Abstract
The coral microbiome conforms a proxy to study effects of changing environmental conditions. However, scarce information exists regarding microbiome dynamics and host acclimation in response to environmental changes associated to global-scale disturbances. We assessed El Niño Southern Oscillation (ENSO)-derived thermal anomalies shifts in the bacterial microbiome of Pacifigorgia cairnsi (Gorgoniidae: Octocorallia) from the remote island of Malpelo in the Tropical Eastern Pacific. Malpelo is a hot spot of biodiversity and lacks direct coastal anthropogenic impacts. We evaluated the community composition and predicted functional profiles of the microbiome during 2015, 2017 and 2018, including different phases of ENSO cycle. The bacterial community diversity and composition between the warming and cooling phase were similar, but differed from the neutral phase. Relative abundances of different microbiome core members such as Endozoicomonas and Mycoplasma mainly drove these differences. An acclimated coral holobiont is suggested not just to warm but also to cold stress by embracing similar microbiome shifts and functional redundancy that allow maintaining coral’s viability under thermal stress. Responses of the microbiome of unperturbed sea fans such as P. cairnsi in Malpelo could be acting as an extended phenotype facilitating the acclimation at the holobiont level.
Similar content being viewed by others
Introduction
Climate change negatively impacts coral ecosystems globally, causing dramatic ecological transformations1. Increasing seawater temperatures associated with global warming represent a severe threat to corals (either Scleractinia or Octocorallia) worldwide since there are upper thermal limits that once exceeded, corals experience significant declines2,3. Among the most common consequences of thermal stress are coral bleaching together with growth, calcification and abundance decline which have lead to massive coral die-offs worldwide4,5,6.
Global warming impacts over properties and dynamics of local but also global-scale phenomena such as ENSO events7. Predictions state that ENSO events may increase in frequencies and severity over the next decades under conditions of climate change8,9. This is of great concern since these events have driven global bleaching events and mortality of coral reefs affecting the spatial refuges and viability of these ecosystems10,11.
The coral holobiont is an ecological unit that constitutes the coral itself and an associated microbial community (the microbiome), which includes protists, bacteria, archaea, viruses and fungi12,13. The microbiome, fundamental for the health and resilience of coral reefs12,14 plays key functions to maintain the fitness and homeostasis of the holobiont15,16,17. Microbial communities provide nutrient acquisition and disease resistance through the production of antibiotic compounds18,19. Additionally, the microbiome is potentially involved in different coral adaption and acclimation processes, maintaining intimate associations of high bacterial diversity with wide metabolic repertories and fast generation rates that may contribute to adaptive responses of the holobiont20,21,22. Then, being highly sensitive to environmental changes, the microbiome is capable of responding under disturbed conditions like thermal stress through microbiome restructuring16,23,24,25. The relationship between coral physiology and its microbiome may reveal why some corals are more resilient to global-scale conditions26. Thermal stress generates coral responses, via microbiome changes that allow the coral holobiont to increase its fitness whereas maintaining the homeostasis16,24,27,28. In this sense, understanding the bacterial community mechanisms that contribute to rapid coral acclimation is crucial to elucidate the holobiont responses to thermal stress under the climate change context22.
Gorgonian corals (Anthozoa, Octocorallia) present in shallow waters are among the most vulnerable organisms of coral ecosystems6,29,30. Being long-lived, sessile and slow-growing species, gorgonians are particularly sensitive to environmental changes and anthropogenic disturbances31,32. One example is Pacifigorgia cairnsi, an azooxanthellate sea fan inhabiting the remote and pristine island Malpelo located in the Colombian Tropical Eastern Pacific (TEP)6,33,34. This species dominates the seascape of the island forming dense aggregations on rocky reefs and walls up to 30 m depth35,36. Malpelo Fauna and Flora Sanctuary is a marine protected area and a World Heritage Site, considered a hot spot of biodiversity under pristine conditions34,37. Gorgonian populations in Malpelo cope with cold waters and high levels of suspended matter during seasonal upwelling (from December to March), as well as anomalous increases in seawater temperature during sporadic ENSO events6,38,39. Recently, the vulnerability of P. cairnsi growth rates facing anomalous increases in seawater temperature during sporadic El Niño–Southern Oscillation (ENSO) events in Malpelo has been registered6. This is of concern since this sea fan is considered a keystone species around the island by providing habitat and structural complexity to the benthic seascapes, and by coupling the bentho-pelagic system thanks to its strict filter feeding condition40.
Although numerous studies have evaluated coral’s microbiomes in general41,42,43,44, microbial community dynamics of corals under global-scale climate anomalies,, such as ENSO events, are not easily accessible and thus are scarce45. Actually, few studies of microbiome changes associated to ENSO-derived thermal anomalies affecting gorgonian corals exist46,47. Therefore, elucidating this kind of knowledge to gorgonian corals from remote places with absence of direct human-related stressors, as is the case of P. cairnsi in Malpelo Island, is highly valuable. The core microbiome of this sea fan species and its shifts associated with the necrotic patch disease (NPD) has been recently assessed43. However, microbiome changes driven by ENSO events of this keystone species remain elusive. Understanding micro-ecological processes, especially from the microbial component, is crucial to identify coral responses under thermal stress and hence, to evaluate the resilience capacity of these benthic organisms facing global changing conditions.
To understand the dynamics involving the holobiont and the responses to ENSO-derived thermal anomalies of corals removed from local-scale disturbances, we evaluated the composition and predicted functions of the bacterial microbiome of P. cairnsi sea fans through three years including different phases of ENSO cycle. This is, (1) El Niño-warming phase (year 2015), (2) La Niña-cooling phase (year 2018) and (3) the Neutral phase (year 2017) (Fig. 1). During the warm phase of ENSO (El Niño), positive anomalies of Seawater Surface Temperature (SST) are registered, while during the cooling phase of ENSO (La Niña) negative anomalies are present in the SST48. This study provides crucial data on how corals largely removed from direct human impacts respond to thermal stress associated with these global-scale events and may reveal why some corals are more resilient to these global change conditions. Finally, this may serve as key baseline information to understand microbiome dynamics from corals under the effects of direct-anthropogenic impacts and may provide valuable knowledge to assess coral responses according to future predictions on the climate change scenario.
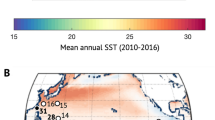
Study site and Pacifigorgia cairnsi sampling information. Geographical location of Malpelo Island and the sampling locality (Arrecife). Map modified from Sánchez and Ballesteros 2014, Revista de Biología Tropical and edited by Adobe Illustrator CS5 version 15.1.1 (A). Pacifigorgia cairnsi colony at Arrecife. Photo by Juan Armando Sánchez (B). Sampling information and associated ENSO phases according to the National Oceanic and Administration (NOAA *https://origin.cpc.ncep.noaa.gov) (C). ENSO phases dynamics between 2013 and 2021 years (D).
Results
16S rRNA sequences of 2015 were taken from Quintanilla et al.43 dataset. Specifically, we used the sequences from healthy proximal tissues in order to make it comparable to the tissues sampled in 2017 and 2018. After filtering, the final dataset consisted of 31 individual samples, 234 ASVs, a minimum of 16,760 reads and a maximum of 40,000 reads per sample and 69 recognized genera in total. Finally, 64 different ASVs accounted for the cumulative 99% of the total abundance. Rarefaction curves for all samples plateaued at 11,000 sequences, indicating a good representation of bacterial diversity (Fig. S1).
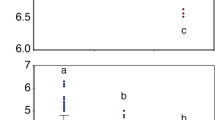
Among the three years, Shannon and Simpson diversity indexes were significantly different. In the pairwise comparisons the diversity between La Niña year (2018) and the neutral year (2017) was significantly different for Simpson index and between 2018 and El Niño year (2015) for Simpson and Shannon indexes. On the other hand, Chao 1 and observed ASVs indexes did not show significant statistical differences between years (Fig. 2a; Supplementary Table 1).
Regarding beta-diversity analyses, the first two coordinates of the PCoA plot showed three clusters of samples. Samples from abnormal thermal conditions (2015 and 2018) clustered separately from samples of the neutral thermal condition (2017) (Fig. 2b). Significant differences in bacterial community compositions were obtained between each pair of compared sampling years (PERMANOVA analyses, Supplementary Table 2). The homogeneity observed in multivariate dispersions among the sampling groups (Supplementary Table 2) confirmed that the significant differences obtained with PERMANOVA were due to differences in bacterial community compositions49. Between years, samples of 2017 differed from those of 2015 in 92.52% and from those of 2018 with 91.41%. However, samples between 2015 and 2018 years differed 63.10% (Supplementary Table 3).
In general, the most abundant bacterial phylotypes were Mycoplasma (42.5 ± 0.3%, relative abundance of total sequences), four Endozoicomonas ASVs (34.1 ± 0.08%). A set of ASVs mainly conformed by Mycoplasma and Endozoicomonas bacteria members drove the differentiation between 2015 and 2018 on one hand, and 2017 on the other hand. Some of these ASVs were absent in 2017 while were highly abundant in 2015 and 2018, and the other way around (Fig. 3a, Supplementary Table S3). Specifically, in 2017 Mycoplasma (ASV8, ASV9), Endozoicomonas (ASV12 and ASV21), relative abundances were highly abundant, while in 2015 and 2018 were not present (Fig. 3a). On the other hand, in 2015 and 2018 the coral microbiome showed high relative abundances of Mycoplasma (ASV1), Endozoicomonas (ASV2, ASV3, ASV4, ASV5 and ASV7), Nitrincolaceae (ASV6) and Spirochaeta (ASV13). Accordingly, 2015 and 2018 shared 63% of the ASVs, while 2015 and 2017 shared 0.3%, and 2018 and 2017 shared 0.2% (Fig. 3).
Main ASVs driving the dissimilarity and similarity between Pacifigorgia cairnsi colonies during sampling years. Heatmap of normalized abundances of ASVs mainly contributed to the differentiation between Pacifigorgia cairnsi samples according to the SIMPER analyses (A). Venn diagram showing the percentage of ASVs shared between Pacifigorgia cairnsi samples (B).
Finally, no clustering groups were observed in predicted metabolic functions according to sampling years. A group of samples including 2015, 2017 and 2018 years were enriched in dehalogenation, ammonia oxidizer, sulfide oxidizer, sulfate reducer, nitrite reducer (Fig. 4).
Taxonomy-based functional profiling of bacterial communities in Pacifigorgia cairnsi samples. Shifts in potential functional differences are represented by a relative abundance scale showing the enrichment (red colour) and depletion (blue colour) in different metabolic profiles mapped to the corresponding taxonomic information by METAGENassist. Hierarchical clustering of samples and functions was performed by a single linkage algorithm using Euclidean distance measurements.
Discussion
We tested for shifts in the bacterial microbiome of gorgonian corals, without direct anthropogenic impacts, during different phases of ENSO events. Although there may exist potential limitations due to our sampling size, our results revealed similar bacterial communities between the warming (El Niño) and the cooling (La Niña) phases of the ENSO cycle, but different bacterial microbiomes between those phases, and the neutral phase. These differences were driven by a differentiation in relative abundances of a set of bacteria mainly constituted by members of Mycoplasma and Endozoicomonas taxa, with functional redundancy acting as an extended phenotype and leading acclimation of the coral under global-scale thermal anomalies, and thus potentially contributing to maintain the homeostasis at the holobiont level despite temperature regime changes.
Pacifigorgia cairnsi colonies showed evident changes in their microbiome structure in response to global thermal conditions derived from ENSO cycle phases. Less microbial diversity was observed in anomalous thermal years (El Niño and La Niña, 2015 and 2018, respectively) in comparison to the neutral thermal year (2017). Thermal changes affect vulnerable sessile organisms such as corals in many aspects. Diminished immune system leading to coral bleaching, disease outbreaks and dysbiosis have been linked to rising seawater temperatures50. Likewise, changes in coral-associated microbiomes have been observed in response to thermal stress, leading to coral die-offs24,26,51. The increase of bacterial diversity associated with disrupted microbiomes is a common trend found in corals affected by diseases52,53. In fact, microbiome shifts induced by disturbances are considered stochastic and thus dysbiotic individuals have more variability in microbial community compositions than healthy individuals in what is known as the ‘Anna Karenina principle’54. Increased diversity has been observed in microbiomes of heat-stressed and diseased individuals of corals such as Orbicella faveolata, Pavona duerdeni and Porites lutea and sea anemones55,56,57. However, this trend contrasts with our findings, likely indicating that P. cairnsi holobiont is under an acclimation process rather than a dysbiotic state.
Evident changes in microbiome compositions were observed between sampling years, revealing that thermal shifts derived from ENSO events have effects on P. cairnsi microbiomes. Changes of microbiome structures associated to thermal anomalies have been largely observed in corals in detriment of the holobiont’s viability58,59. However, these kinds of shifts affecting gorgonian corals remain largely elusive. Interestingly, we registered closer bacterial compositions between el Niño and La Niña phases (2015 and 2018, respectively) than from those in the neutral phase (2017). To our knowledge this is the first study reporting comparable in situ microbiome shifts for heat and cold stress for the same gorgonian coral species. Usually, findings are related with heat-induced microbiome shifts in detriment of coral’s viability (i.e. leading corals towards disease-prone states, coral bleaching, etc.)59. Some studies have shown no microbiome variations, suggesting coral’s resilience to short-term thermal stress46,60. Thus, our results suggest an acclimated coral holobiont not just to warm but also to cold stress by embracing similar microbiome shifts that can potentially maintain coral’s viability. The latter is evidenced by the absence of disease (NPD) symptoms and NPD-associated opportunistic microbial consortium (see Quintanilla et al.43).
Changes in P. cairnsi microbiome compositions between thermal anomalies and thermal normality mainly occurred within the same bacterial taxa. Redundancy and plasticity in core members of the microbiome may act as an extended phenotype, allowing acclimation during temperature changes61,62. This is, members from Endozoicomonas, Mycoplasma and Spirochaeta taxonomic groups were contrarily present in the warming and cooling phase of ENSO on one side, and in the neutral phase on the other side. Interestingly, Endozoicomonas and Mycoplasma bacterial members were found to be dominant core microbiome members of healthy P. cairnsi colonies43 and are known to typically constitute dominant endosymbionts in scleractinian and gorgonian core microbiomes42,63,64. Some Endozoicomonas produce antimicrobial compounds against pathogens, thus contributing to maintain the coral health state19,65. Mycoplasma, although is a dominant member of the microbiome of healthy colonies of gorgonians and cold-water scleractinians, its specific role within the coral holobiont remains unclear43,47. Therefore, the fact that we found an enrichment of specific Endozoicomonas and Mycoplasma members in corals under thermal anomalies may be indicative of possible protection of the holobiont against environmental-thermal stress. Moreover, Nitrincolaceae is a family found enriched in disease corals and hypoxic coral samples66,67, environments under stress conditions. These findings are consistent with the high levels of Nitrincolaceae members found in the abnormal thermal years, the cool and warm phases of ENSO. Finally, Spirochaeta is a chemioheterotrophic bacteria found in different environments68. Spirochaetes members are reported in healthy corals such as Muricea californica42, Paragorgia arborea69, Corallium rubrum70, Thouarella superba71 but also in diseased corals55,72,73. Members of Spirochaetes were suggested as potential nitrogen fixers74 and carbon fixers75. We found Spirochaeta in all samples of thermal anomalies (2015, 2018) in higher abundances than in neutral thermal conditions (2017). Due to its important role in host metabolism76, it is probable that this bacteria is key in restructuring the P. cairnsi microbiome in order to prevent a dysbiosis state during thermal anomalies.
Pacifigorgia cairnsi harbouring different bacterial members from the same taxonomic group under thermal anomalies may indicate that the holobiont is choosing a best-suited microbiome to face the environmental disturbances. Three concepts are related with the latter. The first one, the ‘Coral Probiotic hypothesis’, which states that corals can improve coral health and resilience by shifting the abundance and diversity of their microbial partners and thus selecting the most advantageous coral holobiont in response to changing environmental conditions77. The second concept, the ‘symbiont switching’, which states that coral acquires novel bacteria from the environmental pool under altered environmental conditions, triggering coral acclimation78. For instance, symbiont switching to thermally resistant Symbiodinium clades has been reported in zooxanthellate corals79,80,81,82. Additionally, some bacteria have been registered to facilitate resilience in corals during warm temperature anomalies77,83. The third concept related with our results is ‘symbiont shuffling’, which states that microbial frequency shifts are observed during environmental stress to acclimate the coral holobiont78. See Fig. 5. Specifically, Endozoicomonas (ASV4, ASV5 and ASV7) were enriched in the SST anomaly years compared with the neutral phase (Fig. 5). In fact, proliferation of Endozoicomonas after warm periods has been related to the ability to mitigate holobiont dysbiosis to thermal stress and to the acclimation and resilience capacity of coral microbiomes84. Therefore, these azooxanthellate sea fans may be extending their phenotypes by selecting the most advantageous holobiont under different ENSO phases (warming and cooling) by shifting the structure and diversity of associated bacterial communities of their core microbiome for others of the same taxon, thus allowing the acclimation and adaptation of the holobiont under thermal stress.
Proposed bacterial community dynamics potentially supporting holobiont acclimation of Pacifigorgia cairnsi via shuffling (blue) and switching (purple). Coral holobiont under neutral phase maintains specific microorganisms. The microbial shifts help the coral to acquire more flexibility under SST anomalies (El Niño and La Niña phases) and acclimate via functional redundancy. Labels of relevant bacterial members driving microbiome shifts and acclimation and their corresponding ASV numbers are shown in right.
Results in the microbiome composition are consistent with the trend observed in the predicted functional profiling of the microbiome. Although the microbiome composition changed, no differences were observed in the predicted functionality between the three years of the study. Vital functions for the viability of the coral holobiont85, such as those related with nitrogen and sulfur cycles seem to be equally expressed in P. cairnsi microbiomes in neutral year and thermal anomaly years. This suggests that P. cairnsi exhibits metabolic redundancy in their microbiome despite its composition changes under thermal stress, meaning that different bacterial species fulfil similar functions within the coral holobiont. By shifting some core microbiome members (potentially for more thermally resistant ones), the sea fan secures to maintain the core microbiome functions and thus achieves holobiont plasticity and stability for rapid acclimation under thermal anomalies28,86,87,88,89. Additionally, the fact that these are azooxanthellate corals (i.e., no dependency on autotrophy) could also support their acclimation potential and resilient capacity to environmental changes90,91. The patterns found here are particularly interesting since numerous reef-building coral species worldwide have shown vulnerability and decay patterns when facing thermal stress92,93,94. Therefore, our findings highlight that resilience and adaptability capacity of gorgonian corals, possibly enhanced by the pristine conditions of the environment.
Overall, our results suggest coral holobiont acclimation not just to thermal but also to cold stress by embracing similar microbiome shifts. These findings may be indicating that this coral holobiont has a well-established relationship with their microbial partners, all together potentially representing a solid acclimated and resilient mechanism under thermal and cold stress.
By restructuring microbial communities and by maintaining functional redundancy, the gorgonian holobiont shows adaptation to thermal stress derived from global-scale disturbances such as ENSO events. This study reveals that the coral microbiome has the ability to adapt rapidly, acting as an extended plastic phenotype, during thermal stress under pristine conditions in order to maintain fitness at the holobiont level.
In further studies we encourage to increase sample size and to incorporate omic approaches (e.g. metagenomics and metatranscriptomics) in order deepen and confirm microbiome mechanisms of P. cairnsi acclimation to ENSO-derived thermal anomalies. We also suggest studying abiotic changes related to ENSO events that may also modulate the microbiome shifts in order to obtain an overview of different processes that may affect the response of the coral holobiont under thermal stress conditions. Finally, we suggest assessing coral microbiome dynamics under future predictions of ENSO events in order to understand ongoing responses and the adaptability threshold of coral holobionts under climate change scenarios.
Materials and methods
Study site and sample collection
Pacifigorgia cairnsi sampling was conducted in Malpelo (Arrecife reef), an oceanic remote island of difficult access about 500 km off the Colombian coast in the Tropical Eastern Pacific (3° 58′ 30″ N, 81° 34′ 48″ W) (Fig. 1). Malpelo Fauna and Flora Sanctuary is a marine protected area and a World Heritage Site, considered a hot spot of biodiversity under pristine conditions34,37. Samples were collected in September 2015 (n = 19), April 2017 (n = 5) and April 2018 (n = 7) (Fig. 1). Due to the remote condition and difficult access to Malpelo Island, having a large number of samples from different years, is particularly valuable. Sampling description is detailed in Quintanilla et al.43. After collection each sample was gently rinsed with 100 ml filtered fresh water to remove transient microorganisms loosely associated with the coral tissue. All samples were taken from colonies visibly healthy (i.e. absence of NPD symptoms, see Quintanilla et al.43). Samples were stored in RNAlater (Thermo Fisher Scientific, Waltham, USA) for 2015 samples and in DMSO for 2017 and 2018. All samples were preserved at − 80 °C until subsequent DNA extraction. Collections were made possible with research permit No. 105 (2013), issued by the Autoridad Nacional de Licencias Ambientales-ANLA, Ministerio de Ambiente y Desarrollo Sostenible, Colombia, Contrato de Acceso a Recursos Genéticos para Investigación Científica Sin Interés Comercial No. 106, 20 (2014) RGE0114 [1] and through resolution 1177 of October 9th, 2014-IBD 0359 from Universidad de los Andes.
Regarding global thermal conditions and according to the National Oceanic and Administration (NOAA) (http://www.cpc.ncep.noaa.gov), September 2015 corresponded to the El Niño-warming phase warming phase (+ 1.8/ + 2.1 °C), April 2017 corresponded to the Neutral phase (+ 0.1/ + 0.3 °C) and April 2018 to La Niña-cooling phase (− 0.6/− 0.4 °C). See Fig. 1. Sea surface temperatures were provided by Fundación Malpelo.
DNA extraction and 16S rRNA gene sequencing
Sequences of 2015 were taken from Quintanilla et al.43 (peripheral tissues from healthy colonies-HP). Sequences of 2017 and 2018 were obtained following those author’s descriptions. In brief, the variable V4 region of the 16S rRNA gene was sequenced using the 515F/806R PCR primers and Illumina flowcell adapter sequences according to the earth microbiome protocol95 (http://www.earthmicrobiome.org/emp-standard-protocols/16s/). The Takara Taq DNA polymerase premix was used for PCR amplifications as referred in Quintanilla et al.43. Barcoded amplicons were pooled and sequenced on the Illumina MiSeq platform (Illumina, San Diego, USA), implementing 2 × 250 bp paired end read libraries.
Microbiome data analyses
Amplicon sequence data were demultiplexed, assembled and analysed using QIIME2 version 2023.5)96 to identify Amplicon Sequence Variants (ASVs). Denoising, chimera filtering, and trimming was performed in Deblur97. Taxonomy was assigned using the trained classifier SILVA 138 at 99% of similarity (silva-138-nb-classifer)98. Singleton ASVs were removed to minimize false ASVs, as well as ASVs identified as eukaryotes, mitochondria or chloroplasts. Subsequently, the table was filtered to remove uninformative and low quality features to keep ASVs with at least 3 counts in at least 3 samples (~ 10% of the total samples).
Alpha diversity metrics analysis (total number of observed ASVs, species richness Chao1, Shannon and Simpson diversity index) and rarefaction curves were generated through phyloseq package99 in R version 4.3.1 in order to obtain a comprehensive description of the within-sample bacterial community. Differences in alpha diversity metrics between sampling years were tested with Kruskal–Wallis and ANOVA tests.
To assess differences in bacterial community compositions between samples from different sampling years (beta diversity) we considered taxa accounting for the cumulative 99% of total abundance. We conducted multivariate analyses using PRIMER 6 & PERMANOVA+ software100. In order to visualize differences in bacterial community compositions between sampling years, principal coordinate analysis (PCoA) was performed, applying a square-root transformation to relative abundances and calculating Bray–Curtis dissimilarity matrices. Permutational Analyses of Variance (PERMANOVA) were conducted to test differences in bacterial community compositions between sampling years (9999 permutations). Additionally, Permutational Analysis of Multivariate Dispersions (PERMDISP) were used to test for homogeneity of multivariate dispersions (9999 permutations) between sampling groups. Similarity percentage (SIMPER) analyses were used to identify the taxa contributing to the greatest extent to the observed patterns, taking into account a cutoff greater than 1% of contribution. Accordingly, a heat map was elaborated using the package phyloseq with the function plot_taxa_heatmap using log10 as normalizing parameter. Venn diagrams showing shared and unique ASVs per sampling year (percentage of the relative abundances) were performed using the package ampvis2 with the function amp_venn with an abundance cutoff of 0.1 and frequency cutoff of 10.
Putative functional differences associated with shifts in bacterial community compositions among sampling years were assessed by using METAGENassist101. ASVs filtering and normalization parameters were used as described by102. Euclidean distance measure (single linkage algorithm) was used to visualize functional profiles in heatmap mapped to the microbial communities.
Accession numbers
The sequences were deposited in the NCBI Sequence Read Archive (SRA) under BioProject number PRJNA403829 for 2015, BioProject number PRJNA722752 for 2017 samples (SAMN18791299-SAMN18791303) and Bioproject number PRJNA737787 for 2018 samples (SAMN19712929-SAMN19712935).
Data availability
All data are available in the main text or the supplementary materials. The sequences were deposited in the NCBI Sequence Read Archive (SRA) under BioProject number PRJNA403829 for 2015, BioProject number PRJNA722752 for 2017 samples (SAMN18791299-SAMN18791303) and Bioproject number PRJNA737787 for 2018 samples (SAMN19712929-SAMN19712935).
References
Hoegh-Guldberg, O. & Bruno, J. F. The impact of climate change on the world’s marine ecosystems. Science (80–) 328, 1523–1528 (2010).
Bruno, J. F. et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 5, 25 (2007).
Garrabou, J. et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Chang. Biol. 15, 1090–1103 (2009).
Erez, J., Reynaud, S., Silverman, J., Schneider, K. & Allemand, D. Coral Reefs: An Ecosystem in Transition 151–176 (Springer, 2011).
Hetzinger, S., Pfeiffer, M., Dullo, W.-C., Zinke, J. & Garbe-Schönberg, D. A change in coral extension rates and stable isotopes after El Niño-induced coral bleaching and regional stress events. Sci. Rep. 6, 32879 (2016).
Quintanilla, E., Madurell, T., Wilke, T. & Sánchez, J. A. Dynamic interplay of ENSO events and local hydrodynamic parameters drives demography and health status of Gorgonian sea fan populations on a remote tropical Eastern Pacific Island. Front. Mar. Sci. 6, 694 (2019).
Turkington, T., Timbal, B. & Rahmat, R. The impact of global warming on sea surface temperature based El Niño-Southern Oscillation monitoring indices. Int. J. Climatol. 39, 1092–1103 (2019).
Santoso, A. et al. Late-twentieth-century emergence of the El Niño propagation asymmetry and future projections. Nature 504, 126–130 (2013).
Cai, W. et al. Increasing frequency of extreme El Niño events due to greenhouse warming. Nat. Clim. Change 4, 111–116 (2014).
Heron, S. F., Maynard, J. A., van Hooidonk, R. & Eakin, C. M. Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci. Rep. 6, 38402 (2016).
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017).
Rohwer, F., Seguritan, V., Azam, F. & Knowlton, N. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Prog. Ser. 243, 1–10 (2002).
Knowlton, N. & Rohwer, F. Multispecies microbial mutualisms on coral reefs: The host as a habitat. Am. Nat. 162, 51–62 (2003).
Bourne, D. G. et al. Microbial disease and the coral holobiont. Cell Press 17, 554–562 (2009).
Tout, J. et al. Variability in microbial community composition and function between different Niches within a Coral Reef. Microb. Ecol. 20, 540–552 (2014).
Bourne, D. G., Morrow, K. M. & Webster, N. S. Insights into the coral microbiome: Underpinning the health and resilience of reef ecosystems. Annu. Rev. Microbiol. 70, 317–340 (2016).
van de Water, J. A. J. M., Allemand, D. & Ferrier-Pagès, C. Host-microbe interactions in octocoral holobionts—recent advances and perspectives. Microbiome 6, 25 (2018).
Rosenberg, E., Koren, O., Reshef, L., Efrony, R. & Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 5, 355–362 (2007).
Rypien, K. L., Ward, J. R. & Azam, F. Antagonistic interactions among coral-associated bacteria. Environ. Microbiol. 12, 28–39 (2010).
Bosch, T. C. G. & McFall-Ngai, M. J. Metaorganisms as the new frontier. Zoology 114, 185–190 (2011).
Bosch, T. C. G. Cnidarian-microbe interactions and the origin of innate immunity in metazoans. Annu. Rev. Microbiol. 67, 499–518 (2013).
Torda, G. et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 7, 627–636 (2017).
Röthig, T., Yum, L. K., Kremb, S. G., Roik, A. & Voolstra, C. R. Microbial community composition of deep-sea corals from the Red Sea provides insight into functional adaption to a unique environment. Sci. Rep. 7, 44714 (2017).
Ziegler, M., Seneca, F. O., Yum, L. K., Palumbi, S. R. & Voolstra, C. R. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 8, 14213 (2017).
Gardner, S. G. et al. Coral microbiome diversity reflects mass coral bleaching susceptibility during the 2016 El Niño heat wave. Ecol. Evol. 9, 938–956 (2019).
Grottoli, A. G. et al. Coral physiology and microbiome dynamics under combined warming and ocean acidification. PLoS One 13, e0191156 (2018).
Ziegler, M. et al. Coral bacterial community structure responds to environmental change in a host-specific manner. Nat. Commun. 10, 3092 (2019).
Marangon, E., Laffy, P. W., Bourne, D. G. & Webster, N. S. Microbiome-mediated mechanisms contributing to the environmental tolerance of reef invertebrate species. Mar. Biol. 168, 89 (2021).
Goldberg, J. & Wilkinson, C. In Status of Coral Reefs of the World (ed. Wilkinson, C.) 67–92 (2004).
Sánchez, J. A. et al. In Mesophotic coral ecosystems, coral reefs of the world (ed. Loya Y, Puglise KA, B. T.) 729–747 (2019).
Solan, M. et al. Extinction and ecosystem function in the marine benthos. Science (80–) 306, 1177–1180 (2004).
Linares, C., Doak, D. F., Coma, R., Díaz, D. & Zabala, M. Life history and population viability of a long-lived marine invertebrate: The octocoral Paramuricea clavata. Ecology 88, 918–928 (2007).
Zapata, F. A. & Vargas-Ángel, B. In Latin American Coral Reefs (ed. Cortés, J.) 419–447 (Elsevier, 2003).
Quimbayo, J. P., Mendes, T. C., Kulbicki, M., Floeter, S. R. & Zapata, F. A. Unusual reef fish biomass and functional richness at Malpelo, a remote island in the Tropical Eastern Pacific. Environ. Biol. Fishes 100, 149–162 (2016).
Sánchez, J. A., Gómez, C. E., Escobar, D. & Dueñas, L. F. Diversidad, abundancia y amenazas de los octocorales de la Isla Malpelo, Pacífico Oriental Tropical, Colombia. Bol. Investig. Mar. Costeras 40, 139–154 (2011).
Sánchez, J. A. et al. Octocoral densities and mortalities in Gorgona Island, Colombia. Trop. Eastern Pac. Rev. Biol. Trop. 62, 209–219 (2014).
Zapata, F. A. & Vargas-Ángel, B. In Latin American Coral Reefs 419–447 (2003). https://doi.org/10.1016/B978-044451388-5/50019-9.
Glynn, P. W. & Colgan, M. W. Sporadic disturbances in fluctuating coral reef environments: El Niño and coral reef development in the Eastern Pacific. Am. Zool. 32, 707–718 (1992).
Zapata, F. A., Rodríguez-Ramírez, A., Caro-Zambrano, C. & Garzón-Ferreira, J. Mid-term coral-algal dynamics and conservation status of a Gorgona Island (Tropical Eastern Pacific) coral reef. Rev. Biol. Trop. 58, 81–94 (2010).
Sánchez, J. A. et al. The masquerade game: Marine mimicry adaptation between egg-cowries and octocorals. PeerJ 4, e2051 (2016).
Correa, H., Haltli, B., Duque, C. & Kerr, R. Bacterial communities of the Gorgonian octocoral Pseudopterogorgia elisabethae. Invertebr. Microbiol. 66, 972–985 (2013).
Holm, J. B. & Heidelberg, K. B. Microbiomes of Muricea californica and M. fruticosa: Comparative analyses of two co-occurring Eastern Pacific Octocorals. Front. Microbiol. 7, 25 (2016).
Quintanilla, E. et al. Local confinement of disease-related microbiome facilitates recovery of gorgonian sea fans from necrotic-patch disease. Sci. Rep. 8, 14636 (2018).
van de Water, J. A. J. M. et al. Seasonal stability in the microbiomes of temperate Gorgonians and the Red Coral Corallium rubrum across the Mediterranean Sea. Microb. Ecol. 75, 274–288 (2018).
Randall, C. J., Toth, L. T., Leichter, J. J., Maté, J. L. & Aronson, R. B. Upwelling buffers climate change impacts on coral reefs of the eastern tropical Pacific. Ecology 101, e02918 (2020).
Wessels, W., Sprungala, S., Watson, S., Miller, D. J. & Bourne, D. G. The microbiome of the octocoral Lobophytum pauciflorum: Minor differences between sexes and resilience to short-term stress. FEMS Microbiol. Ecol. 93, 25 (2017).
McCauley, M., Jackson, C. R. & Goulet, T. L. Microbiomes of caribbean octocorals vary over time but are resistant to environmental change. Front. Microbiol. 11, 25 (2020).
Wang, C. & Fiedler, P. C. ENSO variability and the eastern tropical Pacific: A review. Prog. Oceanogr. 69, 239–266 (2006).
Anderson, M. J., Ellingsen, K. E. & McArdle, B. H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 9, 683–693 (2006).
Ruiz-Moreno, D. et al. Global coral disease prevalence associated with sea temperature anomalies and local factors. Dis. Aquat. Organ. 100, 249–261 (2012).
Leggat, W. P. et al. Rapid coral decay is associated with marine heatwave mortality events on reefs. Curr. Biol. 29, 2723–2730 (2019).
Roder, C., Arif, C., Daniels, C., Weil, E. & Voolstra, C. R. Bacterial profiling of White Plague Disease across corals and oceans indicates a conserved and distinct disease microbiome. Mol. Ecol. 23, 965–974 (2014).
Meyer, J. L. et al. Epimicrobiota associated with the decay and recovery of Orbicella corals exhibiting dark spot syndrome. Front. Microbiol. 7, 1–9 (2016).
Zaneveld, J. R., McMinds, R. & Thurber, R. V. Stress and stability: Applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2, 25 (2017).
Closek, C. J. et al. Coral transcriptome and bacterial community profiles reveal distinct Yellow Band Disease states in Orbicella faveolata. ISME J. 8, 2411–2422 (2014).
Roder, C., Bayer, T., Aranda, M., Kruse, M. & Voolstra, C. R. Microbiome structure of the fungid coral Ctenactis echinata aligns with environmental differences. Mol. Ecol. 24, 3501–3511 (2015).
Ahmed, H. I., Herrera, M., Liew, Y. J. & Aranda, M. Long-term temperature stress in the coral model aiptasia supports the ‘Anna Karenina Principle’ for bacterial microbiomes. Front. Microbiol. 10, 25 (2019).
Wang, L. et al. Corals and their microbiomes are differentially affected by exposure to elevated nutrients and a natural thermal anomaly. Front. Mar. Sci. 5, 25 (2018).
Pootakham, W. et al. Heat-induced shift in coral microbiome reveals several members of the Rhodobacteraceae family as indicator species for thermal stress in Porites lutea. Microbiologyopen 8, e935 (2019).
Epstein, H. E., Smith, H. A., Torda, G. & van Oppen, M. J. Microbiome engineering: Enhancing climate resilience in corals. Front. Ecol. Environ. 17, 100–108 (2019).
Bailey, N. W. Evolutionary models of extended phenotypes. Trends Ecol. Evol. 27, 561–569 (2012).
Hunter, P. The revival of the extended phenotype. EMBO Rep. 19, 25 (2018).
Bayer, T. et al. Bacteria of the genus Endozoicomonas dominate the microbiome of the Mediterranean gorgonian coral Eunicella cavolini. Mar. Ecol. Prog. Ser. 479, 75–84 (2013).
Vezzulli, L., Pezzati, E., Huete-Stauffer, C., Pruzzo, C. & Cerrano, C. 16SrDNA pyrosequencing of the mediterranean gorgonian Paramuricea clavata reveals a link among alterations in bacterial holobiont members, anthropogenic influence and disease outbreaks. PLoS One 8, 25 (2013).
Rua, C. P. J. et al. Diversity and antimicrobial potential of culturable heterotrophic bacteria associated with the endemic marine sponge Arenosclera brasiliensis. PeerJ 2, e419. https://doi.org/10.7717/peerj.419 (2014).
Johnson, M. D. et al. deoxygenation on a Caribbean coral reef. Nat. Commun. 12, 25 (2021).
Huntley, N. et al. Experimental transmission of Stony Coral Tissue Loss Disease results in differential microbial responses within coral mucus and tissue. ISME Commun. 2, 46 (2022).
Ludwig, W., Euzéby, J. & Whitman, W. B. In Bergey’s Manual® of Systematic Bacteriology: Volume Four The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes (eds Krieg, N. R. et al.) 1–19 (Springer, 2010).
Weiler, B. A., Verhoeven, J. T. P. & Dufour, S. C. Bacterial communities in tissues and surficial mucus of the cold-water coral Paragorgia arborea. Front. Mar. Sci. 5, 25 (2018).
van de Water, J. A. J. M. et al. Spirochaetes dominate the microbial community associated with the red coral Corallium rubrum on a broad geographic scale. Sci. Rep. 6, 1–7 (2016).
Gray, M. A., Stone, R. P., McLaughlin, M. R. & Kellogg, C. A. Microbial consortia of gorgonian corals from the Aleutian islands. FEMS Microbiol. Ecol. 76, 109–120 (2011).
Frias-lopez, J., Zerkle, A. L., Bonheyo, G. T. & Fouke, B. W. Partitioning of bacterial communities between seawater and healthy. Black Band Dis. Dead Coral Surf. 68, 2214–2228 (2002).
Sekar, R., Kaczmarsky, L. T. & Richardson, L. L. Microbial community composition of black band disease on the coral host Siderastrea siderea from three regions of the wider Caribbean. Mar. Ecol. Prog. Ser. 362, 85–98 (2008).
Lawler, S. N. et al. Coral-associated bacterial diversity is conserved across two deep-sea anthothela species. Front. Microbiol. 7, 1–18 (2016).
Leadbetter, J. R., Schmidt, T. M., Graber, J. R. & Breznak, J. A. Acetogenesis from H2 plus CO2 by spirochetes from termite guts. Science 283, 686–689 (1999).
Ainsworth, T. D. et al. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J. 9, 2261–2274 (2015).
Reshef, L., Koren, O., Loya, Y., Zilber-rosenberg, I. & Rosenberg, E. The coral probiotic hypothesis. Environ. Microbiol. 8, 2068–2073 (2006).
Webster, N. S. & Reusch, T. B. H. Microbial contributions to the persistence of coral reefs. ISME J. 11, 2167–2174 (2017).
Oliver, T. A. & Palumbi, S. R. Many corals host thermally resistant symbionts in high-temperature habitat. Coral Reefs 30, 241–250 (2011).
Silverstein, R. N., Cunning, R. & Baker, A. C. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Chang. Biol. 21, 236–249 (2015).
Boulotte, N. M. et al. Exploring the Symbiodinium rare biosphere provides evidence for symbiont switching in reef-building corals. ISME J. 10, 2693 (2016).
Núñez-Pons, L., Bertocci, I. & Baghdasarian, G. Symbiont dynamics during thermal acclimation using cnidarian-dinoflagellate model holobionts. Mar. Environ. Res. 130, 303–314 (2017).
Tracy, A. M., Koren, O., Douglas, N., Weil, E. & Harvell, C. D. Persistent shifts in Caribbean coral microbiota are linked to the 2010 warm thermal anomaly. Environ. Microbiol. Rep. 7, 471–479 (2015).
Maher, R. L. et al. Coral microbiomes demonstrate flexibility and resilience through a reduction in community diversity following a thermal stress event. Front. Ecol. Evol. 8, 25 (2020).
Thompson, J. R., Rivera, H. E., Closek, C. J., Medina, M. & Van Oppen, M. J. H. Microbes in the coral holobiont: Partners through evolution, development, and ecological interactions. Front. Cell. Infect. Microbiol. 4, 1–20 (2015).
Ra, N., Pogoreutz, C., Voolstra, C. R. & Wild, C. Nitrogen cycling in corals: The key to understanding holobiont functioning?. Trends Microbiol. 23, 25 (2015).
Pogoreutz, C. et al. Nitrogen fixation aligns with nifH abundance and expression in two coral trophic functional groups. Front. Microbiol. 8, 25 (2017).
Louca, S. et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2, 936–943 (2018).
Voolstra, C. R. & Ziegler, M. Adapting with microbial help: Microbiome flexibility facilitates rapid responses to environmental change. BioEssays 42, 25 (2020).
Lasker, H. R., Bramanti, L., Tsounis, G. & Edmunds, P. J. The rise of octocoral forests on Caribbean reefs. In Advances in Marine Biology (ed. Sad, D.) 361–410 (Academic Press, 2020).
Derviche, P., Menegotto, A. & Lana, P. Carbon budget trends in octocorals: A literature review with data reassessment and a conceptual framework to understand their resilience to environmental changes. Mar. Biol. 169, 159 (2022).
Putnam, H. M., Barott, K. L., Ainsworth, T. D. & Gates, R. D. The vulnerability and resilience of reef-building corals. Curr. Biol. 27, R528–R540 (2017).
Lough, J. M., Anderson, K. D. & Hughes, T. P. Increasing thermal stress for tropical coral reefs: 1871–2017. Sci. Rep. 8, 6079 (2018).
Cant, J. et al. The projected degradation of subtropical coral assemblages by recurrent thermal stress. J. Anim. Ecol. 90, 233–247 (2021).
Gilbert, J. A., Jansson, J. K. & Knight, R. The Earth Microbiome project: Successes and aspirations. BMC Biol. 12, 69 (2014).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Amir, A. et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2, e00191-e216 (2017).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8, e61217 (2013).
Anderson, M., Gorley, R. & Clarke, K. P. For PRIMER: Guide to software and statistical methods. Prim. Plymouth UK 20, 20 (2008).
Arndt, D. et al. METAGENassist: A comprehensive web server for comparative metagenomics. Nucleic Acids Res. 40, 88–95 (2012).
Hadaidi, G. et al. Stable mucus-associated bacterial communities in bleached and healthy corals of Porites lobata from the Arabian Seas. Sci. Rep. 7, 1–11 (2017).
Acknowledgements
We thank the Fundación Malpelo and Parques Nacionales Naturales (PNN) in Colombia for organizing the expeditions to Malpelo Island, especially Sandra Bessudo and Nancy Murillo for their valuable support. We also acknowledge the colleagues and students from the Laboratorio de Biología Molecular Marina (BIOMMAR, Universidad de los Andes) for their support in the field work and laboratory stage. Finally, we thank the HPC support team of Universidad de Los Andes.
Funding
This study was funded by: Fundación para la Promoción de la Investigación y la Tecnología (Grant No. 3744). University of Los Andes (project P17.160322.001/01-B1017 and Vicerrectoria de Investigaciones: Programas de investigación and proyectos de finalización). Molecular Marine Biology (BIOMMAR) laboratory. COLCIENCIAS (Grant No. 1204-521-29002). Center of Excellence in Marine Sciences (CEMarin) in Bogotá.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.M.S., E.Q. Methodology: S.M.S., E.Q. Interpretation of results: S.M.S., E.Q., J.A.S. Writing: S.M.S., E.Q. Revision of the manuscript: S.M.S., E.Q., J.A.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montaño-Salazar, S., Quintanilla, E. & Sánchez, J.A. Microbial shifts associated to ENSO-derived thermal anomalies reveal coral acclimation at holobiont level. Sci Rep 13, 22049 (2023). https://doi.org/10.1038/s41598-023-49049-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49049-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.