Abstract
Calcareous soils are characterized by a high calcium carbonate content (calcite), which plays a crucial role in the soil structure, plant growth, and nutrient availability. The high content of CaCO3 leads to the increment of the soil alkalinity, which results in a lowering of the nutrient availability causing a challenge for the agriculture in these soils. In this study, the calcite-solubilizing potential of the diazotrophic Azotobacter salinestris YRNF3 was investigated in vitro as a probable bio-agent for enhancing the calcareous soils properties such as soil pH and nutrient availability. Twelve diazotrophic bacterial strains were isolated from wheat rhizosphere collected from different wheat-cultivated fields in five Egyptian governorates. Using Nessler’s reagent, all isolated bacterial strains were found to have the ability to produce ammonia. By amplification of nifH gene, a PCR product of 450 bp was obtained for all isolated bacterial strains. For each isolate, three biological and three technical replicates were applied. All isolated diazotrophic bacteria were qualitatively screened for their calcite-solubilizing ability. To quantitatively investigate the calcite-solubilizing potential of A. salinestris YRNF3 in vitro, changes in the contents of soluble calcium (Ca2+), bicarbonate (HCO3−), total nitrogen (TN), total protein (TP), and pH were daily measured in its culture filtrate along 10 days of incubation. The results showed that the pH values in the culture filtrate ranged from 5.73 to 7.32. Concentration of Ca2+ and HCO3− in the culture filtrate significantly decreased with the increment in the incubation time, while concentration of TN increased along the time. The highest TN concentration (0.0807 gL−1) was observed on days 4 and 5, compared to that of the day 0 (0.0014 gL−1). Content of TP in the culture filtrate also significantly increased along the incubation period. The highest TP content was recorded in day 4 (0.0505%), while no TP content was recorded on day 0. Furthermore, data obtained revealed that A. salinestris YRNF3 produced acid phosphatase at low activity (5.4 U mL−1). HPLC analysis of the culture filtrate indicated production of different organic acids, namely lactic acid (82.57 mg mL−1), formic acid (46.8 mg mL−1), while acetic acid was detected in a low quantity (3.11 mg mL−1). For each analysis, three replicates of each treatment were analyzed. Means of the tested treatments were compared using Tukey's HSD test at p ≤ 0.05. In conclusion, findings of this work suggested that A. salinestris YRNF3 has the potential to be a probable bioagent to be used for the reclamation of the calcareous soils by solubilizing CaCO3, improving soil fertility, and promoting plant growth. However, further studies are needed to investigate its field application and their long-term effects on the soil properties and plant productivity. To the best of the author's knowledge, this is the first study reporting the calcite-solubilizing ability of a nitrogen-fixing bacteria. Having these two abilities by one microorganism is a unique feature, which qualifies it as a promising bioagent for reclamation of the calcareous soils.
Similar content being viewed by others
Introduction
Calcareous soils are widespread in arid and semiarid regions, covering about 30% of the world's land area, including Egypt1. These soils have a high content of calcium carbonate (CaCO3), which makes them highly alkaline and reduces the nutrient availability for plants. Calcite solubilization is a natural process that occurs in soil environments, where microorganisms can dissolve the calcite leading to the release of calcium and carbonate ions2. Microbial calcite solubilization can decrease soil pH releasing the nutrients from the soil matrix and making them available for the plant uptake3.
Various methods have been used for soil reclamation, including chemical and physical methods. Chemical methods such as sulfuric acid and gypsum application have been used to reduce the soil pH and improve the nutrient availability4. However, these methods have drawbacks such as the high cost, negative environmental impacts, and long-term effects on the soil properties. Physical methods such as the deep plowing and subsoiling have been used to improve soil structure and reduce soil alkalinity5. However, these methods may cause soil erosion and compaction6.
Microbial calcite solubilization is a potential, eco-friendly, and sustainable alternative to the chemical and physical methods, as it is a natural process that can improve the soil properties without any negative effects. Several studies have investigated the ability of microorganisms to precipitate calcite for soil stabilization and improving soil strength and stiffness7,8,9. However, reports dealing with microbial calcite solubilization are limited. In this regard, Tamilselvi et al.,10 reported the calcite-solubilizaing ability of Brevibacterium sp. SOTI06 released 18.6% of the native CaCO3 solution. In another study, Peper et al.3 isolated 65 calcite-solubilizing bacterial (CSB) isolates belonging to 10 genera from a peanut pegging zone in Georgia. The main responsible mode of action for calcite-solubilization is the production of organic acids such as citric, gluconic, and acetic acids10. In addition, the production of sanazine pigment was also reported11.
The use of microorganisms to enhance the soil fertility and plant growth has gained attention in the recent years due to their potential to provide sustainable solutions to the agricultural challenges12,13,14,15. Diazotrophic bacteria are capable to fix the atmospheric nitrogen to the inorganic form (NH4+) which is available to the plant via the nitrogenase enzyme16. Members of various bacterial genera, known as nitrogen fixers, are categorized into two types. The first type is the symbiotic bacteria which live in a mutualistic relationship with specific plants such as Rhizobium spp. that live in association with the leguminous plants17. The second type is the free-living (asymbiotic) bacteria such as Azotobacter spp., Beijerinckia spp. and cyanobacteria18. Furthermore, nitrogen fixing bacteria are known to have another growth promoting effects on the plant via production of phytohormones, signaling molecules, and siderophores, trigging plant tolerance to the biotic and abiotic stresses, as well as minerals solubilization19,20,21. Members of the genus Azotobacter are the most prevalent asymbiotic nitrogen-fixing and plant growth-promoting bacteria22. Among them, A. salinestris is known to pose a high potential nitrogen-fixing ability, tolerance to salinity stress up to 8% NaCl and the common pesticides, and an efficient antifungal potential23. As an initial phase within a long-term project aimed at the development of a multifunctional bio-product for the reclamation of calcareous soils and the enhancement of the soil fertility, this study had the following objectives: (1) to isolate a variety of free-living nitrogen-fixing bacteria from diverse soil samples, (2) to assess their calcite-solubilization activity, (3) to identify the most efficient isolate, (4) to estimate its in vitro calcite-solubilization potential, and (5) to investigate the proposed mode of action..
Results and discussion
Isolation of the diazotrophic bacteria
In this study, twenty-five soil samples from different agricultural fields located at five Egyptian governorates representing varied climatic conditions and agricultural practices and soil types were collected. Twelve bacterial isolates, with varied colony shape and color, were isolated from the collected samples using the nitrogen-free (NF) medium. In this regard, the highest number of isolated bacteria was recorded in the New Valley and Al-Behera governorates (4 bacterial strains), followed by Alexandria and Sinai governorates (2 bacterial strains), while no bacterial strains were isolated from the soil samples from Port Said governorate.
Using the Nessler’s reagent, all isolated bacterial strains were found to be positive for ammonia production which may support their ability to fix the atmospheric nitrogen. To confirm the diazotrophic property, all isolated bacterial strains were subjected to amplification of nifH gene using PCR, which is universally used as a biomarker for the nitrogen-fixation ability. In this concern, a PCR product of 450 bp was obtained for all isolated bacterial strains confirming their diazotrophic potential. The PCR-amplified products of nifH gene of all isolated bacterial strains are shown in Fig. 1.
Diazotrophic microorganisms are globally significant because they are the only biological source of the fixed nitrogen in their ecosystems. Nitrogen fixation is an enzymatic process which has been carried out via the nitrogenase reductase, coded by nifH gene, by which the atmospheric nitrogen (N2) transforms to ammonium equivalents that are available to the plants24. nifH has become the marker gene of choice for studying biodiversity, distribution, ecology and phylogeny of the diazotrophic organisms25.
According to Delgado-Baquerizo et al.26, soil pH significantly affects abundance and diversity of their content of bacterial communities. However, diazotrophic bacteria tend to be affected by other physicochemical properties of the soil. For example, nitrogen fertilization suppresses their abundance and taxon composition, while potassium and phosphorus fertilization enhances it27. Song et al.28 found that the community structure of the nitrogen-fixing bacteria considerably varied based on the moisture, pH, salinity, contents of nitrogen, carbon, and sulphur. Severin et al. reported the negative effect of the high salinity on the nitrogenase activity and nifH expression in Cyanobacteria28, while other diazotrophs were found to inhabit the sea and ocean water29.
Screening the isolated bacterial strains for their calcite-solubilization ability
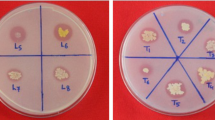
All isolated diazotrophic bacteria were qualitatively screened for their calcite-solubilization ability. Results obtained revealed that the isolated diazotrophic bacterial strains showed medium to low solubilization index values (0.41–3.04), except one strain (YRNF3) which showed a maximum solubilization index value 5.9 (Table 1). This strain was selected for the next evaluation tests. Calcite-solubilization potential of the diazotrophic bacterial strain YRNF3 is illustrated in Fig. 2. In general, the diazotrophic bacteria differ in their bioactivities and abilities, due to their high genetic diversity, which qualify them to adapt with different types of ecosystems. In addition to the nitrogen fixation, some diazotrophic bacteria have the ability to dissolute the rock phosphates29. Assistance in phytoremediation of heavy metals has been also reported for the diazotrophic bacteria30. On the contrary, to the best of the author's knowledge, no studies have been conducted on diazotrophic bacteria with a calcite-solubilization ability. Therefore, this study was aimed to isolate an efficient diazotrophic bacteria with a calcite-solubilization ability.
Molecular identification of the diazotrophic bacteria YRNF3
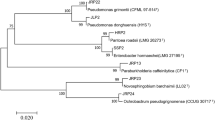
The diazotrophic bacteria YRNF3 was molecularly identified based on its 16S rDNA region. The result indicated that the amplified nucleotide sequence (1393 bp) of the diazotrophic bacteria YRNF3 had 99.78% similarity with A. salinestris (CP045302). Based on this result, the diazotrophic bacteria YRNF3 was identified as A. salinestris YRNF3 and its nucleotide sequence was deposited in the GenBank database under the accession number (OQ605418). Phylogenetic analysis of A. salinestris YRNF3 with different species in genus Azotobacter from the GenBank database revealed that all species were clustered into two distinct clads. One of them contained two species A. beijerinckii (FNYO01000222) and A. chroococcum (SWKB01000103) with a 86% bootstrap support, while the other species were clustered in the other clade under two subclades, one of them contained A. salinestris YRNF3 (OQ605418), A. salinestris (JN641802) and A. vinelandii (OQ415270) with a bootstrap support (92%). In the other subclade, four species were grouped, namely A. tropicalis (MW586885), A. nigricans (LN874291), A. armeniacus (OP978160), and A. bryophylli (MF078077) with a 88% bootstrap support (Fig. 3).
A Phylogenetic tree indicates the relationship between the diazotrophic bacteria Azotobacter salinestris YRNF3 and eight different species from genus Azotobacter from the GenBank database. Bootstrap values are shown on the nodes. The scale bar represents the number of nucleotide substitutions per site.
Estimation of the calcite-solubilization potential under in-vitro conditions
Calcite-solubilization potential of A. salinestris YRNF3 was estimated along 10 days of incubation under in-vitro conditions as follows:
pH
The pH value ranged from 5.73 to 7.32, compared with the initial pH 7.24 (Fig. 4). The pH significantly (p < 0.01) fluctuated throughout the experiment, reaching a minimum of 5.98 on the day 4, compared to the control (day 0). The pH then increased gradually until day 7, followed by a slight decrease on day 8 (Fig. 1). As shown in Fig. 4, application of A. salinestris YRNF3 led to a lowering in the pH of the culture filtrate with the increment in the incubation period. The pH value at day 8 recorded the lowest value, while no significant differences (p < 0.01) were observed among days 3, 4, 6, and 8–10. The pH at days 0–2 showed the highest values. No significant difference was observed between the pH value at days 5 and 7.
Variation in pH values of the culture filtrate of Azotobacter salinestris YRNF3 along 10 days of incubation. Values superscripted with the same letter are not significantly different according to Tukey’s HSD test at p ≤ 0.01. Each value represents the mean of three replicates. Error bars represent standard errors. LSD = least significant difference.
The observed changes in pH values can be attributed to several factors such as changes in the dissolved CO2 level, production of acidic or alkaline metabolites, or to the bacterial activity2,3,31. However, the pH values observed in this study were within the acceptable range for the bacterial growth, which is typically ranged between 6.5 and 8.5. During the bacterial growth, various acidic or alkaline metabolites can be produced and modulate the pH of the culture32. In particular, production of ammonia and organic acids, in this case, as noticed in the next results. In addition, level of the dissolved CO2 in the culture can also affect the pH value, as the dissolved CO2 can react with water to form carbonic acid, which can lower the pH value of the culture. In the same time, the pH value of the soil may affect the type and quantity of the bacterial population that can grow in this soil32. Reaction of the dissolved CO2 and water to form carbonic acid proceeds according to the following equation:
This reaction can affect the pH of the culture as the carbonic acid can donate H+ ions, leading to a decrease in the pH33,34. While solubilization of calcium carbonate in water proceeds as follows:
This reaction results in an increment in the concentration of the bicarbonate ion leading to a decrease in the pH. On the other hand, the calcareous soils typically have a high pH due to the high content of calcium carbonate. This pH increment can affect the type and population of the inhabitant microorganisms2,3,31. In this case, addition of acids-producing bacteria can potentially help in lowering the pH to a more neutral range. This may create a more favorable environment for the growth of various microorganisms as well as increase the nutrients availability to the growing plants. However, it is important to note that the specific effects of the inhabiting bacteria on the calcareous soil will depend on other factors such as the composition of the soil and the type of the present microorganisms10.
Furthermore, the existing microorganisms in the soil, including both bacteria and other microbial communities, contribute to the overall soil ecosystem. Interactions between different microorganisms can be cooperative or competitive, and they can have synergistic or antagonistic effects on each other. The presence of specific microorganisms can influence the activities and functions of bacteria in the soil, including nutrient cycling, organic matter decomposition, and disease suppression10.
In summary, while the presence of bacteria in the calcareous soil can has effects on the soil processes, the specific outcomes will be influenced by other factors such as soil composition, nutrient availability, pH, and the interactions with other microorganisms. Understanding these complex interactions is crucial for comprehending the overall dynamics of the soil ecosystem and its implications for the plant growth, nutrient cycling, and soil health32.
Calcium concentration
Calcium concentration ranged from 0.17 to 1.76 gL−1. The initial calcium concentration in the bacterial culture on day 0 was 0.21 gL−1, and the highest concentration was observed on day 10 recording 1.76 gL−1 (Fig. 5).
Gradual increment in the calcium concentration in the culture of Azotobacter salinestris YRNF3 along the time of the experiment. Values superscripted with the same letter are not significantly different according to Tukey’s HSD test at p ≤ 0.01. Each value represents the mean of three replicates. Error bars represent standard errors. LSD = least significant difference.
Figure 5 illustrates the impact of A. salinestris YRNF3 on the calcium carbonate solubilization in the culture filtrate. Results showed that culturing of A. salinestris YRNF3 led to a significant increment (p < 0.01) in the calcium concentration in the culture medium from day 4 to 10, compared to the control. No significant difference was observed in the calcium concentration among days 1–3. However, the calcium concentration at days 6–10 had the highest values without any significant difference (p < 0.01) between them.
The fluctuation in the calcium concentration can be attributed to the solubilization of calcium-bearing minerals and cellular metabolic processes10. The observed fluctuation in calcium concentration along the studied period suggests that calcium is a dynamic and complex nutrient. The initial calcium concentration on day 0 was relatively low. However, as the experiment progressed, the calcium concentration gradually increased, reaching the maximum on day 10. This increment in the calcium concentration indicates the calcite-solubilization by A. salinestris YRNF3.
On the other hand, the observed fluctuations in the calcium concentration can provide significant implications for the calcareous soil reclamation. Calcareous soils are characterized by a high content of calcium carbonate, which causes soil alkalinity and reduces the availability of certain nutrients to the plants2. Depending on the solubility product of CaCO3, this solubilization results in a high HCO3− concentration that buffers the soil pH in the range of 7.5 to 8.5 which can enhance the nutrients availability in the soil34. Therefore, addition of CSB can potentially enhance the soil fertility and promote the plant growth13. Modulation of the bicarbonate ion in the soil by calcium is achieved according to the following equation:
Calcium is a crucial nutrient for maintaining of the healthy plants and fertile soils, influencing vital physiological processes such as cell division, nutrient uptake, and cell wall development, in addition to its contribution into the properties of soil aggregates35,36.
Bicarbonate concentration
The obtained results revealed that the bicarbonate concentration in the culture medium ranged between 0.37 and 2.59 gL−1. The highest concentration (2.59 gL−1) was observed on day 10. The initial bicarbonate concentration on day 0 was 1.09 gL−1 (Fig. 6). The bicarbonate concentration showed the highest values on days 8–10 compered to days 0–7, without any significant difference (p < 0.01) between them. While it had the lowest value in days 0–5 without any significant difference between them at (p < 0.01). Bicarbonate is an important buffer system that regulates pH levels in water bodies34. Bicarbonate content can neutralize the soil pH, as illustrated in the following equation:
Fluctuation of the bicarbonate concentration in the culture of Azotobacter salinestris YRNF3 along the time of the experiment. Values superscripted with the same letter are not significantly different according to Tukey’s HSD test at p ≤ 0.01. Each value represents the mean of three replicates. Error bars represent standard errors. LSD = least significant difference.
This chemical reaction generates carbon dioxide (CO2) and water (H2O), which provide plants with a source of carbon., Bicarbonate-based amendments can enhance the overall quality of the calcareous soil by balancing the soil pH and providing carbon for the plant growth. However, the specific effects of bicarbonate addition to the calcareous soil depend on various factors such as the composition of the soil and the type of the grown plants. The obtained results in this study suggested that application of A. salinestris YRNF3 may be effective in the calcareous soil reclamation. By adding the organic acids, the soil pH can be decreased leading to an improvement of the nutrients availability for the grown plants. In addition, incorporating the diazotrophic bacteria to the soil can be a viable solution for improving the quality of calcareous soil33,37.
The total nitrogen content
The total nitrogen (TN) content encompasses various forms of nitrogen, including nitrate (NO3−), nitrite (NO2−), ammonia (NH3), ammonium (NH4+), and organic nitrogen. This relationship can be expressed by the following equation20,21
Results obtained indicated that the TN content in the bacterial culture ranged from 0.0014 to 0.0807 gL−1. The highest concentration was observed with a high significance (p < 0.01) on days 4 and 5, compared with the other days (Fig. 7). On other hand, the TN content in day 0 had the lowest value, compared to the all days. The incubation time from days 1–3, 9,10 and 6–8 showed no significant differences between them, compared with days 4 and 5. This confirms the nitrogen-fixing ability of A. salinestris YRNF3. Conversion of the atmospheric nitrogen (N2) into ammonia (NH3) is known as the biological process of nitrogen fixation. This process is carried out by certain microorganisms that called diazotrophic bacteria such as A. salinestris YRNF3 and can be represented by the following equation38
Fluctuation of total nitrogen (TN) concentration in the culture of Azotobacter salinestris YRNF3 along the time of the experiment over time. Values superscripted with the same letter are not significantly different according to Tukey’s HSD test at p ≤ 0.01. Each value represents the mean of three replicates. Error bars represent standard errors. LSD = least significant difference.
Nitrogen fixation is a critical reaction in the nitrogen cycling and is essential for the growth and survival of microorganisms and plants39. However, it is important to note that the effects of soil nitrogen levels on the bacterial growth depend on various factors, such as the composition of the soil and the type of microorganisms present. However, the excessive nitrogen content in the bacterial culture can lead to downregulation of the nitrogenase gene resulting in the suppression of the nitrogen fixation process as follows16:
Furthermore, application of the diazotrophic bacteria for soil improvement should be carefully managed to avoid excessive nitrogen accumulation and the associated negative effects on the soil and water quality13,20.
The total protein content
The total protein (TP) content produced by A. salinestris YRNF3 along the ten days of incubation ranged between 0.0009 and 0.0505% (Fig. 8). The highest content was recorded in the day 4 (0.0505%), while no TP content was recorded on the day 0. From the data presented in Fig. 8, we can see that the TP content reached its highest value on the day 4. After which its value gradually decreased from day 5 to 8. While the TP content values were constant on days 9–10 without any significant difference at (p < 0.01) between them.
Total protein content in the culture of Azotobacter salinestris YRNF3 along the time of the experiment. Values superscripted with the same letter are not significantly different according to Tukey’s HSD test at p ≤ 0.01. Each value represents the mean of three replicates. Error bars represent standard errors. LSD = least significant difference.
Proteins are essential for the bacterial growth and playing an important role in many physiological processes including the growth and development. The observed fluctuation in the TP can be attributed to several factors such as the nitrogen fixation rate, nutrient uptake by the bacteria, and the release of proteins from the dead cells. The increment in TP in day 4 can be attributed to the growth and replication of bacteria that utilize proteins as a nutrient source. As the bacterial growth and reproduction increase, more proteins are consumed, leading to a decrease in the TP. Proteins can be synthesized from nitrogen-containing amino acids, which can be obtained from a variety of sources, including nitrogen gas (N2) in the air. Ammonia (NH3) produced by the nitrogen fixation can be assimilated into amino acids and other nitrogen-containing compounds as follows40:
Synthesis of proteins from nitrogen is a critical process in the growth and development of living organisms, and the ability to fix atmospheric nitrogen provides an important source of nitrogen for this process16.
Quantification of the organic acids produced by A. salinestris YRNF3
Results obtained from the HPLC analysis revealed that A. salinestris YRNF3 produced three organic acids in its culture filtrate, at varying extents, including formic, lactic, and acetic acids (Fig. 9, and Table 2). The major produced organic acid was lactic acid (82.57 mg mL−1), followed by formic acid (46.8 mg mL−1), while acetic acid was detected in a low quantity (3.11 mg mL−1). Based on this result, the calcite-solubilization ability of A. salinestris YRNF3 can be attributed mainly to the produced lactic acid. Solubilization of calcium carbonate by acidification has been widely studied in the last years41,42. Acidic solubilization of calcite is proceeded according to the following equation:
Gray et al. (2018) found that the solubilization of calcite by hydrochloric acid (HCl) in porous media is primarily controlled by the transport of acid into the media, with the increment in the solubilization rate the acid concentration increases and the pore size decreases. The authors also observed the formation of etch pits and channels on the surface of the calcite crystals, suggesting that the solubilization process is selective and influenced by the crystallographic orientation of the calcite. The study provides valuable insights into the chemical mechanisms involved in the solubilization of calcite in the porous media, with important implications for understanding the geochemical processes in the natural and engineered systems.
The solubilization of calcite by hydrochloric acid (HCl) in porous media can be represented by the following chemical equation:
Organic acids are characterized with low corrosivity and slow reaction rates, which qualify them for the calcite solubilization. However, lactic acid has a significant advantage over other organic acids, which is its high solubility at high temperatures, forming calcium lactate43. Formic acid has the same strength of lactic acid, and a dissociation constant higher ten times than the acetic acid44.
Al-Khaldi et al.40 described a study that investigated the reaction of citric acid with calcite. The authors used a variety of experimental techniques to measure the reaction kinetics and mass transfer of citric acid with calcite under different conditions such as temperature, pH, and concentration. The results of the study showed that the reaction rate of citric acid with calcite is influenced by several factors, including the mineral surface area, the concentration of citric acid, and the pH of the solution. The reaction of citric acid with calcite can be represented by the following chemical equation:
The production of the organic acids by A. salinestris YRNF3 is likely a survival mechanism for the bacterium in its natural environment. Organic acids can act as chelators, which are molecules that bind to metal ions and increase their solubility41,42. By producing organic acids such as lactic acid, A. salinestris YRNF3 may be able to access and utilize nutrients such as calcium, which are typically not readily available in its environment. In addition, the production of organic acids may help A. salinestris YRNF3 to compete with other microorganisms by lowering the pH in its environment, which can inhibit the growth of other bacteria20,21.
Furthermore, the specific organic acids produced by A. salinestris YRNF3 may be influenced by various environmental factors such as temperature, pH, and nutrient availability. For instance, lactic acid production may be favored at higher temperatures due to its high solubility, while formic acid may be produced in response to low nutrient availability44,57.
Rabie et al.41 found that the reaction rate of lactic acid with calcite and dolomite is influenced by several factors, including the mineral surface area, the concentration of lactic acid, and the pH of the solution. The study showed that the reaction rate increased with increasing temperature and decreasing pH, and the calcite was more reactive than dolomite under the same conditions. The results have important implications for understanding the geochemical processes that occur in subsurface environments, and may have practical applications in areas such as acidizing and hydraulic fracturing of carbonate reservoirs41,42.
The reaction of lactic acid with calcite and dolomite can be represented by the following chemical equation:
Overall, the study provides valuable insights into the reaction kinetics and mass transfer of lactic acid with calcite and dolomite, which may help to improve our understanding of carbonate solubilization mechanisms and their effects on subsurface fluid flow and transport.
Activity of acid phosphatase produced by A. salinestris YRNF3
Data obtained revealed that A. salinestris YRNF3 produced acid phosphatase at low activity (5.4 U.mL−1). This result can be discussed in the light of the quantity and form of the phosphorus content in the culturing medium, where it exists in a low quantity and in the form of orthophosphate, which is readily available for the microbial uptake. In this case, the bacteria are not in need to the activity of phosphatase. Phosphorus is a vital nutrient for plants and microorganisms and involved in many physiological processes such as energy transfer, metabolism, and membrane transport. Under soil conditions, the high weathering rate and the geochemical binding of phosphorus to iron and aluminum result in an orthophosphate devoid soil45. In order to alleviate the phosphorus limitation, some of the soil microorganisms such as arbuscular mycorrhizal fungi and phosphorus-dissolving bacteria are important to regulate the phosphorus acquisition by production of acid and alkaline phosphatases46. However, phosphorus availability is a highly dependent on many factors such as soil pH, sorption–desorption and solubilization–precipitation equilibriums. Acid and alkaline phosphatases can solubilize phosphorus from these minerals47.
Conclusions
Azotobacter salinestris YRNF3 was found to has a calcite-solubilization potential. Based on the obtained results, it can be concluded that A. salinestris YRNF3 is a promising candidate for application in reclamation of the calcareous soils. However, further studies are required to evaluate application of A. salinestris YRNF3 in the open large-scale field. The findings of this work provide insights into the calcite-solubilization property of the diazotrophic A. salinestris YRNF3 and contribute to understand the role of biological systems in CaCO3 solubilization. To the best of the author's knowledge, this is the first study dealing with the calcite-solubilization ability of a diazotrophic bacteria.
Materials and methods
Soil samples
Soil samples were collected from different agricultural fields in different Egyptian governorates representing variable climatic conditions and soil types, namely Alexandria, Al-Behera, New Valley, Port Said, and Sinai during 2021. The sampling sites were georeferenced using the global positioning system (GPS) to ensure accurate location data. For each site, 250 g of soil was carefully collected using a sterile spatula at a depth of 10 cm. The sampling process aimed to obtain representative soil samples from the rhizosphere, the region of soil surrounding plant roots where active nutrient exchange and microbial activity occur. The agricultural fields, from which the soil samples were taken, were cultivated indicating that they were actively used for agricultural purposes. The specific crop grown in these cultivated soils was wheat.
To maintain the integrity and quality of the soil samples, they were collected in sterile plastic bags, labeled in the field, and immediately transferred to the laboratory. The samples were stored at 4 °C until further analysis and investigation48.
Isolation of the diazotrophic bacteria
Ten grams of each soil sample were suspended in 90 mL sterile water and shaken at 120 rpm using a rotary at room temperature for 15 min. The suspension was then serially diluted and 1 mL was spread onto NF medium and incubated at 30 °C for 5 days. The used medium composed of mannitol (20 gL−1), CaCO3 (5 gL−1), NaCl (0.2 gL−1), K2HPO4 (0.2 gL−1), K2SO4 (0.1 gL−1), MgSO4.7H2O (0.2 gL−1), and agar (20 gL−1) (Merck KGaA, Darmstadt, Germany). The pH of the medium was adjusted at 6.5. After the incubation period, colonies of the grown bacteria were picked according to the shape, color, and size of the colony and purified before storing in glycerol (20%) at − 20 °C until use49.
Ammonia production test
All isolated bacteria were qualitatively tested for ammonia production using Nessler’s reagent. Each bacterial isolate was inoculated in a sterilized glass tube containing 4% peptone broth and incubated for a week at 30 °C. After the incubation period, 0.5 ml of Nessler’s reagent was added to each tube. Ammonia production was positively detected by the development of a brown to yellow color50.
Amplification of nitrogenase gene
Total DNA of the bacterial isolate was extracted using a QiAamp DNA Mini Kit (Qiagen, Hilden, Germany). To amplify the nitrogenase gene (nifH), the primers nifH-F 5’-AAAGGYGGWATCGGYAARTCCACCAC-3’ and nifH-R 5’-TTGTTSGCSGCRTACATSGCCATCAT-3’ were used. The PCR reaction mixture (25µL) composed of 5 × reaction buffer (10 µL), dNTPs (2 mmol L−1, 5 µL), primers F and R (10 µM, 1 µL for each primer), DNA (2 µL), DNA polemerase (2 U µL−1, 0.25 µL), and dd H2O (8.75 µL). The PCR program was performed as follows: 5 min at 95 °C, 40 cycles (5 s at 95 °C, 30 s at 57 °C, and 40 s at 72 °C). The amplified product was electrophoresed on an agarose gel and imaged using a gel documentation system51.
Screening of the isolated bacteria for calcite-solubilizing activity
The isolated bacteria were screened for their calcite-solubilization activity. Each bacteria was cultured on DBA medium which consisted of glucose (5 gL−1), yeast extract (1 gL−1), peptone (1 gL−1), CaCO3 (5 gL−1), NaCl (5 gL−1), K2HPO4 (0.4 gL−1), (NH4)2SO4 (0.05 gL−1), MgSO4 (0.01 gL−1), and agar (20 gL−1)52. The positive isolate was selected based on the clear zone formation around the colony. The solubilization index of an isolate was determined by measuring diameters of the clear zone and the colony size as follows:
Molecular identification of the selected isolate
Total DNA of the selected isolate based on its calcite-solubilization potential using the QiAamp DNA Mini Kit (Qiagen, Hilden, Germany). Amplification of the 16S rDNA region was performed using the primers 16S-27F 5’-AGAGTTTGATCMTGGCTCAG-3’ and 16S-1492R 5’-CGGTTACCTTGTTACGACTT-3’ as described by White et al.53. Sequence of the amplified product was aligned and compared to the GenBank database via BLAST. Phylogeny tree of the selected isolate compared to the closest sequences from the GenBank database was generated using the maximum likelihood method using MEGA X software (10.2.4).
Estimation of the calcite-solubilization potential under the in-vitro conditions
Calcite-solubilization potential of the selected diazotroph YRNF3 was estimated along 10 days of incubation under the in-vitro conditions. The bacterial isolate was cultured on NF broth (see 2.2.) and incubated under shaking at 120 rpm at 30 °C for 10 days. At each day, the bacterial culture was centrifuged at 3000 rpm for 15 min and the cell free supernatant was analyzed for the soluble calcium54, bicarbonate55, total nitrogen by Kjeldahl method56, total protein using Bradford reagent57, and pH using a pH meter. All analyses were performed in triplicates.
High-performance liquid chromatography (HPLC)
A cell-free culture filtrate of the diazotrophic bacteria YRNF3 was assessed for the organic acids production using a HPLC system (Agilent Technologies, Santa Clara, CA, USA) with a UV detector. The chromatographic separation was done using a reversed-phase column (Extend-C18, column, 4.6 mm × 250 mm, 5 μm particle size) with acetonitrile in H2SO4 as a mobile phase. The mixture standard solution (formic acid, lactic acid, acetic acid, and citric acid) was used for the organic acids detection10.
Quantification of the acid phosphatase activity
The cell-free supernatant of the diazotrophic bacteria YRNF3 was used as a crude enzyme. Activity of the acid phosphatase was estimated as described by Abdelgalil et al.58. The reaction mixture, composed of the crude enzyme, 100 mM sodium acetate buffer pH 4, and 1 mM ρ-nitrophenylphosphate, was incubated at 65 °C. The color was measured using a UV–visible spectrophotometer at 405nm.
Statistical analyses
The data obtained were analyzed using the statistical software CoStat (version 6.4). One-way analysis of variance (ANOVA) was used to test for significant differences between the means of the experimental groups. Tukey's HSD test at p ≤ 0.05 was used for the post-hoc analysis to determine the differences between the individual groups.
Data availability
Data that support these results are available from the corresponding author upon request. Nucleotide sequence of A. salinestris YRNF3 was deposited in the GenBank database under the accession number (OQ605418).
References
Wassif, M. M. & Wassif, O. M. Types and Distribution of Calcareous Soil in Egypt. In Springer Water (eds Elkhouly, A. A. & Negm, A.) 51–88 (Springer International Publishing, 2021).
Johnston, V. E., Martín-Pérez, A., Skok, S. & Mulec, J. Microbially-mediated carbonate dissolution and precipitation; towards a protocol for ex-situ, cave-analogue cultivation experiments. Int. J. Speleol. 50, 137–155 (2021).
Peper, A., Brenneman, T. & Yang, L. Calcite dissolving bacteria from peanut (Arachis hypogaea) pegging zone influences soil calcium level. Front. Microbio. https://doi.org/10.3389/frmbi.2022.1019134 (2022).
Tavakkoli, E., Uddin, S., Rengasamy, P. & McDonald, G. K. Field applications of gypsum reduce pH and improve soil C in highly alkaline soils in southern Australia’s dryland cropping region. Soil Use Manag. 38, 466–477 (2022).
Alcántara, V., Don, A., Well, R. & Nieder, R. Deep ploughing increases agricultural soil organic matter stocks. Glob. Chang. Biol. 22, 2939–2956 (2016).
Yang, P., Dong, W., Heinen, M., Qin, W. & Oenema, O. Soil compaction prevention, amelioration and alleviation measures are effective in mechanized and smallholder agriculture: A meta-analysis. Land https://doi.org/10.3390/land11050645 (2022).
Bu, C. et al. Soil improvement by microbially induced calcite precipitation (MICP): A review about mineralization mechanism, factors, and soil properties. Arab. J. Geosci. 15, 863 (2022).
Mujah, D., Shahin, M. A. & Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol. J. 34, 524–537 (2017).
Hadi, Z. S. & Saeed, K. A. Effect of microbial-induced calcite precipitation (MICP) on the strength of soil contaminated with lead nitrate. J. Mech. Behav. Mater. 31, 143–149 (2022).
Tamilselvi, S. M., Thiyagarajan, C. & Uthandi, S. Calcite dissolution by Brevibacterium sp. SOTI06: A futuristic approach for the reclamation of calcareous sodic soils. Front. Plant Sci. 7, 1828 (2016).
Rana, G., Mandal, T., Mandal, N. K., Sakha, D. & Meikap, B. C. Calcite solubilization by bacteria: A novel method of environment pollution control. Geomicrobiol. J. 32, 846–852 (2015).
El-Sharkawy, H. H. A., Rashad, Y. M. & Elazab, N. T. Synergism between Streptomyces viridosporus HH1 and Rhizophagus irregularis effectively induces defense responses to fusarium wilt of pea and improves plant growth and yield. J. Fungi https://doi.org/10.3390/jof8070683 (2022).
Hafez, M., Abdallah, A. M., Mohamed, A. E. & Rashad, M. Influence of environmental-friendly bio-organic ameliorants on abiotic stress to sustainable agriculture in arid regions: A long term greenhouse study in northwestern Egypt. J. King Saud Univ. - Sci. 34, 102212 (2022).
Al-Askar, A. A. et al. Streptomyces griseorubens E44G: A Potent Antagonist Isolated from Soil in Saudi Arabia. J. Pure Appl. Microbiol. 8, 221–230 (2014).
Rashad, Y. M., Abbas, M. A., Soliman, H. M., Abdel-Fattah, G. G. & Abdel-Fattah, G. M. Synergy between endophytic Bacillus amyloliquefaciens GGA and arbuscular mycorrhizal fungi induces plant defense responses against white rot of garlic and improves host plant growth. Phytopathol. Mediterr. 59, 169–186 (2020).
Pajares, S. & Bohannan, B. J. M. Ecology of nitrogen fixing, nitrifying, and denitrifying microorganisms in tropical forest soils. Front. Microbiol. https://doi.org/10.3389/fmicb.2016.01045 (2016).
Marek-Kozaczuk, M. et al. Host-dependent symbiotic efficiency of Rhizobium leguminosarum bv. trifolii strains isolated from nodules of Trifolium rubens. Int. J. Gen. Mol. Microbiol. 110, 1729–1744 (2017).
Zhan, J. & Sun, Q. Diversity of free-living nitrogen-fixing microorganisms in the rhizosphere and non-rhizosphere of pioneer plants growing on wastelands of copper mine tailings. Microbiol. Res. 167, 157–165 (2012).
Katiyar, P., Kumar, S. & Arora, N. K. Interactions of Nitrogen-Fixing Bacteria and Cereal Crops: An Important Dimension. In (eds Maheshwari, D. K., Dobhal, R. & Dheeman, S.) 169–194 (Springer Nature Singapore, 2022). doi:https://doi.org/10.1007/978-981-19-4906-7_8.
Hafez, M., Abo El-Ezz, S. F., Popov, A. I. & Rashad, M. Organic amendments combined with plant growth-promoting rhizobacteria (Azospirillum Brasilense) as an eco-friendly by-product to remediate and enhance the fertility of saline sodic-soils in Egypt. Commun. Soil Sci. Plant Anal. 52, 1416–1433 (2021).
Hafez, M., Ge, S., Tsivka, K. I., Popov, A. I. & Rashad, M. Enhancing calcareous and saline-sodic soils fertility by increasing organic matter decomposition and enzyme activities: An incubation study. Commun. Soil Sci. Plant Anal. 53, 2447–2459 (2022).
Wakarera, P. W., Ojola, P. & Njeru, E. M. Characterization and diversity of native Azotobacter spp. isolated from semi-arid agroecosystems of Eastern Kenya. Biol. Lett. 18, 20210612 (2022).
Chennappa, G., Naik, M. K., Adkar-Purushothama, C. R., Amaresh, Y. S. & Sreenivasa, M. Y. PGP potential, abiotic stress tolerance and antifungal activity of Azotobacter strains isolated from paddy soils. Indian J. Exp. Biol. 54, 322–331 (2016).
Pi, H. W. et al. Origin and evolution of nitrogen fixation in prokaryotes. Mol. Biol. Evol. 39, msac1816 (2022).
Gaby, J. C. & Buckley, D. H. A comprehensive evaluation of PCR primers to amplify the nifH gene of nitrogenase. PLoS One 7, e42149 (2012).
Delgado-Baquerizo, M. et al. Ecological drivers of soil microbial diversity and soil biological networks in the Southern Hemisphere. Ecology 99, 583–596 (2018).
Wang, C. et al. Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol. Biochem. 113, 240–249 (2017).
Severin, I., Confurius-Guns, V. & Stal, L. J. Effect of salinity on nitrogenase activity and composition of the active diazotrophic community in intertidal microbial mats. Arch. Microbiol. 194, 483–491 (2012).
Pierella Karlusich, J. J. et al. Global distribution patterns of marine nitrogen-fixers by imaging and molecular methods. Nat. Commun. 12, 4160 (2021).
Ullah, A. et al. Diazotrophs-assisted phytoremediation of heavy metals: a novel approach. Environ. Sci. Pollut. Res. 22, 2505–2514 (2015).
Hafez, M., Rashad, M. & Popov, A. I. The biological correction of agro-photosynthesis of soil plant productivity. J. Plant Nutr. 43, 2929–2980 (2020).
Ratzke, C. & Gore, J. Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 16, e2004248 (2018).
Rashad, M., Hafez, M., Popov, A. I. & Gaber, H. Toward sustainable agriculture using extracts of natural materials for transferring organic wastes to environmental-friendly ameliorants in Egypt. Int. J. Environ. Sci. Technol. https://doi.org/10.1007/s13762-022-04438-8 (2022).
Taalab, A. S., Ageeb, G. W., Siam, H. S. & Mahmoud, S. A. Some characteristics of calcareous soils. A review AS Taalab1, GW Ageeb2, Hanan S. Siam1 and Safaa A Mahmoud1. Middle East J. 8, 96–105 (2019).
Thor, K. Calcium—nutrient and messenger. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.00440 (2019).
Vargas, G. et al. The effect of four calcium-based amendments on soil aggregate stability of two sandy topsoils. J. Plant Nutr. Soil Sci. 182, 159–166 (2019).
Turk-Kubo, K. A. et al. Nitrogenase (nifH) gene expression in diazotrophic cyanobacteria in the Tropical North Atlantic in response to nutrient amendments. Front. Microbiol. https://doi.org/10.3389/fmicb.2012.00386 (2012).
Goyal, R. K., Schmidt, M. A. & Hynes, M. F. Molecular biology in the improvement of biological nitrogen fixation by rhizobia and extending the scope to cereals. Microorganisms 9, 1–24. https://doi.org/10.3390/microorganisms9010125 (2021).
Aasfar, A. et al. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. https://doi.org/10.3389/fmicb.2021.628379 (2021).
Katz, A. & Orell, O. Protein Synthesis and the Stress Response. In Cell-Free Protein Synthesis Vol. 7 (ed. Biyani, M.) (IntechOpen, Cham, 2012).
Gray, F., Anabaraonye, B., Shah, S., Boek, E. & Crawshaw, J. Chemical mechanisms of dissolution of calcite by HCl in porous media: Simulations and experiment. Adv. Water Resour. 121, 369–387 (2018).
Al-Khaldi, M. H., Nasr-El-Din, H. A., Mehta, S. & Al-Aamri, A. D. Reaction of citric acid with calcite. Chem. Eng. Sci. 62, 5880–5896 (2007).
Rabie, A. I., Shedd, D. C. & Nasr-El-Din, H. A. Measuring the reaction rate of lactic acid with calcite and dolomite by use of the rotating-disk apparatus. SPE J. 19, 1192–1202 (2014).
Al-Otaibi, M. B., Al-Moajil, A. M. & Nasr-El-Din, H. A. In-situ acid system to clean up drill-in fluid damage in high-temperature gas wells. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference 2006 - Meeting the Value Challenge: Performance, Deliverability and Cost vol. 2006 418–434 at https://doi.org/10.2118/103846-ms (2006).
Dalling, J. W., Heineman, K., Lopez, O. R., Wright, S. J. & Turner, B. L. Nutrient Availability in Tropical Rain Forests: The Paradigm of Phosphorus Limitation. In (eds Goldstein, G. & Santiago, L. S.) 261–273 (Springer International Publishing, 2016). doi:https://doi.org/10.1007/978-3-319-27422-5_12.
Rashad, Y. M., Fekry, W. M. E., Sleem, M. M. & Elazab, N. T. Effects of mycorrhizal colonization on transcriptional expression of the responsive factor JERF3 and stress-responsive genes in banana plantlets in response to combined biotic and abiotic stresses. Front. Plant Sci. 12, 742628. https://doi.org/10.3389/fpls.2021.742628 (2021).
Cabugao, K. G. et al. Root and rhizosphere bacterial phosphatase activity varies with tree species and soil phosphorus availability in puerto rico tropical forest. Front. Plant Sci. https://doi.org/10.3389/fpls.2017.01834 (2017).
Kifle, M. H. & Laing, M. D. Isolation and screening of bacteria for their diazotrophic potential and their influence on growth promotion of maize seedlings in greenhouses. Front. Plant Sci. 6, 1225 (2016).
Döbereiner, J. Isolation and identification of root associated diazotrophs. In Nitrogen Fixation with Non-Legumes (eds Skinner, F. A. et al.) 103–108 (Springer Netherlands, 1989).
Ahmad, F., Ahmad, I. & Khan, M. S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 163, 173–181 (2008).
Turk, K. A. et al. Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic. ISME J. 5, 1201–1212 (2011).
Cacchio, P. et al. Involvement of microorganisms in the formation of carbonate speleothems in the Cervo cave (L’Aquila-Italy). Geomicrobiol. J. 21, 497–509 (2004).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics. In PCR Protocols (eds Innis, M. A. et al.) 315–322 (Academic Press, 1990).
Jackson, M. Soil chemical analysis 498 (Prentice-Hall, 2005).
Loeppert, R. H. & Suarez, D. L. Carbonate and gypsum. Methods Soil Anal. Part 3 Chem. Methods. https://doi.org/10.2136/sssabookser5.3.c15 (2018).
Manickam, S. A. Estimation of nitrogen by Micro-Kjeldahl. In Biochemical methods for agricultural sciences. 34–37 (Wiley Eastern Limited, 1992).
Bradford, M. M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Abdelgalil, S. A., Kaddah, M. M. Y., Duab, M. E. A. & Abo-Zaid, G. A. A sustainable and effective bioprocessing approach for improvement of acid phosphatase production and rock phosphate solubilization by Bacillus haynesii strain ACP1. Sci. Rep. 12, 8926 (2022).
Acknowledgements
Authors would like to thank Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB) for providing the open access fees.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Y.R. and M.H.; Conceptualization, Methodology, Validation, Formal analysis, Data Curation, Writing—Original Draft, and Writing – Review & Editing. M.R.; Methodology, Review & Editing. All authors revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rashad, Y.M., Hafez, M. & Rashad, M. Diazotrophic Azotobacter salinestris YRNF3: a probable calcite-solubilizing bio-agent for improving the calcareous soil properties. Sci Rep 13, 20621 (2023). https://doi.org/10.1038/s41598-023-47924-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47924-w
This article is cited by
-
Eggshell waste bioprocessing for sustainable acid phosphatase production and minimizing environmental hazards
Journal of Biological Engineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.