Abstract
Lumbar puncture (LP) is recommended in patients with thunderclap headache and negative computed tomography to rule out spontaneous subarachnoid haemorrhage (SAH). Blood contamination of cerebrospinal fluid (CSF) due to traumatic LP poses a diagnostic dilemma. Therefore, routine CSF parameters were investigated to distinguish between SAH and a traumatic LP. CSF red blood cell (RBC), white blood cell (WBC) count, total protein, CSF colour and supernatant were used for group comparisons of patients with SAH and ‘symptomatic controls’. Due to variable time intervals between bleeding onset and LP in SAH patients in contrast to patients with traumatic LP, where blood contamination of CSF occurs at the time of LP, CSF variables were adjusted for decay in time to allow comparability. Logistic regression analysis identified bloody CSF [odds ratio (OR) 32.6], xanthochromic supernatant [OR 15.5] and WBCadjusted [OR 4.5 (per increase of 100/µl)] as predictors of SAH, while age, sex and CSF total proteinadjusted were no predictors. Optimal cut-point of RBCadjusted (determined at day 1 after bleeding) was > 3667/µl to identify SAH patients with a 97% sensitivity and 94% specificity. Combination of low RBC and clear CSF supernatant was found in none of SAH patients. Combined CSF RBC count and CSF supernatant reliably distinguished traumatic LP from SAH.
Similar content being viewed by others
Introduction
Spontaneous subarachnoid haemorrhage (SAH) is a severe life-threating neurological disease which accounts for approximately 5–10% of all strokes and bears the risk of significant morbidity and mortality1,2. Cerebral computed tomography (CT) scan is the first investigation if SAH is suspected. Its diagnostic sensitivity is high in the first hours after the bleeding, but sharply decreases thereafter3,4. As patients frequently present hours or even days after symptom onset, lumbar puncture (LP) should be performed in case of normal CT scans to detect the low but clinically significant percentage of CT negative SAH patients5. Under physiological conditions, cerebrospinal fluid (CSF) does not contain red blood cells (RBC) and, thus, can rule out SAH. However, RBC are artificially introduced into the CSF in up to one third of patients as a result of needle trauma mostly due to puncturing spinal venous plexus6. This hampers the differentiation between patients with SAH and traumatic LP.
Prior studies have investigated various CSF biomarkers to identify SAH patients, e.g., RBC count, xanthochromia, oxyhaemoglobin, methaemoglobin, ferritin or siderophages, but reported only moderate diagnostic value due to various reasons7. Furthermore, some of these biomarkers are not in wide clinical use. While different temporal dynamics of CSF biomarkers in SAH patients have been acknowledged and certain time frames defined for their detection, e.g., bilirubin > 12 h after the bleeding7, none of these studies considered that the magnitude of CSF changes might also depend on disease duration.
The objective of this study was to investigate the diagnostic value of widely available CSF parameters to discriminate patients with SAH from patients with diseases other than SAH but with traumatic LP applying a multivariable approach and considering disease duration as a covariate in particular. In addition, a literature review was conducted to provide an overview of current knowledge on the ability of CSF parameters to differentiate between SAH and traumatic LP.
Methods
Patients and samples
We have stored the results of CSF and serum sample analyses performed for routine diagnostic purposes in the Innsbruck CSF Database from patients with mainly neurological diseases. We screened for samples applying the following criteria: CSF collection by LP and sample processing within 24 h after withdrawal (done on Nov 6th, 2022). Medical records of the remaining patients were studied and patients classified into a spontaneous SAH group and control group. The authors had access to information that could identify individual participants during or after data collection.
Patients with spontaneous SAH were subdivided into patients with evidence of subarachnoid blood on the CT scan (termed ‘SAH’) and patients without evidence of intrathecal blood on the CT scan (termed ‘CT negative SAH’). Within the SAH and CT negative SAH group, patients with re-bleeding or ventriculitis before LP were excluded to ensure that adjustment for disease duration (see below) is not biased by these complications, which might lead to changes in RBC, WBC count and CSF total protein (Fig. 1).
Patients fulfilling the definition of ‘symptomatic controls’ according to a recent consensus8 or patients without any neurological disease were used as control group. These patients should show normal routine CSF findings (i.e. normal WBC count and CSF total protein). Diagnoses of the control group are given in Table S1.
Definition of CT negative SAH
Patients classified as ‘CT negative SAH’ had typical clinical presentation (thunderclap headache), normal CT scan, but further clinical/ paraclinical findings, which led to the diagnosis of ‘CT negative SAH’ by the treating physicians, e.g., MRI with evidence of bleeding, signs of erythrophagocytosis in the CSF (detection of erythrophages, siderophages, hematoidin and/ or hemosiderin).
CSF analysis
CSF WBC and CSF RBC were counted within a Fuchs-Rosenthal chamber, which has a volume of 3.2 μL9. Division by 3.2 allowed reporting of cell counts per μL according to International System of units (SI). CSF total protein concentration was measured by spectrophotometry after incubation of centrifuged CSF with 3% trichloroacetic acid10. CSF colour (clear, bloody) and CSF supernatant (clear vs. xanthochromic) were classified by visual inspection.
Statistical analysis
Data were displayed as mean ± standard deviation (SD) or as median, interquartile range (IQR), 5th-95th percentile, as appropriate.
As CSF of patients with SAH is usually taken hours or days after the bleeding, in contrast to patients with traumatic LP, where blood contamination of CSF occurs at the time of LP, the variables RBC count, WBC count and CSF total protein were corrected for their decay in time to make them comparable between patients. They were adjusted according to the decay law previously published11.
where \(y\) denotes RBC, WBC or CSF total protein respectively, \({T}_{i}\) is the disease duration (days since symptom onset) for patient \(i\), and \(\widehat{\lambda }\) is the estimated constant time decay parameter. For our purpose the three variables (RBC, WBC or CSF total protein, respectively) were adjusted to \({T}_{i}=1\), and we used only patients who had LP less or equal than 22 days after disease onset (Fig. 1), as the decay parameter was estimated for this time period in the previous study11. The \(\widehat{\lambda }\) for RBC, WBC or CSF total protein were -0.281, -0.217 and -0.06311. E.g., a patient with a RBC count of 2000/μl determined in CSF collected three days after SAH onset has an RBCadjusted of 3508/μl, calculated as
With a binary logistic regression model the probability for both SAH & CT negative SAH was modelled using RBCadjusted, WBCadjusted, CSF total proteinadjusted, CSF supernatant, CSF colour, sex and age as independent variables. As sample size was small a likelihood ratio test was used to test for statistical significance of each variable.
We calculated the optimal cut-off point in the logistic regression of the probability of both SAH & CT negative SAH dependent on RBCadjusted, using the sum of specificity and sensitivity as optimization criterion.
The significance level was 5%. All computations were done with R Core Team (2017) and the package cutpoint12,13.
Ethics
The conduct of the study was approved by the Ethics Committee of the Medical University of Innsbruck (approval number 1269/2022). Informed consent was not needed because this was a retrospective analysis of existing data that were obtained in routine diagnostic procedures.
Literature search
A literature search in PubMed using the search terms “cerebrospinal fluid” AND “subarachnoid haemorrhage” AND “traumatic tap” or “red blood cell count” or “white blood cell count” or “total protein” or “opening pressure” or “three tube test” or “xanthochromia” or “colour” or “supernatant” or “ferritin” or “oxyhaemoglobin” or “bilirubin” or “methaemoglobin” limited to August 1st, 2022 offered 18, 39, 35, 80, 32, 7, 92, 25, 17, 24, 70, 119, and 17 references. Abstracts that did not primarily deal with the use of CSF to differentiate between SAH and traumatic LP were excluded. In addition, articles identified in reference lists of individual papers were selected if considered appropriate. Only original articles written in English were considered.
Results
Routine CSF parameters reliably discriminate SAH from traumatic LP
A total of 471 samples comprising 27 patients with SAH, 5 patients with CT negative SAH and 439 controls were included in the study. For inclusion criteria see Fig. 1. Demographic and clinical characteristics of the three groups are detailed in Table 1, the main CSF findings are shown in Table S2, a detailed characterisation of the CT negative SAH patients is provided in Table S3.
The median disease duration, i.e. the time interval from symptom onset to LP, in patients with SAH was 9 days and in patients with CT negative SAH 6 days. We adjusted RBC, WBC count and CSF total protein for the individual disease duration, i.e. the time interval from symptom onset until LP. For comparison of the measured and adjusted values, please refer to Table S2.
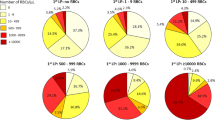
RBCadjusted, WBCadjusted as well as CSF total proteinadjusted were significantly higher in SAH patients and CT negative SAH patients compared to controls. SAH patients compared to controls showed more frequently bloody CSF (97% vs. 29%) and xanthochromic CSF supernatant (84% vs. 3%) (Fig. 2).
Various CSF parameters discriminating between SAH and traumatic LP. RBC count, WBC count and CSF total protein concentration were adjusted for a fixed disease duration of one day in patients with SAH and CT negative SAH. SAH and CT negative SAH patients showed higher RBCadjusted, WBCadjusted and CSF total proteinadjusted compared to controls. Bloody CSF and xanthochromic supernatant were more frequently observed in patients with SAH and CT negative SAH than in controls. CSF cerebrospinal fluid, CT computed tomography, RBC red blood cells, SAH subarachnoid haemorrhage, WBC white blood cells.
Multivariable logistic regression analyses identified bloody CSF colour [odds ratio (OR) 32.6, p = 0.013], xanthochromic CSF supernatant [OR 15.5, p < 0.001] and WBCadjusted [OR 4.5 (per increase of 100/μl), p < 0.001] as predictors, while age, sex and CSF total proteinadjusted were not statistically significant predictors (Table 2). The high correlation between RBCadjusted and WBCadjusted is responsible for the lacking significance of RBCadjusted as a predictor of SAH (Table S4). In univariate analysis, RBCadjusted was a statistically significant predictor of SAH [OR 1.4 (per increase of 10,000/μl), p < 0.001] (Table S5). An optimal cut-point of RBCadjusted count to identify SAH (both SAH and CT negative SAH) was obtained at 3667/µl (Fig. 3). Twenty-six of 27 (96.3%) SAH patients, and all CT negative SAH patients had a high RBCadjusted (> 3667/µl), while 413 (94.1%) of 439 controls had low RBCadjusted (≤ 3667/µl). The negative predictive value (NPV) of low RBC count was 99.8% in controls, while the positive predictive value (PPV) of high RBC was 54.4% considering all SAH patients (Table S6). Overall, patients who showed the combination of high RBC and CSF xanthochromia had SAH in 78.8% of cases, while all patients who showed low RBC and clear CSF supernatant were allocated to the control group (Table S6).
Using RBCadjusted (i.e. RBC counts adjusted for disease duration) instead of RBCmeasured resulted in an improved classification of SAH patients versus controls of 11 percentage points (Table S7). The comparison of our RBCadjusted cut-point with a previously suggested cut-point (for RBCmeasured of 2000/μl) showed an increase in this classification by 15 percentage points (Table S8).
Literature search
A total of 575 articles were screened. Of those, 29 focused on the diagnostic value of different CSF parameters in SAH for its differentiation from traumatic LP14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. Two studies were excluded due to non-reporting appropriate results20,38. For a comprehensive overview, refer to Table S9. The majority of studies addressed RBC counts and xanthochromia with variable results14,15,16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,40,41,42. Two recent studies not accounting for disease duration suggested a RBC cut-point of approximately 2,000 RBC/µl for detection of SAH and reported that low RBC in combination with clear CSF supernatant reliably excluded SAH14,15. There are also studies that assessed the decrease in RBC count between different CSF collection tubes15,29,31 with one study reporting that an approximately 60% decrease from first to final tube could be the optimal threshold to discriminate traumatic LP from SAH29. A few studies focused on erythrocyte degradation products such as CSF ferritin39,40,43 or bilirubin30,32,33,34,35,36,37,40. The latter has been recommended in clinical use in the U.K.44.
Discussion
Bloody CSF obtained by LP of patients with suspected SAH but normal CT scan constitutes a diagnostic dilemma, as there is no single CSF parameter that allows a reliable discrimination between true SAH and traumatic LP. One of the main challenges in conducting studies and interpreting CSF results is that CSF biomarkers show different temporal dynamics7. Here, for the first time, we adjusted routine CSF findings for the individual disease duration at the time of LP and used the estimated values in a multivariable approach to identify predictors of SAH and traumatic tap, respectively. All patients with low adjusted CSF RBC counts and clear CSF supernatant were identified as traumatic LP and none as true SAH.16. This means that SAH can be excluded with very high certainty in patients fulfilling this CSF criterion.
To date, several studies have assessed the diagnostic value of different CSF parameters to identify SAH patients (Table S9)14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,45. SAH occurs when blood is extravasated, in the majority of patients due to the aneurysm rupture1, and degradation of intrathecal blood leads to aseptic inflammation46. WBC are hypothesized to cross the arterial walls and to infiltrate the subarachnoid blood clot, secreting cytokines and initiating different processes47,48. Finally, blood and to a certain extent intrathecal inflammation lead to decreased CSF flow49,50. These main pathophysiological processes in SAH are mirrored in the CSF. Accordingly, we observed higher levels of RBC, WBC count and CSF total protein in SAH patients as compared to the control group confirming previous studies14,15,16,26,28,51.
Although different temporal dynamics of certain CSF biomarkers, e.g., of bilirubin or ferritin7, have been acknowledged as relevant factor in determining their diagnostic utility, none of the above-mentioned studies (Table S9) did this for routine CSF parameters such as RBC, WBC or CSF total protein. A recent study using several longitudinally collected CSF samples of SAH patients showed that RBC count is highest shortly after bleeding and gradually decreases over weeks. Similarly, WBC count and CSF total protein tend to normalize with advancing disease course11. We hypothesized that timing of LP impacts on the magnitude of the change of different CSF parameters. In the present study, CSF was taken a median of 9 days after disease onset in patients with SAH (mostly due to hydrocephalus) and after 6 days in patients with CT negative SAH. This is in contrast to patients with traumatic LP, where blood contamination of CSF occurs at the time of LP. In patients with SAH and CT negative SAH, RBC count at the time of the bleeding was estimated from the RBC count determined at the time of LP, using the previously published decay law11 and the respective time from symptom onset, i.e. adjusting for the in vivo cell degradation over time. Indeed, the measured and adjusted variables showed considerable differences (Table S2). This is clinically relevant because a significant proportion of patients with thunderclap headache—at risk of having suffered an intrathecal hemorrhage—present themselves with delay, sometimes several days52. Not considering disease duration would result in an underestimation of CSF changes due to the bleeding.
We identified an adjusted RBC cut point of approximately 3600/μl which was higher than previously suggested cut points14,15. Mark et al. observed that SAH patients (only 33% with LP performed within the first 12 h) had RBC counts above 2000/μl and/or xanthochromia15. Perry et al. included 15 CT negative SAH patients, which had LP up to 5 days after symptom onset and identified a RBC cut-off of 2450/μl14. However, both studies did not consider the different disease duration and, thus, used non-adjusted, potentially false-low RBC counts. In our study, when we used RBCadjusted or RBCmeasured as predictor of SAH versus controls, an improved classification was achieved by the RBCadjusted approach.
In clinical practice, our approach would allow first to adjust the RBC count for the patient’s individual disease duration (i.e. time since symptom onset) and then to assess whether it is above or below the cut-off (i.e. to assess whether intrathecal bleeding is likely or not), instead of uncritically applying non-adjusted RBC counts, i.e. independent of disease duration, to a fixed cut-off. Despite of the obvious advantages of our approach, prospective studies are required to validate these results and to show superiority.
There are some limitations to our study. First, this was a retrospective study with all inherent limitations. Secondly, the decay rates used to adjust CSF parameters for individual disease duration were estimated in longitudinally collected CSF obtained by ventricular drainage11. However, we assume that cell degradation, which occurred in a non-linear manner, should be independent of the exact location within the CSF space and the site of the sample obtainment. Thirdly, we included CSF samples processed within 24 h after LP. Longer intervals to laboratory processing might result in decreased RBC and WBC count9. This means that shorter processing time would have led to higher RBC cut-off values. As the main conclusion of our study is that patients with RBC count below the cut-off are most likely traumatic LP, higher RBC cut-off would not impair the negative predictive values. Furthermore, we were not able to consider other CSF parameters such as bilirubin, or the “three-tube test” in our multivariable analysis, as we did not routinely perform or document these measurements. Also, some of the patients had an intervention (i.e. either coiling or clipping) before CSF withdrawal. It cannot be excluded that this had an influence on CSF parameters. We have to state that we adjusted CSF parameters in our analyses for a fixed disease duration of one day in SAH patients; an earlier time point would not be valid, as we also considered CSF supernatant, and colour change (into xanthochromia) needs at least 12 h as bilirubin has to be formed in vivo. Considering the disease duration within the SAH group of median 7.5 days (3. quartile: 16 days), it might be that at a different (earlier) time point of LP, a higher percentage of SAH patients would have shown CSF xanthochromia. However, it is not possible to adjust the variable “CSF xanthochromia” for disease duration, as in case of a negative status, a positive status before cannot be extrapolated. This means that the diagnostic value of CSF supernatant might have been underestimated. Another limitation of our study is that we could only include 5 patients with CT-negative SAH, therefore, the results on CT-negative SAH patients (e.g., the cut-off of RBCadjusted) need to be replicated by further studies.
Altogether, we present a tool, which has the potential to be widely used in clinical practice, to identify patients with traumatic LP by RBC count and inspection of CSF supernatant. Even though assessment of CSF supernatant is rater-dependent and anecdotal reports of SAH in patients with very low RBC counts53 cannot be excluded, the herein applied criterion (of an adjusted RBC count of less than 3667/μl combined with clear CSF supernatant) identified only patients with traumatic LP and none of the SAH patients. It has to be clearly stated that in case of high RBC counts and/ or xanthochromia, the differentiation between SAH and traumatic LP is still not reliable. Altogether, we think that the results of our study contribute to further understanding. Correct interpretation of CSF findings is of utmost importance, as SAH—if misdiagnosed—is a severe neurological disease with significant morbidity and mortality.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Lawton, M. T. & Vates, G. E. Subarachnoid hemorrhage. N. Engl. J. Med. 377(3), 257–266. https://doi.org/10.1056/NEJMcp1605827 (2017).
Etminan, N. et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: A systematic review and meta-analysis. JAMA Neurol. 76(5), 588–597. https://doi.org/10.1001/jamaneurol.2019.0006 (2019).
Perry, J. J. et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 343, d4277. https://doi.org/10.1136/bmj.d4277 (2011).
Backes, D., Rinkel, G. J., Kemperman, H., Linn, F. H. & Vergouwen, M. D. Time-dependent test characteristics of head computed tomography in patients suspected of nontraumatic subarachnoid hemorrhage. Stroke 43(8), 2115–2119. https://doi.org/10.1161/STROKEAHA.112.658880 (2012) (epub 20120719).
Edlow, J. A. et al. Clinical policy: Critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann. Emerg. Med. 52(4), 407–436. https://doi.org/10.1016/j.annemergmed.2008.07.001 (2008).
Shah, K. H., Richard, K. M., Nicholas, S. & Edlow, J. A. Incidence of traumatic lumbar puncture. Acad. Emerg. Med. 10(2), 151–154 (2003).
Nagy, K. et al. Cerebrospinal fluid analyses for the diagnosis of subarachnoid haemorrhage and experience from a Swedish study. What method is preferable when diagnosing a subarachnoid haemorrhage?. Clin. Chem. Lab. Med. 51(11), 2073–2086. https://doi.org/10.1515/cclm-2012-0783 (2013).
Teunissen, C. et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult. Scler. 19(13), 1802–1809. https://doi.org/10.1177/1352458513488232 (2013) (epub 2013/05/21).
Deisenhammer, F. et al. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur. J. Neurol. 13(9), 913–922. https://doi.org/10.1111/j.1468-1331.2006.01493.x (2006).
Meulemans, O. Determination of total protein in spinal fluid with sulphosalicylic acid and trichloroacetic acid. Clin. Chim. Acta 5, 757–761. https://doi.org/10.1016/0009-8981(60)90020-6 (1960).
Zinganell, A. et al. Longitudinal ventricular cerebrospinal fluid profile in patients with spontaneous subarachnoid hemorrhage. Front. Neurol. 13, 861625. https://doi.org/10.3389/fneur.2022.861625 (2022) (epub 20220726).
Team RC. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2017).
Thiele, C. cutpointr: Determine and Evaluate Optimal Cutpoints in Binary Classification Tasks (2019).
Perry, J. J. et al. Differentiation between traumatic tap and aneurysmal subarachnoid hemorrhage: Prospective cohort study. BMJ. 350, h568 (2015) (epub 2015/02/18).
Mark, D. G. et al. Validation of cerebrospinal fluid findings in aneurysmal subarachnoid hemorrhage. Am. J. Emerg. Med. 33(9), 1249–1252. https://doi.org/10.1016/j.ajem.2015.05.012 (2015) (epub 2015/05/15).
Rankin, S., McGuire, J., Chekroud, M., Alakandy, L. & Mukhopadhyay, B. Evaluating xanthochromia in the diagnosis of subarachnoid haemorrhage in Scotland in the era of modern computed tomography. Scott Med. J. 67(2), 71–77. https://doi.org/10.1177/00369330211072264 (2022) (epub 20220201).
Arora, S., Swadron, S. P. & Dissanayake, V. Evaluating the sensitivity of visual xanthochromia in patients with subarachnoid hemorrhage. J. Emerg. Med. 39(1), 13–16. https://doi.org/10.1016/j.jemermed.2007.09.052 (2010) (epub 2008/06/24).
Hann, A., Chu, K., Greenslade, J., Williams, J. & Brown, A. Benefit of cerebrospinal fluid spectrophotometry in the assessment of CT scan negative suspected subarachnoid haemorrhage: A diagnostic accuracy study. J. Clin. Neurosci. 22(1), 173–179. https://doi.org/10.1016/j.jocn.2014.07.025 (2015) (epub 20141028).
Perry, J. J. et al. Should spectrophotometry be used to identify xanthochromia in the cerebrospinal fluid of alert patients suspected of having subarachnoid hemorrhage?. Stroke 37(10), 2467–2472. https://doi.org/10.1161/01.STR.0000240689.15109.47 (2006) (epub 2006/08/31).
Goyale, A., O’Shea, J., Marsden, J., Keep, J. & Vincent, R. P. Analysis of cerebrospinal fluid for xanthochromia versus modern computed tomography scanners in the diagnosis of subarachnoid haemorrhage: Experience at a tertiary trauma referral centre. Ann. Clin. Biochem. 53(Pt 1), 150–154. https://doi.org/10.1177/0004563215579454 (2016) (epub 20150312).
Ahmed, F., Gibbons, S. & El-Kadiki, A. CSF xanthochromia: Correlation with brain imaging and its usefulness as an out-of-hours test. J. Clin. Pathol. 67(8), 736–738. https://doi.org/10.1136/jclinpath-2014-202193 (2014) (epub 20140516).
Dupont, S. A., Wijdicks, E. F., Manno, E. M. & Rabinstein, A. A. Thunderclap headache and normal computed tomographic results: Value of cerebrospinal fluid analysis. Mayo Clin. Proc. 83(12), 1326–1331. https://doi.org/10.1016/S0025-6196(11)60780-5 (2008).
MacDonald, A. & Mendelow, A. D. Xanthochromia revisited: A re-evaluation of lumbar puncture and CT scanning in the diagnosis of subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 51(3), 342–344. https://doi.org/10.1136/jnnp.51.3.342 (1988).
Wood, M. J., Dimeski, G. & Nowitzke, A. M. CSF spectrophotometry in the diagnosis and exclusion of spontaneous subarachnoid haemorrhage. J. Clin. Neurosci. 12(2), 142–146. https://doi.org/10.1016/j.jocn.2004.05.009 (2005).
Rana, A. K., Turner, H. E. & Deans, K. A. Likelihood of aneurysmal subarachnoid haemorrhage in patients with normal unenhanced CT, CSF xanthochromia on spectrophotometry and negative CT angiography. J. R. Coll. Phys. Edinb. 43(3), 200–206. https://doi.org/10.4997/JRCPE.2013.303 (2013).
Gangloff, A., Nadeau, L., Perry, J. J., Baril, P. & Émond, M. Ruptured aneurysmal subarachnoid hemorrhage in the emergency department: Clinical outcome of patients having a lumbar puncture for red blood cell count, visual and spectrophotometric xanthochromia after a negative computed tomography. Clin. Biochem. 48(10–11), 634–639. https://doi.org/10.1016/j.clinbiochem.2015.03.011 (2015) (epub 20150326).
Wallace, A. N., Dines, J. N., Zipfel, G. J. & Derdeyn, C. P. Yield of catheter angiography after computed tomography negative, lumbar puncture positive subarachnoid hemorrhage [corrected]. Stroke 44(6), 1729–1731. https://doi.org/10.1161/STROKEAHA.113.001234 (2013) (epub 20130425).
Migdal, V.L., Wu, W.K., Long, D., McNaughton, C.D., Ward, M.J. & Self, W.H. Risk-benefit analysis of lumbar puncture to evaluate for nontraumatic subarachnoid hemorrhage in adult ED patients. Am. J. Emerg. Med. 33(11),1597–1601 https://doi.org/10.1016/j.ajem.2015.06.048 (2015)
Czuczman, A. D. et al. Interpreting red blood cells in lumbar puncture: Distinguishing true subarachnoid hemorrhage from traumatic tap. Acad. Emerg. Med. 20(3), 247–256. https://doi.org/10.1111/acem.12095 (2013).
Tsementzis, S. A., Hitchcock, E. R., DeCothi, A. & Gill, J. S. Comparative studies of the diagnostic value of cerebrospinal fluid spectrophotometry and computed tomographic scanning in subarachnoid hemorrhage. Neurosurgery. 17(6), 908–912. https://doi.org/10.1227/00006123-198512000-00007 (1985).
Heasley, D. C., Mohamed, M. A. & Yousem, D. M. Clearing of red blood cells in lumbar puncture does not rule out ruptured aneurysm in patients with suspected subarachnoid hemorrhage but negative head CT findings. AJNR Am. J. Neuroradiol. 26(4), 820–824 (2005).
Gunawardena, H., Beetham, R., Scolding, N. & Lhatoo, S. D. Is cerebrospinal fluid spectrophotometry useful in CT scan-negative suspected subarachnoid haemorrage?. Eur. Neurol. 52(4), 226–229. https://doi.org/10.1159/000082162 (2004) (epub 20041116).
McCarron, M. O. et al. Clinical and diagnostic findings in patients with elevated cerebrospinal bilirubin. Postgrad. Med. J. 91(1082), 675–680. https://doi.org/10.1136/postgradmedj-2015-133360 (2015) (epub 20151021).
Bakr, A., Silva, D., Cramb, R., Flint, G. & Foroughi, M. Outcomes of CSF spectrophotometry in cases of suspected subarachnoid haemorrhage with negative CT: Two years retrospective review in a Birmingham hospital. Br. J. Neurosurg. 31(2), 223–226. https://doi.org/10.1080/02688697.2016.1265089 (2017) (epub 20161208).
Horstman, P., Linn, F. H., Voorbij, H. A. & Rinkel, G. J. Chance of aneurysm in patients suspected of SAH who have a “negative” CT scan but a “positive” lumbar puncture. J. Neurol. 259(4), 649–652. https://doi.org/10.1007/s00415-011-6228-1 (2012) (epub 20110908).
Martin, S. C. et al. Defending a traditional practice in the modern era: The use of lumbar puncture in the investigation of subarachnoid haemorrhage. Br. J. Neurosurg. 29(6), 799–803. https://doi.org/10.3109/02688697.2015.1084998 (2015) (epub 20150916).
Falconer, H. L., Walker, S. A. & Peter, A. J. Specificity of elevated cerebrospinal fluid bilirubin in the investigation of subarachnoid haemorrhage. Ann. Clin. Biochem. 52(Pt 3), 404–406. https://doi.org/10.1177/0004563214554463 (2015) (epub 20140923).
Birch, K. et al. Cerebrospinal fluid total protein cannot reliably distinguish true subarachnoid haemorrhage from other causes of raised cerebrospinal fluid net bilirubin and net oxyhaemoglobin absorbances. Ann. Clin. Biochem. 51(Pt 6), 657–661. https://doi.org/10.1177/0004563214538949 (2014) (epub 20140520).
Watson, I. D., Beetham, R., Fahie-Wilson, M. N., Holbrook, I. B. & O’Connell, D. M. What is the role of cerebrospinal fluid ferritin in the diagnosis of subarachnoid haemorrhage in computed tomography-negative patients?. Ann. Clin. Biochem. 45(Pt 2), 189–192. https://doi.org/10.1258/acb.2007.007043 (2008).
O’Connell, D. M. & Watson, I. D. Definitive angiographic detection of subarachnoid haemorrhage compared with laboratory assessment of intracranial bleed in CT-negative patients. Ann. Clin. Biochem. 40(Pt 3), 269–273. https://doi.org/10.1258/000456303321610592 (2003).
Lang, D. T., Berberian, L. B., Lee, S. & Ault, M. Rapid differentiation of subarachnoid hemorrhage from traumatic lumbar puncture using the d-dimer assay. Am. J. Clin. Pathol. 93(3), 403–405. https://doi.org/10.1093/ajcp/93.3.403 (1990).
Juliá-Sanchis, M. L., Estela-Burriel, P. L., Lirón-Hernández, F. J. & Guerrero-Espejo, A. Rapid differential diagnosis between subarachnoid hemorrhage and traumatic lumbar puncture by d-dimer assay. Clin. Chem. 53(5), 993. https://doi.org/10.1373/clinchem.2007.085613 (2007).
Petzold, A. et al. Cerebrospinal fluid ferritin level, a sensitive diagnostic test in late-presenting subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 20(6), 489–493. https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.02.021 (2011) (epub 2010/08/17).
Cruickshank, A. et al. Revised national guidelines for analysis of cerebrospinal fluid for bilirubin in suspected subarachnoid haemorrhage. Ann. Clin. Biochem. 45(Pt 3), 238–244. https://doi.org/10.1258/acb.2008.007257 (2008).
China, M., Matloob, S. A., Grieve, J. P. & Toma, A. K. The value of repeated lumbar puncture to test for xanthochromia, in patients with clinical suspicion of subarachnoid haemorrhage, with CT-negative and initial traumatic tap. Br. J. Neurosurg. https://doi.org/10.1080/02688697.2021.1875398 (2021) (epub 20210202).
Rass, V. & Helbok, R. How to diagnose delayed cerebral ischaemia and symptomatic vasospasm and prevent cerebral infarction in patients with subarachnoid haemorrhage. Curr. Opin. Crit. Care 27(2), 103–114. https://doi.org/10.1097/MCC.0000000000000798 (2021).
Hegen, H., Auer, M. & Deisenhammer, F. Vascular diseases and bleedings. Handb. Clin. Neurol. 146, 207–236. https://doi.org/10.1016/B978-0-12-804279-3.00013-7 (2017).
Macdonald, R. L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 10(1), 44–58. https://doi.org/10.1038/nrneurol.2013.246 (2014).
Simon, M. J. & Iliff, J. J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 1862(3), 442–451. https://doi.org/10.1016/j.bbadis.2015.10.014 (2016) (epub 20151022).
Reiber, H. Flow rate of cerebrospinal fluid (CSF)—A concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 122(2), 189–203. https://doi.org/10.1016/0022-510x(94)90298-4 (1994).
Koopman, I., Zuithoff, N. P. A., Rinkel, G. J. E. & Vergouwen, M. D. I. The course of cerebrospinal fluid parameters ≤ 20 days after aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 415, 116899. https://doi.org/10.1016/j.jns.2020.116899 (2020) (epub 2020/05/19).
Jakobsson, K. E. et al. Warning leak and management outcome in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 85(6), 995–999. https://doi.org/10.3171/jns.1996.85.6.0995 (1996).
Walton, J. Subarachnoid Haemorrhage (E. & S. Livingstone Ltd., 1956).
Author information
Authors and Affiliations
Contributions
A.Z.: data collection, draft manuscript preparation. K.B.: review of the manuscript for intellectual content. G.B.: review of the manuscript for intellectual content. F.D.P.: review of the manuscript for intellectual content. V.R.: data collection, review of the manuscript for intellectual content. R.H.: review of the manuscript for intellectual content. J.W.: statistical analysis. F.D.: review of the manuscript for intellectual content. H.H.: study conception and design, analysis and interpretation of results, draft manuscript preparation. All authors have approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zinganell, A., Berek, K., Bsteh, G. et al. Subarachnoid haemorrhage or traumatic lumbar puncture. Differentiation by cerebrospinal fluid parameters in a multivariable approach. Sci Rep 13, 22310 (2023). https://doi.org/10.1038/s41598-023-49693-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49693-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.