Abstract
Chlamydophila pneumoniae is a cause of community-acquired pneumonia (CAP) and responsible for 1–2% of cases in paediatric patients. In Mexico, information on this microorganism is limited. The aim of this study was to detect C. pneumoniae using two genomic targets in a real-time PCR and IgM/IgG serology assays in paediatric patients with CAP at a tertiary care hospital in Mexico City and to describe their clinical characteristics, radiological features, and outcomes. A total of 154 hospitalized patients with diagnosis of CAP were included. Detection of C. pneumoniae was performed by real-time PCR of the pst and arg genes. Complete blood cell count, C-reactive protein measurement and IgM and IgG detection were performed. Clinical-epidemiological and radiological data from the patients were collected. C. pneumoniae was detected in 25 patients (16%), of whom 88% had underlying disease (P = 0.014). Forty-eight percent of the cases occurred in spring, 36% in girls, and 40% in children older than 6 years. All patients had cough, and 88% had fever. Interstitial pattern on chest-X-ray was the most frequent (68%), consolidation was observed in 32% (P = 0.002). IgM was positive in 7% and IgG in 28.6%. Thirty-six percent presented complications. Four percent died. A high proportion showed co-infection with Mycoplasma pneumoniae (64%). This is the first clinical report of C. pneumoniae as a cause of CAP in Mexican paediatric patients, using two genomic target strategy and serology. We found a frequency of 16.2% with predominance in children under 6 years of age. In addition; cough and fever were the most common symptoms. Early detection of this pathogen allows timely initiation of specific antimicrobial therapy to reduce development of complications. This study is one of the few to describe the presence of C. pneumoniae in patients with underlying diseases.
Similar content being viewed by others
Introduction
Community-acquired pneumonia (CAP) is an acute infection of the lower respiratory tract and one of the main causes of childhood morbidity and mortality worldwide. Each year, it causes the death of about one million children younger than 5 years old. This number represents approximately 15% of all deaths in this age group, and between 90 and 95% of these deaths occur in developing countries1.
Chlamydophila pneumoniae is an obligate intracellular bacterium that causes respiratory tract infections, such as pharyngitis (5%), bronchitis (0.3–5%), sinusitis (13%), exacerbations of chronic bronchitis, and CAP2,3,4,5. C. pneumoniae is responsible for approximately 1–2% of all cases of CAP in children, mainly affecting those over 3 years of age6. Other reports indicate that the frequency varies between 5 and 20% in school-age children and adolescents7. Epidemiological studies suggest a cyclical pattern (every 4 years) of pneumonia caused by C. pneumoniae. In a worldwide study, the frequency of CAP due to C. pneumoniae, was 8% in North America, 7% in Europe, 6% in Latin America, and 5% in the Asia-Africa region2.
As diagnosis of C. pneumoniae infection is complicated, so its frequency may be underestimated. Despite its low sensitivity (50–70%), culture is still considered the gold standard. Unfortunately, C. pneumoniae grows slowly, and use of McCoy or HeLa cell cultures, specialized laboratory and trained personnel are necessary2,8. Microimmunofluorescence is the serological test of choice, but it is a complex technique, its interpretation is subjective and cross-reaction with other species of Chlamydia spp. also occurs. In addition, it is necessary to collect serum in the initial and convalescent phases to detect an acute infection, with sensitivity of 60–82% and specificity of 40–77%9.
Due to their high analytical sensitivity (50–100 fg) and specificity (100%), molecular techniques, such as real-time PCR are recommended by the Centers for Disease Control and Prevention (CDC) for diagnosis of C. pneumoniae10,11.
In addition, two or more tests are usually used to diagnose this infection as real-time PCR, specific anti-C. pneumoniae IgM, IgG and IgA antibodies or immunohistochemistry12,13,14. Several targets have been used to detect this microorganism including ompA gene,12,15,16, tyrosine tRNA gene in a Pan-Chlamydia real-time PCR17, 16S ribosomal RNA genes15 and 23S ribosomal RNA genes in a novel digital microfluidic RT-qPCR Platform18. The arg gene encodes the 147 amino acid arginine repressor protein that regulates the glnPQ operon which expresses a putative arginine transport system in an arginine-responsive manner. The arg gene has been used to detect and differentiate C. pneumoniae from other species of the genus Chlamydophila10,17. On the other hand, pst gene encodes DNA-directed RNA polymerase subunit beta, a 75 kDa protein10,11.
Some studies have considered that a positive PCR test is sufficient to diagnose a C. pneumoniae infection13.
Timely detection allows for administration of adequate antimicrobial treatment, as chronic or recurrent infections by C. pneumoniae have been associated with development of complications such as chronic obstructive pulmonary disease, asthma, arthritis, heart disease, and neurological disorders (multiple sclerosis and Alzheimer's disease)19,20,21. C. pneumoniae can also increase risk of lung cancer development (odds ratio 1.48–1.6)22.
In Mexico, information on C. pneumoniae as a cause of CAP in the paediatric population is limited. There is only one retrospective report that indicates involvement of C. pneumoniae in adolescents with asthma, in which a seroprevalence of 77.5% was found23.
The aim of this study was to detect C. pneumoniae using two genomic targets in a real-time PCR and IgM/IgG serology assays in paediatric patients with CAP at a tertiary hospital in Mexico City and additionally the study describes their clinical characteristics, radiological features and outcomes.
Material and methods
Location and study population
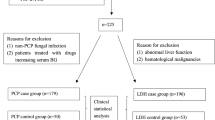
This was a prospective, single-centre study that was conducted at the Instituto Nacional de Pediatria (INP), a tertiary-care hospital in Mexico City. During the study period, November 2015 to March 2017, 154 patients < 18 years old with a diagnosis of CAP that required admission were included.
Definitions and clinical information
Diagnosis of CAP was considered in patients who presented fever (≥ 37.5 °C), cough, polypnea, or respiratory distress and who had hypoventilation, crackles, and/or effusion by clinical examination or chest X-ray. Respiratory distress was defined per guidelines of the Infectious Diseases Society of America24. For the patients included in the study, the above signs and symptoms started in an outpatient setting, and the patient was not hospitalized for at least 2 weeks before the onset of symptoms. Underlying disease was defined as a medical condition that involved any organ or system and that required specialized care25. Acute disease was defined as a short and relatively severe course (< 1 week); chronic disease was any that continued for more than a week. Wheezing was defined as a continuous, adventitious, high-pitched sound that originated in airways narrowed by spasm, thickened mucosa, and/or luminal obstruction. Crackles were discontinuous cracking sounds caused by the passage of air through secretions of the airway due to sudden equalization of gas pressure. A decrease in the level of oxygen in the blood (SpO2) < 92% was recorded as desaturation.
A normal radiological pattern was defined as a chest X-ray with no abnormal findings in the lung parenchyma and pleural spaces. The interstitial pattern was defined as an image resulting from oedema and inflammation, with cellular infiltrates located predominantly in the interstitial tissue of the lung. The bronchopneumonic pattern was characterized by suppurative inflammation distributed in patches around bronchi, which may or may not be localized to a single lobe of the lung. The consolidation pattern was a homogeneous increase in the density of the parenchyma, which erased the contours of vessels and the walls of the airway and could include air bronchogram. The atelectasis represented an increased density with ipsilateral traction of the trachea and mediastinum structures. The multifocal pattern was the presence of a homogeneous increase in density at multiple sites. The overdistention pattern was defined as air trapping, with increased intercostal spaces and/or flattening of the hemidiaphragms. Pleural effusion was defined as the presence of fluid in the pleural cavity resulting from excessive transudation or exudation from the pleural surface26,27. The radiological patterns were independently interpreted by two different infectious diseases paediatricians.
We categorized the age range as follows: term neonatal (birth–27 days), infant (28 days–12 months), toddler (13 months–2 years), early childhood (2–5 years), middle childhood (6–11 years), and early adolescence (12– < 18 years)28.
Biological samples and DNA extraction
A nasopharyngeal swab was taken from each patient with a nylon swab (FLOQ Swabs; COPAN Murrieta, CA, USA). The swab was introduced in 1.5 mL of 0.85% NaCl and transported under refrigeration (4–8 °C), as soon as possible. Samples were centrifuged at 6000g for 10 min. The supernatant was decanted and 180uL of ATL buffer and 20uL of proteinase K (0.5 mg/mL) were added to the pellet. Samples were incubated at 56 °C for 1.5 h. DNA was extracted with QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The DNA was eluted in 200 μL of nuclease-free water and stored at − 20 °C until use.
Two peripheral blood samples were taken, one with EDTA for a complete blood cell count and another without anticoagulant for quantification of C-reactive protein (elevated: > 0.8 mg/L) and detection of IgM and IgG by ELISA. Haematological results were interpreted based on the age range of the patient29.
C. pneumoniae DNA standard curves
By using the reported sequence of C. pneumoniae ATCC 53592D, primers were designed with the program Primer3 (4.0.0)30,31,32 for PCR amplification of a fragment of the pst gene (F: ACATCCATAACGACGCCTTC; R: GGGTGATGTGATTGCTGATG; 456 bp) and the arg gene (F: CGGCAACTCAGGAGGAATTA; R: TTTGCAATCGAAACCATCAA; 361 bp). The reaction was performed using an AB9700 thermocycler (Applied Biosystems, Foster City, CA, USA) with 12.5 mL AmpliTaq Gold® 360 MasterMix (Applied Biosystems, Foster City CA, USA) and 0.4 pmol/μL primers. Fragments were purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany) and cloned into the pDrive vector (Qiagen, Hilden, Germany) according to the manufacturer's instructions. To determine the real-time PCR detection limit, dilutions of recombinant plasmids of the pst and arg genes (107–100 DNA molecules/µL) were prepared; reactions were performed using an ABI-7500-FAST (Applied Biosystems, Foster City, CA, USA). The reaction mixture included 12.5 μL TaqMan Universal Master Mix II with UNG (Applied Biosystems, Foster City, CA, USA), 0.1 μM each primer for pst and arg, TaqMan probes for pst (0.2 μM) and arg (0.1 μM), and 4 mL DNA. We utilized primers and probes previously reported10,11.
Detection of C. pneumoniae in respiratory samples
Detection of C. pneumoniae was performed for the pst and arg genes using real-time PCR with previously reported primers and probes10,11. The human RNase P gene was used as a DNA extraction and amplification control33. The reaction mixture consisted of 12.5 μL TaqMan Universal Master Mix II with UNG (Applied Biosystems, Foster City, CA, USA), 0.1 μM each primer for pst and arg, 0.3 mM primers for RNase P, TaqMan probes for pst (0.2 µM), arg and RNase P (0.1 µM), and 4 µL DNA. The reactions were performed in triplicate using an ABI-7500-FAST (Applied Biosystems, Foster City, CA, USA).
Serological test
Anti-C. pneumoniae IgM and IgG antibodies were detected in 10 µL of serum with anti-C. pneumoniae IgM-IgG human ELISA kits (Abcam, Cambridge, UK) according to the manufacturer's instructions. The results were interpreted as follows: positive: > 11 standard units; indeterminate: 9–11; and negative: < 9.
Statistical analysis
The demographic characteristics, such as gender and age of the patients were described using both absolute frequencies and percentages, as well as presented in terms of medians and ranges. Furthermore, the distribution of underlying diseases, clinical symptoms, and radiographic findings was compared between C. pneumoniae-positive and C. pneumoniae-negative patients employing χ2 or Mann–Whitney U tests. Effect sizes were determined using the Phi index or Kramer's v index in the case of χ2 test comparisons and the r index for comparisons conducted through the Mann–Whitney U test. Effect sizes were categorized as small (S), medium (M), or large (L) based on the criteria "0.1," "0.3," and "0.5," respectively, with values below 0.1 considered presenting null effect (N). A significance level of p < 0.05 was employed for statistical tests. Data analysis was executed utilizing the R software and the functions provided by the R package "effectize"34,35,36.
Ethical approval
This study was approved by the Research and Ethics Committees of the INP (IRB: 00008064, IRB: 00008065) with registration number 2014/058; following the guidelines of the Declaration of Helsinki. Written informed consent was obtained from the parent or guardian of each child enrolled in the study. Patient data were deidentified.
Results
The detection limit of the real-time PCR was 100 DNA molecules/μL for the pst [cycle threshold (Ct) 34.407] and arg (Ct 34.361) genes. During the study period, 154 paediatric patients with CAP were included, and C. pneumoniae was detected in 25 (16.2%). The two genomic targets (pst and arg) were amplified in 13 samples (8.5%), the pst gene alone in three (1.9%), and the arg gene alone in nine (5.8%). RNase P gene amplification was obtained in all samples (median Ct. 28.1, range 19.1–35) (Table 1).
Thirty-six percent of the patients with C. pneumoniae were female and 64% were male. Forty percent were older than 6 years. The median age of patients with C. pneumoniae was 4.7 years (range 0.4–14.8 years). By age group, toddlers were the most frequent (28%), followed by middle childhood (24%) and early childhood (20%). The proportions with and without C. pneumoniae were similar within each age group. CAP most often occurred during spring (n = 12, 48%), followed by autumn (n = 7, 28%) (Table 2).
A total of 88% of the patients with C. pneumoniae had underlying diseases (P = 0.014), mainly congenital (36%) and neurological (16%) (Table 1).
The course of the disease was acute in 56% of the patients. All patients had cough, 88% had fever, and 48% had rhinorrhoea. The median duration of cough was 5 days (range 1–90 days), and for fever was 2.5 days (range 1–10 days). Eight percent presented with headache, and 24% experienced vomiting. Regarding respiratory signs, 93% presented with desaturation, 52% with wheezing, and 68% with crackles. None of these symptoms or clinical signs showed statistically significant associations with C. pneumoniae (Table 3). Thirty per cent of the patients received community treatment with antibiotics, mainly beta-lactams (Table 3). The median duration of illness was 22 days (range 1–98). Median hospital stay was 16 days (range 2–42). For the radiological findings, the interstitial pattern was the most frequent (68%); however, consolidation was significantly associated with infection (32%; P = 0.002). Twenty-eight percent showed a bronchopneumonic pattern, and 16% had overdistention. Twelve patients presented a single radiological pattern, and 13 with mixed pattern (Table 3).
Complete blood count results were obtained for 23 patients with C. pneumoniae. A total of 17.4% had leukocytosis, 39.1% neutrophilia, 69.6% lymphopenia, and 52.2% monocytosis. C-reactive protein was measured in 20 patients: and was reported elevated (> 0.8 mg/L) in 80% (n = 16). No statistical significance was observed for any of these data.
Of the 25 patients with C. pneumoniae, serology was performed in 14. One patient had positive IgM and IgG, and three were only positive for IgG. In 10 patients (71.4%), no anti-C. pneumoniae antibodies were detected.
Thirty-six percent of the patients presented complications, most of them (n = 8) required admission to the paediatric intensive care unit (PICU) for a median of 7 days (range 3–21 days) with mechanical ventilation (median 5 days, range 3–19 days) (P = 0.031). In this series, two patients developed pleural effusion, one of them also presented with autoimmune hemolytic anemia. Another patient developed pericarditis and one patient died with myocarditis (4%). Fifteen patients (64%) had coinfection with M. pneumoniae and three with other bacteria (Streptococcus pyogenes n = 1, Pseudomonas aeruginosa and Escherichia coli n = 1, and methicillin-sensitive Staphylococcus aureus n = 1). No respiratory viral co-infections were analysed.
Discussion
The present work describes the contribution of C. pneumoniae as a causal agent of CAP in Mexican paediatric patients treated in a tertiary care hospital, with a frequency of 16.2%. A retrospective study carried out in Chinese paediatric patients found a similar result (21.8%)37. Frequencies ranging from 0.2 to 18% have been reported for other countries13,20,38,39,40,41,42,43,44,45. These differences may be due to the number of patients included in each study, the diagnostic method used, and the target genes detected. The tests applied in these studies included nested PCR, serology, real-time PCR, and a combination of methods20,38,39,40,41,42,43,44,45. In a study from Brazil, 18% of paediatric patients with CAP were infected by C. pneumoniae based on real-time PCR with the 16S rRNA gene as the genomic target20; in Poland, 5.5% had positive serology (IgM)38 and 8.8% by nested PCR in Peru40. On the other hand, in a study from India in patients with lower respiratory tract infection (LRTI), greater positivity was found by serology (13.3%) than nested PCR (2.6%)46. In the present study, the arg gene was positive in 22 samples and the pst gene in 16. The real-time PCR assay using the arg gene has been validated by the CDC and is the recommended method for detection of C. pneumoniae10. A combination of methods might increase the probability of detection.
Of the patients with CAP due to C. pneumoniae, 64% were boys (P = 0.27). In two studies from India and Japan that included 11 and 42 patients, respectively, with LRTI due to C. pneumoniae, a higher frequency in males was also reported (73 and 58.8%, respectively)46,47. In contrast, it was more frequent in females in Korean paediatric patients (57%)13, whereas no sex predominance was observed in a Peruvian study40. It seems that the gender factor is not decisive for the infection.
Traditionally, it has been established that infections due to atypical agents occur more frequently in children over 5 years of age; however, in our study, 60% of children with C. pneumoniae were under 6 years of age (P = 0.12). Overall, data regarding age distribution differ even in the same country. For example, in China, three independent reports showed different frequencies. In a multicentre study carried out from 2008 to 2018, a frequency of 52.9% was informed in the group aged 6–10 years47, but a study carried out in a single-centre hospital from January 2019 to December 2020 reported that C. pneumoniae was predominant in children older than 6 years (64.8%)48. In a third study that included 81 patients with C. pneumoniae, patients were most often under 1 year of age (49.4%)49. In Peru, C. pneumoniae predominated in the group of 29 days to 2 months old (26.8%), followed by the group of 1 to 5 years (25.4%)40. In India, analysis of 11 patients with LRTIs due to C. pneumoniae revealed predominance in children aged two to six months (90.9%)46. These data indicate that unlike other infectious agents that cause CAP or LRTI, such as viruses, which mainly affect children aged 4 months to 5 years50, Streptococcus pneumoniae, which mainly affects children under 5 years51, and M. pneumoniae, that mainly affects children under 6 years52, C. pneumoniae is equally likely to impact any age group.
The seasonality of C. pneumoniae infection has not been established. In this study, it was most often found in spring (48%). In a Chinese study, a higher frequency was reported during winter (34.3%)37; in other cohorts, a uniform distribution of C. pneumoniae throughout the year was reported38,40,48.
There is limited information on C. pneumoniae as a cause of CAP in paediatric patients with underlying diseases39. In our study, 88% had a previous pathology, which was significantly higher than expected (P = 0.014). Congenital and neurological diseases were the most frequent, but they were not significantly more common. This situation occurs from the nature of our hospital: because it is a tertiary-care hospital, patients are referred from institutions with a lower level of specialization, which allowed us to see an epidemiological and clinical panorama of patients with comorbidities and disease development that differed from that in those healthy children with CAP. To our knowledge, only one study has been conducted in a paediatric population with CAP in Vietnam, in which respiratory and cardiac malformations were found to be risk factors for developing severe CAP (odds ratio = 11.1)39. It is necessary to carry out more studies in the paediatric population with underlying diseases to determine whether such conditions predispose acquisition of CAP by C. pneumoniae or represent a risk for complications.
It is important to remark that the clinical signs and symptoms of C. pneumoniae infection are nonspecific and do not differ significantly from those caused by other agents as respiratory viruses and atypical pathogens, such as M. pneumoniae. In our study, the main symptoms included cough (100%, P = 0.174) and fever (88%, P = 0.955) were the most frequent, in accordance with other studies38,53. In contrast, only 52% of Korean children with C. pneumoniae CAP developed fever13. In our study, 48% of the patients had rhinorrhoea, but this was not statistically significant (P = 0.772), contrary to what was reported in a study carried out in 71 Peruvian children with acute respiratory infections. (87.3%, P = 0.01)40. This can be explained of wider selection criteria, where upper and lower respiratory infections were included, contrary to our study where only CAP diagnoses were selected, but could start with a upper tract involvement.
The frequencies of wheezing (52%, P = 0.218) and crackles (68%, P = 0.402) were similar to those reported in Indian children with LRTI due to C. pneumoniae (46.1% and 53.8%, respectively)53. In studies from China and Peru, wheezing was found in 38.3 and 38% of patients with LRTI, respectively40,49. All these findings support the nonspecific clinical manifestations in C. pneumoniae infection, which strengthens the proposal to add molecular detection for diagnosis.
Of the radiological findings, the interstitial pattern was the most frequent among patients with C. pneumoniae (68%, P = 0.562). In a Polish study, this pattern was reported in 88% of children38, though it was reported in only 18.1% of Indian children with LRTI46. In this study, the consolidation pattern was statistically significant (32%; P = 0.002) and was found with greater frequency than that reported in Chinese children with acute respiratory tract infection (10.6%)49. Recently in Korea, 40% of the patients with pneumonia caused by C. pneumoniae showed segmental/lobar consolidation, and it was more common in older children (> 13 years old)13. In the same study, the bronchopneumonic pattern was the main finding (62%)13; in our study, it was found only in 28% of patients. On the other hand, 27.2% (n = 3) of Indian patients with LRTI due to C. pneumoniae had no abnormalities on radiography46. These patterns are also common in other acute infections such as interstitial for viral infections or consolidation in S. pneumoniae, therefore, there is no specific radiological pattern indicative of C. pneumoniae infection.
In general, correct interpretation of the complete blood cell count and inflammation markers can lead to suspicion of an acute viral or bacterial infection, as occurs with S. pneumoniae and the presence of neutrophilia or an increase in lymphocytes in viral infections. However, there are not such clear findings with C. pneumoniae. In our study, 17.4% of the patients had leukocytosis, and 80% had elevated CRP, but these differences were not statistically significant. In studies carried out in children from Brazil and China, no association was found between leukocyte values and CRP with infection by C. pneumoniae20,49. Normal levels of leukocytes have been described in patients with C. pneumoniae, but an increase in the count may be observed in severe cases54.
Of the 14 patients for whom serology was performed, only four had anti-C. pneumoniae antibodies (28.6%). In the rest, the presence of underlying disease or an early stage of the disease might have affected production of antibodies, and it has been described that IgM may appear at 2–3 weeks after the onset of symptoms55. As mentioned above, our hospital cares mainly for the paediatric population with underlying pathologies, which can interfere with the immune response; thus, serology may not be the optimal method for detection of C. pneumoniae in these patients.
Information on C. pneumoniae and the development of complications is scarce. In Spain, of 84 paediatric patients with CAP due to atypical bacteria, 20.2% were admitted to the PICU43. In our study, the most frequent complication was admission to the PICU and mechanical ventilation (32%). Of these patients, six had underlying disease (mainly congenital), which might have influenced the severity of the clinical presentation.
Regarding relevant complications, two patients developed pleural effusion. One of them, a 25-month-old and previously healthy male, presented with a significant hematological alteration, characterized by severe anemia (hemoglobin 3.6 gr/dl and thrombocytopenia (51,000) as well as leukocytosis (47,000 cells, 45,000 neutrophils), a neoplastic process was ruled out, but a Cold-reactive autoimmune hemolytic anemia (cAIHA) was confirmed, characterized by hemolysis, a positive Coombs test and positive C3b. In this case other viruses were negative. The effusion had characteristics of empyema, required pleural drainage and decortication by open thoracic surgery.
It has been described that cAIHA is usually associated with infections, like M. pneumoniae or Epstein–Barr virus (EBV) infection, measles, varicella, influenza and recently with COVID-19 and SARS-CoV-2 vaccination56,57. Because this patient had a co-infection with M. pneumoniae, we could not determine which of the two agents triggered the cAIHA.
A second patient with pleural effusion was a 10-month-old male who had a neurodevelopmental disorder due to perinatal hypoxia, spastic paralysis, and severe malnutrition. He had a very prolonged course of respiratory symptoms, with multiple outpatient antimicrobial treatments. He developed complicated pneumonia with empyema and a tracheobronchial fistula, requiring thoracentesis, partial pneumonectomy, and tracheostomy. This patient was also reported with M. pneumoniae co-infection.
We also report a 30-month-old patient who was admitted for febrile syndrome, pneumonia, and cardiomegaly, after ruling out structural heart disease, moderate pericarditis was documented by transthoracic echocardiography; this effusion did not require pericardial drainage. Viral agents were ruled out as a cause of pericarditis, but we also found that this patient had co-infection with M. pneumoniae.
The presence of pericarditis and even hemorrhagic pericarditis is a well-established entity caused by M. pneumoniae or C. pneumoniae. In a recent systematic review, only six cases of pericarditis associated with C. pneumoniae were reported, of which half of them were paediatric patients58. In this case, given the co-infection with both pathogens and to the fact that pericardial fluid was not obtained, it was not possible to determine which of the two pathogens or both contributed to the development of the pericardial effusion.
Mortality ranges from 3.7 to 15% in patients with CAP due to C. pneumoniae. In Brazil, a mortality rate of 3.7%20 was reported, and it was 15% in Thai children42. In this series, one death was reported, a previously healthy 11-year-old male adolescent, who developed a basal pneumonia, with myocarditis leading to cardiogenic shock and complete AV block, who died 72 h after his admission for the cardiac complication. This patient also had co-infection with M. pneumoniae. Cardiac involvement due to C. pneumoniae is well established, however, the reported cases of myocarditis due to this agent in paediatric age are limited; like the other cases described in this series, it is not possible to establish a direct causal relationship of C. pneumoniae given the co-infection with M. pneumoniae that causes myocarditis as well59 This highlights the low availability of diagnostic methods for atypical bacteria in hospitals in our country, as well as the lack of diagnostic suspicion on the part of clinical staff.
One of the most important findings that we detected in this study is the association with M. pneumoniae, because 15 patients had coinfection with this pathogen (60%). In Peru, this coinfection was detected in three of 146 patients (2.06%)60; in Chinese children with RTI, it was reported in 36.2% of 724 patients37. Another study in Japan reported the simultaneous presence of the two agents in 0.4% of patients, which was associated with a higher proportion of fever (100%) than infection by C. pneumoniae alone (50%)47. In a Korean study, Haemophilus influenzae was the main coinfecting bacterium (62%) followed by S. pneumoniae (29%)13. Recently, coinfection with C. pneumoniae or M. pneumoniae was found in 7.7% of children infected with SARS-CoV-2. This coinfection required that 26% of patients be admitted to the PICU, whereas only 2.7% of those who were only infected with SARS-CoV-2 required intensive care in the PICU61. These results show that simultaneous infection of C. pneumoniae with another pathogen can influence the clinical presentation and lead to a more severe course of the disease. Detection of these coinfections is improved by molecular techniques detecting multiple respiratory pathogens62. In this study, it was not possible to detect co-infection with other respiratory viruses.
Our work has some limitations. Because this study was carried out only in a reference hospital centre, it does not represent the complete epidemiology of CAP due to C. pneumoniae in our country. The INP is a third-level hospital that concentrates the population that is referred from other first- and second-level centres, mainly involving patients with underlying conditions. On the other hand, by clinical condition it was not possible to obtain a blood sample from all patients to perform the complete blood cell count, C-reactive protein measurement, and anti-C. pneumoniae antibody detection.
In the future, we consider to perform a multicentre study in Mexico that includes healthy paediatric population, on the other hand, its necessary to know the role of C. pneumoniae in extrapulmonary conditions and in patients with asthma.
Conclusions
This study represents the first series report in Mexico of C. pneumoniae as a cause of CAP in paediatric patients. It was found in 16.2% of our CAP patients, mainly in children under 6 years of age. This study is one of the few to describe the presence of C. pneumoniae in patients with underlying diseases. Thirty-six percent of the patients presented complications. And we found a high co-infection with M. pneumoniae. Timely detection of this pathogen using molecular techniques allows early administration of appropriate antimicrobial treatment and thus reduces development of associated complications.
Data availability
All the data supporting our findings are contained within the manuscript.
Abbreviations
- CAP:
-
Community-acquired pneumonia
- CDC:
-
Centers for Disease Control and Prevention
- CRP:
-
C-reactive protein
- INP:
-
Instituto Nacional de Pediatria
- LRTI:
-
Lower respiratory tract infection
- PICU:
-
Paediatric intensive care unit
- cAIHA:
-
Cold-reactive autoimmune hemolytic anemia
- EBV:
-
Epstein–Barr virus
- IRB:
-
Institutional Review Board
References
DeAntonio, R. et al. Epidemiology of community-acquired pneumonia and implications for vaccination of children living in developing and newly industrialized countries: A systematic literature review. Hum. Vaccin. Immunother. 12(9), 2422–2440 (2016).
Burillo, A. & Bouza, E. Chlamydophila pneumoniae. Infect. Dis. Clin. N. Am. 24(1), 61–71 (2010).
Park, J. Y. et al. Microorganisms causing community-acquired acute bronchitis: The role of bacterial infection. PLoS ONE. 11(10), e0165553 (2016).
Robinson, J. L. Paediatrics: how to manage pharyngitis in an era of increasing antimicrobial resistance. Drugs Context. 10, 2020-11–6 (2021).
Sawada, S. & Matsubara, S. Microbiology of acute maxillary sinusitis in children. Laryngoscope. 131(10), E2705–E2711 (2021).
Shim, J. Y. Current perspectives on atypical pneumonia in children. Clin. Exp. Pediatr. 63(12), 469–476 (2020).
Kogan, R & Maggiolo, J. Atypical pneumonia. In Pediatric Respiratory Diseases (eds. Bertrand, P. & Sánchez, I.). 309–321 (Springer, 2020).
Hammerschlag, M.R., Kohlhoff, S.A. & Gaydos, C.A. Chlamydia pneumoniae in Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (eds. Bennett, J.E., Dolin, R. & Blaser, M.J.). 2174–2182 (W.B. Saunders, 2015).
Benitez, A. J. et al. Comparison of real-time PCR and a microimmunofluorescence serological assay for detection of Chlamydophila pneumoniae infection in an outbreak investigation. J. Clin. Microbiol. 50(1), 151–153 (2012).
Thurman, K. A., Warner, A. K., Cowart, K. C., Benitez, A. J. & Winchell, J. M. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn. Microbiol. Infect Dis. 70(1), 1–9 (2011).
Ling, C. L. & McHugh, T. D. Rapid detection of atypical respiratory bacterial pathogens by real-time PCR. Methods Mol. Biol. 943, 125–133 (2013).
Jama-Kmiecik, A. et al. Atypical and typical bacteria in children with community acquired pneumonia. Adv. Exp. Med. Biol. 1160, 65–71 (2019).
Han, H. Y. et al. Surge of Chlamydia pneumoniae pneumonia in children hospitalized with community-acquired pneumonia at a single center in Korea in 2016. J. Infect. Chemother. 29(5), 453–457 (2023).
de Higuchi, M. L. et al. Coinfection with Mycoplasma pneumoniae and Chlamydia pneumoniae in ruptured plaques associated with acute myocardial infarction. Arq. Bras. Cardiol. 81(1), 12–22 (2003).
Stivala, A. et al. Comparison of cell culture with three conventional polymerase chain reactions for detecting Chlamydophila pneumoniae in adult’s pharyngotonsillitis. Curr. Microbiol. 77(10), 2841–2846 (2020).
Jiang, X. W. et al. Development of a diagnostic assay by three-tube multiplex real-time PCR for simultaneous detection of nine microorganisms causing acute respiratory infections. Sci. Rep. 12(1), 13306 (2022).
Wolff, B. J. et al. Multiplex real-time PCR assay for the detection of all Chlamydia species and simultaneous differentiation of C. psittaci and C. pneumoniae in human clinical specimens. Ann. Lab. Med. 43(4), 375–380 (2023).
Huang, H. et al. A digital microfluidic RT-qPCR platform for multiple detections of respiratory pathogens. Micromachines (Basel). 13(10), 1650 (2022).
Porritt, R. A. & Crother, T. R. Chlamydia pneumoniae infection and inflammatory diseases. For. Immunopathol. Dis. Ther. 7(3–4), 237–254 (2016).
Alves, M. S. et al. High frequency of Chlamydia pneumoniae and risk factors in children with acute respiratory infection. Braz. J. Microbiol. 51(2), 629–636 (2020).
Piekut, T. et al. Infectious agents and Alzheimer’s disease. J. Integr. Neurosci. 21(2), 73 (2022).
Premachandra, N. M. & Jayaweera, J. Chlamydia pneumoniae infections and development of lung cancer: Systematic review. Infect. Agent Cancer 17(1), 11 (2022).
Garcia, G. et al. Exposure to antibodies anti-Chlamydophila pneumoniae associated to respiratory symptoms of asthma among adolescents. Med. Res. Arch. 9(4), 1–16 (2021).
Bradley, J. S. et al. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53(7), e25-76 (2011).
Lindley, L. C., Cozad, M. J. & Fortney, C. A. Pediatric complex chronic conditions: Evaluating two versions of the classification system. West. J. Nurs. Res. 42(6), 454–461 (2020).
Bertrand, P. Clinical history and physical examination of the respiratory system. In Pediatric Respiratory Diseases (eds. Bertrand, P. & Sánchez, I.). 29–36 (Springer, 2020).
García Bruce, C., Parra Rojas, R. Study of images in respiratory diseases. In Pediatric Respiratory Diseases (eds. Bertrand, P. & Sánchez, I.).107–126 (Springer, 2020).
Williams, K. et al. Standard 6: Age groups for pediatric trials. Pediatrics. 3, 153–160 (2012).
Ahsan, S. & Noether N. J. Hematología. In Manual Harriet Lane de Pediatría: Para la Asistencia Pediátrica Ambulatoria (eds. Robertson, J. & Johns Hopkins Hospital). 322–353 (Elsevier, 2006).
Koressaar, T. & Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 23(10), 1289–1291 (2007).
Untergasser, A. et al. Primer3—New capabilities and interfaces. Nucleic Acids Res. 40(15), e115 (2012).
Koressaar, T. et al. Primer3_masker: Integrating masking of template sequence with primer design software. Bioinformatics. 34(11), 1937–1938 (2018).
Tatti, K. M., Sparks, K. N., Boney, K. O. & Tondella, M. L. Novel multitarget real-time PCR assay for rapid detection of Bordetella species in clinical specimens. J. Clin. Microbiol. 49(12), 4059–4066 (2011).
Cohen , J. The concepts of power analysis. In Statistical Power Analysis for the Behavioral Sciences (ed. Cohen, J.). 1–17 (Lawrence Erlbaum Associates, 1988).
The R Project for Statistical Computing. https://www.r-project.org/. Accessed 28 Oct 2021.
Ben-Shachar, M. S., Lüdecke, D. & Makowski, D. Effect size: Estimation of effect size indices and standardized parameters. JOSS. 5(56), 2815 (2020).
Chen, J. R. & Zhou, X. F. A retrospective survey of Chlamydia pneumoniae infection rates in paediatric patients from a single centre in Wuxi, China. J. Int. Med. Res. 48(10), 300060520961720 (2020).
Kicinski, P., Wisniewska-Ligier, M. & Wozniakowska-Gesicka, T. Pneumonia caused by Mycoplasma pneumoniae and Chlamydophila pneumoniae in children—Comparative analysis of clinical picture. Adv. Med. Sci. 56(1), 56–63 (2011).
Huong Ple, T. et al. First report on prevalence and risk factors for severe atypical pneumonia in Vietnamese children aged 1–15 years. BMC Public Health. 14, 1304 (2014).
Del Valle-Mendoza, J. et al. High prevalence of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with acute respiratory infections from Lima, Peru. PLoS One. 12(1), e0170787 (2017).
Gong, C. et al. Distribution of the atypical pathogens of community-acquired pneumonia to disease severity. J. Thorac. Dis. 10(11), 5991–6001 (2018).
Bunthi, C. et al. Enhanced surveillance for severe pneumonia, Thailand 2010–2015. BMC Public Health. 19(3), 472 (2019).
Otheo, E. et al. Viruses and Mycoplasma pneumoniae are the main etiological agents of community-acquired pneumonia in hospitalized pediatric patients in Spain. Pediatr. Pulmonol. 57(1), 253–263 (2022).
Rueda, Z. V. et al. Induced sputum as an adequate clinical specimen for the etiological diagnosis of community-acquired pneumonia (CAP) in children and adolescents. Int. J. Infect. Dis. 116, 348–354 (2022).
Yun, K. W. et al. Clinical characteristics and etiology of community-acquired pneumonia in US children, 2015–2018. Pediatr. Infect. Dis. J. 41(5), 381–387 (2022).
Kumar, S., Kashyap, B., Kumar, S. & Kapoor, S. Diagnostic utility of serology and polymerase chain reaction for detection of Mycoplasma pneumoniae and Chlamydophila pneumoniae in paediatric community-acquired lower respiratory tract infections. Indian J. Med. Microbiol. 38(2), 152–156 (2020).
Oishi, T. et al. Low prevalence of Chlamydia pneumoniae infections during the Mycoplasma pneumoniae epidemic season: Results of nationwide surveillance in Japan. J. Infect. Chemother. 26(11), 1116–1121 (2020).
Cai, F., Shou, X. & Ye, Q. Epidemiological study on Mycoplasma pneumoniae and Chlamydia pneumoniae infection of hospitalized children in a single center during the COVID-19 pandemic. Front. Cell. Infect. Microbiol. 12, 843463 (2022).
Chen, Z. et al. Epidemiology and associations with climatic conditions of Mycoplasma pneumoniae and Chlamydophila pneumoniae infections among Chinese children hospitalized with acute respiratory infections. Ital. J. Pediatr. 39, 34 (2013).
Cardinale, F., Cappiello, A. R., Mastrototaro, M. F., Pignatelli, M. & Esposito, S. Community-acquired pneumonia in children. Early Hum. Dev. 89(3), S49-52 (2013).
Thadchanamoorthy, V. & Dayasiri, K. Review on pneumococcal infection in children. Cureus 13(5), e14913 (2021).
Merida-Vieyra, J. et al. Detection of Mycoplasma pneumoniae in Mexican children with community-acquired pneumonia: Experience in a tertiary-care hospital. Infect. Drug Resist. 12, 925–935 (2019).
Kumar, S., Saigal, S. R., Sethi, G. R. & Kumar, S. Application of serology and nested polymerase chain reaction for identifying Chlamydophila pneumoniae in community-acquired lower respiratory tract infections in children. Indian J. Pathol. Microbiol. 59(4), 499–503 (2016).
Wang X., Li H. & Xia Z. Chlamydia pneumoniae pneumonia. In Radiology of Infectious Diseases (ed. Li, H.). 69–74 (Springer, 2015).
Miyashita, N. et al. Antibody responses of Chlamydophila pneumoniae pneumonia: Why is the diagnosis of C. pneumoniae pneumonia difficult?. J. Infect. Chemother. 21, 497–501 (2015).
Voulgaridou, A. & Kalfa, T. A. Autoimmune hemolytic anemia in the pediatric. Setting J. Clin. Med. 10(2), 216 (2021).
Fattizzo, B., Pasquale, R., Bellani, V., Barcellini, W. & Kulasekararaj, A. G. Complement mediated hemolytic anemias in the COVID-19 era: Case series and review of the literature. Front. Immunol. 12, 791429 (2021).
Kyriakoulis, K. G et al, Chlamydia pneumoniae-associated pleuropericarditis: A case report and systematic review of the literature. BMC Pulm. Med. 21(1), 380 (2021).
Li, C. M. et al. Age-specific Mycoplasma pneumoniae pneumonia-associated myocardial damage in children. J. Int. Med. Res. 41(5), 1716–1723 (2013).
Del Valle-Mendoza, J. et al. Molecular etiological profile of atypical bacterial pathogens, viruses and coinfections among infants and children with community-acquired pneumonia admitted to a national hospital in Lima, Peru. BMC Res. Notes 10(1), 688 (2017).
Yakovlev, A. S. et al. SARS-CoV-2 infection in children in Moscow in 2020: Clinical features and impact on circulation of other respiratory viruses: SARS-CoV-2 infection in children in Moscow in 2020. Int. J. Infect. Dis. 116, 331–338 (2022).
Tazi, S. et al. Comparative performance evaluation of FilmArray BioFire RP2.1 and MAScIR 2.0 assays for SARS-CoV-2 detection. Adv. Virol. 2022, 4510900 (2022).
Funding
This project was kindly funded by Instituto Cientifico Pfizer (Fondo de Investigación Epidemiologica 2014) and Fiscal Resources Modality A of the National Institute of Paediatrics 2015 under registration INP-058/2014. They had no role in the design of the study; collection, analysis, and interpretation of data; or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
A.A.A. and A.D.C. designed the study and acquired funding. J.M.V. and A.A.A. performed the experiments. J.M.V., A.A.A., A.D.C. and D.P.R. collected the microbiological and epidemiological data. C.H.M. performed the statistical analysis. J.M.V. and A.A.A. analysed the data and wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Merida Vieyra, J., De Colsa Ranero, A., Palacios Reyes, D. et al. Chlamydophila pneumoniae-associated community-acquired pneumonia in paediatric patients of a tertiary care hospital in Mexico: molecular diagnostic and clinical insights. Sci Rep 13, 21477 (2023). https://doi.org/10.1038/s41598-023-48701-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48701-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.