Abstract
We present a single-center retrospective analysis of 228 Japanese patients with peritoneal dialysis, in which we examined whether reduced left ventricular ejection fraction (LVEF) is a risk factor for peritonitis development. Time-dependent multivariable-adjusted Cox proportional hazards models revealed that reduced LVEF (LVEF < 50% vs. preserved LVEF ≥ 50%, hazard ratio (HR) 2.10; 95% confidence interval (CI) 1.16–3.82) was associated with peritonitis. Qualitatively, similar associations with reduced LVEF (< 50%) were observed for enteric peritonitis (adjusted HR 7.68; 95% CI 2.51–23.5) but not for non-enteric peritonitis (adjusted HR 1.15; 95% CI 0.54–2.44). Reduced LVEF is associated with a significantly higher risk of subsequent peritonitis, particularly enteric peritonitis. These results indicate that patients with reduced LVEF may be at risk of enteric peritonitis from bowel sources caused by intestinal involvement due to cardiac dysfunction.
Similar content being viewed by others
Introduction
Peritonitis is a serious complication of peritoneal dialysis (PD) that is associated with significant morbidity, catheter loss, transfer to hemodialysis, transient loss of ultrafiltration, permanent membrane damage, and occasionally death1,2,3,4. Various strategies have been suggested to reduce the risk of peritonitis. However, PD-related peritonitis rates have not adequately improved2,3,4.
Hypokalemia, constipation, and usage of anti-gastric acid agents (H2 receptor antagonists [H2RA] or proton pump inhibitor [PPI]) are some of the previously reported risk factors for PD-related peritonitis5, which can cause impairment of intestinal movement, alterations in the intestinal microbiota, and bacterial translocation, leading to the development of peritonitis6,7,8,9,10. Cardiovascular dysfunction, a major complication in patients undergoing dialysis, is reportedly common in such patients11,12 and is associated with a higher incidence of mortality and hospitalization13,14,15,16,17,18,19. Left ventricular (LV) dysfunction can directly lead to cardiac failure and is strongly associated with poor survival in patients undergoing dialysis with constant hypervolemia20,21.
Hypervolemia due to heart failure impacts the gastrointestinal system by inducing hemodynamic changes affecting the gut morphology, function, and permeability22,23,24. However, no previous studies have assessed the relationship between heart failure and PD-related peritonitis. Therefore, in the present study, we aimed to assess whether patients with PD and reduced left ventricular ejection fraction (LVEF) are vulnerable to developing PD-related peritonitis.
The results of the present study may provide useful clinical information for identifying patients with PD who are at high risk of developing peritonitis by examining their LV function.
Methods
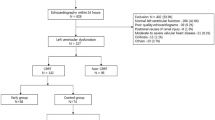
This study included patients aged ≥ 20 years who began PD as renal replacement therapy between January 1997 and December 2017 at Narita Memorial Hospital. Ultrasound echocardiography (UCG) was routinely performed within one month of PD initiation. Among 252 patients, 24 (9.5%) were excluded because of missing UCG data and clinically relevant information, and 228 patients with PD (90.5%) were finally included in the analysis (Fig. 1).
Ethics
The study protocol was approved by the Ethics Committee of the Narita Memorial Hospital (Approval Number 29-12-01). The study was conducted in accordance with Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects enacted by the Ministry of Health, Labour and Welfare of Japan [https://www.mhlw.go.jp/content/001077424.pdf]. Due to the retrospective nature of the study, the need for patients’ informed consent was waived by the Ethics Committee of the Narita Memorial Hospital.
Measurements
The design of the present study has been described in detail in a previous report25. Briefly, baseline characteristics at the start of PD included age, sex, body mass index (BMI), previous atherothrombotic events (coronary heart disease, heart failure, stroke, aortic aneurism and/or peripheral vascular disease requiring intervention or hospital admission), comorbidities (hypertension and diabetes mellitus), cause of kidney disease (diabetic nephropathy, glomerulonephritis, and renal sclerosis), laboratory data (hemoglobin, serum albumin, serum potassium, C-reactive protein, brain natriuretic peptide (BNP) level, and estimated glomerular filtration rate [eGFR], estimated using the equation recently generated by the Japanese Society of Nephrology: eGFR [mL/min/1.73 m2] = 194 × Scr−1.094 × Age−0.287 × 0.739 [if female]26), urine volume (mL/day), peritoneal transport characteristics (dialysate/plasma ratio of creatinine at 240 min during peritoneal equilibration test), daily peritoneal ultrafiltration rate (calculated as the difference between the volume of total dialysate infused and volume drained over 24 h), domestic pets, smoking, constipation (defined as a state of using laxatives), and usage of PPI or H2RA, as previously reported27, cardiothoracic ratio on chest X-ray, and findings of ultrasonic echocardiography. Furthermore, the follow-up data on BMI and urine volume (mL/day) were collected every 12 months.
PD-related peritonitis was diagnosed when at least two of the following conditions were met: (1) abdominal pain and/or cloudy dialysis effluent, which are clinical features of peritonitis; (2) dialysis effluent white cell count > 100/µL or > 0.1 × 109/L (after a dwell time of at least 2 h), with > 50% polymorphonuclear leukocytes; (3) positive dialysis effluent culture5.
The anonymized data set is shown in Table S1.
Echocardiography
Ultrasonic echocardiography was performed according to the American Society of Echocardiography recommendations. LVEF was measured using the modified Sympson method28. We stratified the patients into two LVEF groups, i.e., reduced LVEF group (LVEF < 50%), and preserved LVEF group (LVEF ≥ 50%), as reported previously29,30. LV mass was calculated using the formula recommended by the American Society of Echocardiography28, and indexed based on the body surface area. The diameters of the inferior vena cava (IVC) were measured at approximately 3 cm before merging with the right atrium at end expiration (IVC max) and at inspiration with sniffing (IVC min)31. The collapsibility of the IVC (IVCC) was calculated as IVCmax minus IVCmin divided by IVCmax.
Exposure and outcomes
The primary exposure of interest was LVEF at baseline and the first episode of peritonitis from any cause was the primary outcome of interest. Patients were followed up until the first episode of peritonitis, or censoring events such as loss to follow-up, death (cardiovascular disease, malignancy, infection, and others), PD withdrawal, or end of the follow-up for this study, whichever happened earlier.
Furthermore, we classified peritonitis into “enteric” and “non-enteric” peritonitis corresponding to previous reports32,33. Specifically, we defined enteric peritonitis as being caused by enteric organisms such as enteric bacilli (Escherichia, Klebsiella, Serratia, Proteus, etc.) and enterococcus (Enterococcus faecalis, Enterococcus faecium, etc.)34. We defined other peritonitis cases as non-enteric peritonitis. Incident enteric and non-enteric peritonitis were defined as secondary outcomes.
Additionally, outcomes including PD withdrawal and its causes (PD-related peritonitis, inadequate solute clearance, impairment of activities of daily living, fluid overload, and kidney transplantation) were obtained.
Statistics
Differences in clinical characteristics and outcomes according to the LVEF groups (reduced LVEF (< 50%) and preserved LVEF (≥ 50%)) were compared using the Wilcoxon rank-sum test or Fisher’s exact test.
To identify predictors independently associated with the outcome, we examined potential confounding factors that have previously been reported as clinically important risks for PD-related peritonitis occurrence5 by using unadjusted and time-dependent multivariable-adjusted Cox proportional hazard (CPH) regression models. The models were adjusted for the following potential confounders: baseline data, including age (years), sex, diabetes mellitus, constipation, serum albumin (g/dL), serum potassium (mEq/L), use of PPI, daily ultrafiltration rate (mL), reduced LVEF (< 50%); and follow-up data, including BMI and urine volume (mL/day) at every 12 months.
Furthermore, we employed a stratified analysis to account for each potential confounder, including age, sex, constipation, use of PPI, diabetes mellitus, serum albumin, serum potassium, BMI, urine output, and daily ultrafiltration rate, with reduced LVEF as the exposure of interest. We constructed a forest plot to demonstrate the hazard ratio (HR) for the development of enteric peritonitis in each stratum.
To elucidate the dose-dependent association between LVEF and incidence of peritonitis, restricted cubic spline functions with three knots placed at the 10th, 50th, and 90th percentiles of LVEF were used. Furthermore, we conducted a similar analysis after classifying patients into the enteric and non-enteric peritonitis groups.
The proportional hazard assumption for covariates was tested using scaled Schoenfeld residuals. The cumulative probability for the occurrence of the first episode of peritonitis from any cause, enteric and non-enteric, was calculated using the Kaplan–Meier method and log-rank test.
Continuous variables are expressed as the medians and interquartile ranges, while categorical variables are expressed as numbers and proportions. Significance was set at P < 0.05. Statistical analyses were conducted using the Stata software (version 15.0; StataCorp LP, College Station, TX, USA) and JMP software version 14.0.0 (SAS Institute, Cary, NC, USA).
Results
Study participants and clinical characteristics
This study included 228 PD patients, including 30 (13.2%) patients in the reduced LVEF group (LVEF < 50%) and 198 (86.8%) patients in the preserved LVEF group (LVEF ≥ 50%). The baseline characteristics of the two groups are summarized in Table 1. The reduced LVEF group had a higher proportion of patients with a previous history of coronary heart disease and heart failure, higher BNP, cardiothoracic ratio on chest X-ray, LV mass index, LV end-diastolic dimension, LV end-systolic dimension, and IVC (max and min) on echocardiography than the preserved LVEF group. Conversely, the IVCC in patients with reduced LVEF was lower than that in patients with preserved LVEF. Other baseline factors did not differ significantly between the two groups.
Outcome data
Peritonitis from any cause (primary outcome)
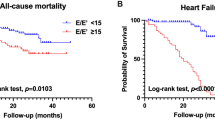
During the follow-up period (median, 36 months; interquartile range, 19–57 months), 17 (56.7%) and 67 (33.8%) patients in the reduced and preserved LVEF groups, respectively, developed peritonitis at least once (Table 2). The incidence of peritonitis was 0.25 and 0.12 person-year in the reduced LVEF and preserved LVEF groups, respectively. The cumulative probabilities of the first episode of peritonitis at 1, 3, and 5 years were 0.27, 0.45, and 0.60, respectively, in the reduced LVEF group, and 0.15, 0.30, and 0.48, respectively, in the preserved LVEF group; this indicated that the reduced LVEF group had a higher risk of developing peritonitis than the preserved LVEF group (log-rank test: P = 0.011; Fig. 2a). In the unadjusted models, diabetes mellitus, lower serum albumin levels, PPI use, and reduced LVEF (LVEF < 50%) were significantly associated with the first episode of peritonitis (Table 3). Time-dependent multivariable-adjusted CPH models further showed that PPI use (HR 1.85; 95% confidence interval [CI] 1.19–2.89), and reduced LVEF (vs. preserved LVEF; HR 2.10; 95% CI 1.16–3.82) were associated with peritonitis (Table 3). A multivariable-adjusted restricted cubic spline model confirmed the nonlinear association between LVEF and incidence of peritonitis (Fig. 3a), suggesting that reduced LVEF was associated with a higher risk of peritonitis.
Restricted cubic spline curve for the association of all-cause peritonitis (a), enteric peritonitis (b), and non- enteric peritonitis (c), adjusted for age (years), sex, diabetes mellitus, constipation, serum albumin (g/dL), serum potassium (mEq/L), use of PPI, and reduced LVEF (< 50%) as covariate. PPI, proton pump inhibitor; LVEF, left ventricular ejection fraction.
Enteric and non-enteric peritonitis (secondary outcomes)
During the follow-up period, 17 (56.7%) and 67 (33.8%) patients developed incident enteric and non-enteric peritonitis, respectively. Among the 17 patients with enteric peritonitis, the proportion of patients with enteric peritonitis in the reduced LVEF group was higher than that of the preserved LVEF group (Table 2).
The pattern of association between LVEF groups and the incidence of enteric peritonitis was qualitatively similar to that with peritonitis from any cause; that is, compared with patients in the preserved LVEF group, those in the reduced LVEF group had a higher cumulative probability of developing enteric peritonitis (log-rank test: P < 0.001; Fig. 2b). Furthermore, unadjusted and time-dependent multivariable-adjusted CPH models demonstrated that the reduced LVEF group was significantly associated with the occurrence of enteric peritonitis (adjusted HR 7.68; 95% CI 2.51–23.5, Table 4). A nonlinear association between LVEF and enteric peritonitis was also verified in a multivariable-adjusted restricted cubic spline model (Fig. 3b), suggesting that reduced LVEF was associated with a higher risk of enteric peritonitis.
In contrast, no significant association was observed between LVEF and non-enteric peritonitis. No significant difference was observed in the cumulative incidence of non-enteric peritonitis between the two groups with respect to LVEF (log-rank test: P = 0.796, Fig. 2c). Unadjusted and time-dependent multivariable-adjusted CPH models showed no significant association between LVEF groups and non-enteric peritonitis (adjusted HR 1.15; 95% CI 0.54–2.44, Table 4). Additionally, no significant association was found between LVEF and non-enteric peritonitis in the multivariable-adjusted restricted cubic spline model (Fig. 3c).
Furthermore, a forest plot demonstrating the HR for the development of enteric peritonitis in each potential confounder indicated similar associations throughout, except for age, sex, and daily ultrafiltration rate (Supplementary Fig. S1).
PD withdrawal
A total of 22 (73.3%) and 145 (73.3%) patients in the reduced and preserved LVEF groups, respectively, withdrew from PD. The reasons for PD withdrawal, such as mortality events of all causes, indicating that the cause of death, were not significantly different between the LVEF groups (Table S2).
Discussion
This study showed that reduced LVEF was significantly associated with the development of PD-related peritonitis. In particular, a significant association was observed with the development of enteric peritonitis and not with non-enteric peritonitis. These results suggest that patients with reduced LVEF may be at risk of developing enteric peritonitis caused by intestinal conditions triggered by cardiac dysfunction, providing clinically useful information for physicians to cautiously monitor peritonitis caused by enteric microorganisms in patients with cardiac dysfunction. To our knowledge, this is the first study to evaluate the association between cardiac function and PD-related peritonitis.
Among the previously reported modifiable risk factors for PD peritonitis6,35, gastrointestinal conditions, such as constipation7, and hypokalemia6,8,9,10, have been reported to be associated with peritonitis due to enteric organisms. Furthermore, emerging data suggests that gastric acid suppression, particularly with H2RA, is a modifiable risk factor for enteric peritonitis in patients undergoing PD, although the risk of peritonitis associated with PPI is sporadically reported25,36. Several mechanisms have been speculated to foster peritonitis in PD, including induction of gastrointestinal dysmotility37 and intestinal bacterial overgrowth38. Consequently, the translocation of bacteria from the intestine to the peritoneal cavity may cause peritonitis. This mechanism is similar to that of spontaneous bacterial peritonitis in cases of liver cirrhosis39.
Therefore, it is important to detect gastrointestinal conditions that may increase vulnerability for development of peritonitis; however, this has not been entirely evaluated.
One retrospective single-center study, which included 580 patients with PD, showed an association between overhydration, as measured by bioimpedance, and a higher incidence of peritonitis and infections from enteric organisms40. Although the results were comparable with those of the present study, the previous study did not evaluate the relationship between echocardiographic cardiac dysfunction and peritonitis.
Previous reports have shown that patients with heart failure experience alterations in the morphology, function, and bacterial flora of the intestine19 through the following pathophysiological mechanisms: increased venous pressure imposes relative ischemia on the intestinal microvilli leaving enterocytes at the villus tip susceptible to ischemic injury. Moreover, ischemic conditions in the intestine may cause a reduced barrier function and the translocation of potentially pathogenic microorganisms, and visceral congestion and the generation of relatively ischemic conditions may cause environmental alterations in the bacterial microbiome of the intestinal lumen41,42,43. Intestinal overgrowth of pathogenic bacteria and increased intestinal permeability may also occur. Given the pathogenic gut flora and increased intestinal permeability, we consider that cardiac dysfunction may be a risk factor for the development of PD-related peritonitis. Previously, there have been three case reports regarding acute peritonitis in patients not undergoing dialysis complicated with heart failure44,45,46, which supported our hypothesis.
Although our study included clinically stable patients (not presenting with overt volume overload status, or dyspnea due to heart failure), while performing UCG, patients with reduced LVEF may have demonstrated a constant hypervolemic status because the patients with reduced LVEF showed a higher cardiothoracic ratio on chest radiography, BNP, IVC, and lower IVCC, which are considered surrogate findings for fluid overload status43. Our results suggest that visceral congestion may be an important cause of enteric peritonitis.
Currently, there are no standardized criteria for the classification of organisms in peritonitis. In this study, enteric organisms were defined based on a previous report33 due to their predilection for intestinal colonization. However, we were unable to determine whether peritonitis caused by enteric organisms was certainly due to a bowel source and whether peritonitis caused by the non-enteric organisms was due to touch contamination or exit-site infection. Therefore, new testing techniques are required to confirm the origin of the causative organism6,33,47. In addition, in the present study, two patients developed peritonitis due to enteric organisms caused by ileus and diverticulitis, and the organisms responsible for it were Escherichia coli and Proteus mirabilis, respectively; these patients were included in the preserved LVEF group. Therefore, if these patients had been excluded from the statistical analysis, our results would remain unchanged. Furthermore, only three patients, one in the reduced LVEF group and two in the preserved LVEF group, had concomitant exit-site infection or tunnel infection-related peritonitis caused by Staphylococcus aureus. Based on these results, we propose that the bowel source may be important for the development of enteric peritonitis with cardiac dysfunction.
The present study had several limitations. First, given the retrospective nature of the study, unmeasured confounding factors associated with reduced LVEF may not have been included in the models. Second, this study had a single-center, small-cohort design; therefore, our results should be validated in studies with other large multicenter well-designed cohorts and longer follow-up periods. Third, heart failure is closely linked to poor dietary intake, malnutrition, low-level physical activity, and poor general condition48; therefore, the association with peritonitis may also be impacted by such conditions. As malnutrition is associated with immune defects, particularly a decrease in T cell function, it also contributes to an increased risk of and a worse outcome in cases of infections49. However, the present study could not evaluate these factors; consequently, the role of other confounding factors on the positive association between cardiac dysfunction and peritonitis cannot be ruled out and warrants further exploration. Fourth, this study could not detect changes in the intestinal microbacterial flora, and the pathomechanism of the development of peritonitis remains unknown. Fifth, the volume status and urine volume of each patient were not assessed during the follow-up. Furthermore, it is not known whether strict control of volume status could prevent the development of peritonitis. Further studies are therefore required to obtain this information.
Despite these methodological issues, to the best of our knowledge, this is the first study describing the relationship between LV function and peritonitis development. These results imply that patients with reduced LVEF may be at risk of enteric peritonitis from bowel sources caused by intestinal involvement due to cardiac dysfunction, which should be verified in different cohorts.
Data availability
All relevant data are presented within the paper and its Supporting Information files.
References
Woodrow, G., Turney, J. H. & Brownjohn, A. M. Technique failure in peritoneal dialysis and its impact on patient survival. Perit. Dial. Int. 17, 360–364 (1997).
Pérez Fontan, M. et al. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit. Dial. Int. 25, 274–284 (2005).
Sipahioglu, M. H. et al. Patient and technique survival and factors affecting mortality on peritoneal dialysis in Turkey: 12 years’ experience in a single center. Perit. Dial. Int. 28, 238–245 (2008).
Bunke, C. M., Brier, M. E. & Golper, T. A. Outcomes of single organism peritonitis in peritoneal dialysis: Gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int. 52, 524–529 (1997).
Li, P. K. et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit. Dial. Int. 42, 110–153 (2022).
Davies, S. J. et al. Low serum potassium levels and clinical outcomes in peritoneal dialysis-international results from PDOPPS. Kidney Int. Rep. 6, 313–324 (2021).
Su, C. Y., Pei, J., Lu, X. H., Tang, W. & Wang, T. Gastrointestinal symptoms predict peritonitis rates in CAPD patients. Clin. Nephrol. 77, 267–274 (2012).
Chuang, Y. W., Shu, K. H., Yu, T. M., Cheng, C. H. & Chen, C. H. Hypokalaemia: An independent risk factor of Enterobacteriaceae peritonitis in CAPD patients. Nephrol. Dial. Transplant. 24, 1603–1608 (2009).
Ribeiro, S. C. et al. Low serum potassium levels increase the infectious-caused mortality in peritoneal dialysis patients: A propensity-matched score study. PLoS One 10, e0127453 (2015).
Liu, D. et al. Degree and duration of hypokalemia associated with peritonitis in patients undergoing peritoneal dialysis. Int. J. Clin. Pract. 75, e14188 (2021).
Goldsmith, D. J. & Covic, A. Coronary artery disease in uremia: Etiology, diagnosis, and therapy. Kidney Int. 60, 2059–2078 (2001).
Yamada, S. et al. Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin. J. Am. Soc. Nephrol. 5, 1793–1798 (2010).
Antlanger, M. et al. Heart failure with preserved and reduced ejection fraction in hemodialysis patients: Prevalence, disease prediction and prognosis. Kidney Blood Press. Res. 42, 165–176 (2017).
Wang, A. Y. et al. Heart failure with preserved or reduced ejection fraction in patients treated with peritoneal dialysis. Am. J. Kidney Dis. 61, 975–983 (2013).
Jankowski, J., Floege, J., Fliser, D., Böhm, M. & Marx, N. Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation 143, 1157–1172 (2021).
Polsinelli, V. B., Sinha, A. & Shah, S. J. Visceral congestion in heart failure: Right ventricular dysfunction, splanchnic hemodynamics, and the intestinal microenvironment. Curr. Heart Fail. Rep. 14, 519–528 (2017).
Gallo, A. et al. The gut in heart failure: Current knowledge and novel frontiers. Med. Princ. Pract. 31, 203–214 (2022).
Pasini, E. et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 4, 220–227 (2016).
Fukui, H. Increased intestinal permeability and decreased barrier function: Does it really influence the risk of inflammation?. Inflamm. Intest. Dis. 1, 135–145 (2016).
Driessen, M. M. et al. Assessment of LV ejection fraction using real-time 3D echocardiography in daily practice: Direct comparison of the volumetric and speckle tracking methodologies to CMR. Neth. Heart J. 22, 383–390 (2014).
Hanachi, M. et al. Micronutrients deficiencies in 374 severely malnourished anorexia nervosa inpatients. Nutrients 11, 792 (2019).
Ikeda, Y. et al. Association between intestinal oedema and oral loop diuretic resistance in hospitalized patients with acute heart failure. ESC Heart Fail. 8, 4067–4076 (2021).
Sroka, N. et al. Show me what you have inside-the complex interplay between SIBO and multiple medical conditions-A systematic review. Nutrients 15, 90 (2022).
Beale, A. L. et al. The gut microbiome of heart failure with preserved ejection fraction. J. Am. Heart Assoc. 10, e020654 (2021).
Maeda, S. et al. Proton pump inhibitor use increases the risk of peritonitis in peritoneal dialysis patients. PLoS One 14, e0224859 (2019).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992 (2009).
Lang, R. M. et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18, 1440–1463 (2005).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 17, 1321–1360 (2016).
Lancellotti, P. et al. Echo-Doppler estimation of left ventricular filling pressure: Results of the multicentre EACVI Euro-Filling study. Eur. Heart J. Cardiovasc. Imaging 18, 961–968 (2017).
Toma, M. et al. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: Insights from the ASCEND-HF Trial. Eur. J. Heart Fail. 16, 334–341 (2014).
Rudski, L. G. et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 23, 685–713 (2010).
Ma, X. et al. Analysis of risk factors and outcome in peritoneal dialysis patients with early-onset peritonitis: A multicentre, retrospective cohort study. BMJ Open 10, e029949 (2020).
Xu, Y. et al. Prevention of peritoneal dialysis-related peritonitis by regular patient retraining via technique inspection or oral education: A randomized controlled trial. Nephrol. Dial. Transplant. 35, 676–686 (2020).
Perl, J. et al. Peritoneal dialysis-related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS). Am. J. Kidney Dis. 76, 42–53 (2020).
Cheetham, M. S. et al. International peritoneal dialysis training practices and the risk of peritonitis. Nephrol. Dial. Transplant. 37, 937–949 (2022).
Zhong, H. J., Lin, D., Lu, Z. Y., Yang, W. Y. & Chen, Y. Use of gastric-acid suppressants may be a risk factor for enteric peritonitis in patients undergoing peritoneal dialysis: A meta-analysis. J. Clin. Pharm. Ther. 44, 209–215 (2019).
Singharetnam, W. & Holley, J. L. Acute treatment of constipation may lead to transmural migration of bacteria resulting in gram-negative, polymicrobial, or fungal peritonitis. Perit. Dial. Int. 16, 423–425 (1996).
Shu, K. H. et al. Intestinal bacterial overgrowth in CAPD patients with hypokalaemia. Nephrol. Dial. Transplant. 24, 1289–1292 (2009).
Scarpellini, E. et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: Is the ring closed?. Am. J. Gastroenterol. 105, 323–327 (2010).
Santhakumaran, T., Samad, N. & Fan, S. L. Hydration status measured by BCM: A potential modifiable risk factor for peritonitis in patients on peritoneal dialysis. Nephrology (Carlton) 21, 404–409 (2016).
Sandek, A. et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol. 64, 1092–1102 (2014).
Mullens, W. et al. Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function?. J. Am. Coll. Cardiol. 51, 300–306 (2008).
Sandek, A. et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 50, 1561–1569 (2007).
Rossiter, J. P., Cunningham, K. & Manley, P. N. Spontaneous bacterial peritonitis: An unusual life-threatening complication of congestive heart failure. Can. J. Cardiol. 31(5), 691.e13–4 (2015).
Higgins, N., Burke, J. P. & McCreery, C. J. Acute peritonitis as the first presentation of valvular cardiomyopathy. Am. J. Emerg. Med. 30(1), 247.e5–6 (2012).
Gonzalez-Cordero, A. F., Malavé-Sánchez, M., Vergara-Gómez, M. & Franqui-Rivera, H. Spontaneous fungal peritonitis: A rare complication of heart failure. JACC Case Rep. 1(3), 372–375 (2019).
Szeto, C. C. et al. Enterobacteriaceae peritonitis complicating peritoneal dialysis: A review of 210 consecutive cases. Kidney Int. 69, 1245–1252 (2006).
LaMonte, M. J. Physical activity and heart failure: Taking steps to control a major public health burden. Am. J. Lifestyle Med. 14, 555–570 (2018).
Chandra, R. K. Nutrition and the immune system from birth to old age. Eur. J. Clin. Nutr. 56(Suppl 3), S73–S76 (2002).
Acknowledgements
We are grateful to K. Nakabayashi for helping in data collection and providing useful suggestions.
Author information
Authors and Affiliations
Contributions
Research idea and study design: M.Y.; data acquisition: all authors; data analysis/interpretation: M.Y., M.A., Y.K., and T.I.; statistical analysis: M.Y., M.A., Y.K., and T.I.; supervision or mentorship: T.I. and Y.I. Each author contributed important intellectual content during manuscript drafting and agrees to be personally accountable for the individual’s own contributions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamaguchi, M., Obayashi, T., Kobayashi, N. et al. Association between reduced left ventricular ejection fraction and peritoneal dialysis related peritonitis: a single center retrospective cohort study in Japan. Sci Rep 13, 22697 (2023). https://doi.org/10.1038/s41598-023-49744-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49744-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.