mitotic cap-dependent protein translation. Proc. Natl. Acad. Sci. USA 112, 5875–5882 (2015).
Kentsis, A., Topisirovic, I., Culjkovic, B., Shao, L. & Borden, K. L. B. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc. Natl. Acad. Sci. USA 101, 18105–18110 (2004).
Pettersson, F. et al. Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B-type breast cancer. Clin. Cancer Res. 17, 2874–2884 (2011).
Perry, R. R., Kang, Y. & Greaves, B. Effects of tamoxifen on growth and apoptosis of estrogen-dependent and -independent human breast cancer cells. Ann. Surg. Oncol. 2, 238–245 (1995).
Karakus, A. et al. In vitro cytotoxic effects of some Covid-19 drugs on lung cancer cells. Trak. Univ. J. Nat. Sci. 22, 173–177 (2021).
Mittal, A., Pate, M. S., Wylie, R. C., Tollefsbol, T. O. & Katiyar, S. K. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. Int. J. Oncol. 24, 703–710 (2004).
Letai, A. G. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat. Rev. Cancer 8, 121–132 (2008).
Brahmbhatt, H., Oppermann, S., Osterlund, E. J., Leber, B. & Andrews, D. W. Molecular pathways: Leveraging the BCL-2 interactome to kill cancer cells-mitochondrial outer membrane permeabilization and beyond. Clin. Cancer Res. 21, 2671–2676 (2015).
Osborne, C. K., Boldt, D. H., Clark, G. M. & Trennt, J. M. Effects of tamoxifen on human breast cancer cell cycle kinetics: Accumulation of cells in early G1 phase. Cancer Res. 43, 3583–3585 (1983).
Otto, A. M., Paddenberg, R., Schubert, S. & Mannherz, H. G. Cell-cycle arrest, micronucleus formation, and cell death in growth inhibition of MCF-7 breast cancer cells by tamoxifen and cisplatin. J. Cancer Res. Clin. Oncol. 122, 603–612 (1996).
Kilker, R. L. & Planas-Silva, M. D. Cyclin D1 is necessary for tamoxifen-induced cell cycle progression in human breast cancer cells. Cancer Res. 66, 11478–11484 (2006).
Stendahl, M. et al. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br. J. Cancer 90, 1942–1948 (2004).
Aaltonen, K. et al. High cyclin B1 expression is associated with poor survival in breast cancer. Br. J. Cancer 100, 1055–1060 (2009).
Li, W. et al. Tamoxifen promotes apoptosis and inhibits invasion in estrogen-positive breast cancer MCF-7 cells. Mol. Med. Rep. 16, 478–484 (2017).
Gong, C. et al. hTERT promotes CRC proliferation and migration by recruiting YBX1 to increase NRF2 expression. Front. Cell Dev. Biol. 9, 1136 (2021).
Liu, H. et al. HTERT promotes cell adhesion and migration independent of telomerase activity. Sci. Rep. 6, 22886 (2016).
Tarazón, E. et al. Mir-138-5p suppresses cell growth and migration in melanoma by targeting telomerase reverse transcriptase. Genes (Basel) 12, 1931 (2021).
Chou, T. C. Preclinical versus clinical drug combination studies. Leuk. Lymphoma 49, 2059–2080 (2008).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408 (2001).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization and design of this work, S.A.F. and R.I.M.; methodology, S.A.F. and Y.A.E.; software, S.A.F. and Y.A.E.; validation, S.A.F. and R.I.M.; data curation, S.A.F. and R.I.M.; writing—original draft preparation, S.A.F., R.I.M., and Y.A.E.; writing—review and editing, S.A.F. and R.I.M.; supervision, S.A.F. and R.I.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fahim, S.A., ElZohairy, Y.A. & Moustafa, R.I. Favipiravir, an antiviral drug, in combination with tamoxifen exerts synergistic effect in tamoxifen-resistant breast cancer cells via hTERT inhibition. Sci Rep 14, 1844 (2024). https://doi.org/10.1038/s41598-024-51977-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51977-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.