Abstract
Surgical intervention in the setting of cardiogenic shock (CS) is burdened with high mortality. Due to acute condition, detailed diagnoses and risk assessment is often precluded. Atrial fibrillation (AF) is a risk factor for perioperative complications and worse survival but little is known about AF patients operated in CS. Current analysis aimed to determine prognostic impact of preoperative AF in patients undergoing heart surgery in CS. We analyzed data from the Polish National Registry of Cardiac Surgery (KROK) Procedures. Between 2012 and 2021, 332,109 patients underwent cardiac surgery in 37 centers; 4852 (1.5%) patients presented with CS. Of those 624 (13%) patients had AF history. Cox proportional hazards models were used for computations. Propensity score (nearest neighbor) matching for the comparison of patients with and without AF was performed. Median follow-up was 4.6 years (max.10.0), mean age was 62 (± 15) years and 68% patients were men. Thirty-day mortality was 36% (1728 patients). The origin of CS included acute myocardial infarction (1751 patients, 36%), acute aortic dissection (1075 patients, 22%) and valvular dysfunction (610 patients, 13%). In an unadjusted analysis, patients with underlying AF had almost 20% higher mortality risk (HR 1.19, 95% CIs 1.06–1.34; P = 0.004). Propensity score matching returned 597 pairs with similar baseline characteristics; AF remained a significant prognostic factor for worse survival (HR 1.19, 95% CI 1.00–1.40; P = 0.045). Among patients with CS referred for cardiac surgery, history of AF was a significant risk factor for mortality. Role of concomitant AF ablation and/or left atrial appendage occlusion or more aggressive perioperative circulatory support should be addressed in the future.
Similar content being viewed by others
Introduction
Cardiogenic shock (CS)-related condition at the time of cardiac surgery procedures is a common cause of mortality and its management remains a major challenge despite advances in therapeutic options including mechanical cardiovascular support (MCS)1,2,3. Some of the reversible causes of cardiogenic shock can be successfully managed surgically, provided they are diagnosed quickly before damage to the myocardium is permanent and recovery unlikely4,5,6. Regardless, cardiac surgery in patients with cardiogenic shock is often burdened with excessive risk7,8,9, for the following reasons: (1) detailed diagnostic process might have been not performed due to extremely compromised patient hemodynamic condition; (2) surgery tends to focus on the main objective which is to reverse the CS with borderline coronary lesions or moderate valve insufficiencies seldom addressed; (3) risk of postoperative complications is much higher due to end-organ hypoperfusion and dysfunction at baseline; and finally; (4) post-cardiotomy shock from low cardiac output syndrome (LCOS) is more likely to develop in these patients and postoperative MCS, such as veno-arterial extracorporeal membrane oxygenation (V-A ECMO) and other type of temporary or durable ventricle assist device (VAD), may be necessary alongside pharmacological support to stabilize the patients in this critical condition10.
Atrial fibrillation (AF) is the most common arrhythmia worldwide and its prevalence is higher in patients with coronary artery—(CAD) or valve disease11. The effect of untreated AF on long-term prognosis, both in patients who need cardiac surgery and in patients who do not, is well known12,13,14. On the other hand, the available evidence on whether and how pre-existing AF is complicating cardiogenic shock is limited to acute myocardial infarction (AMI) induced CS (AMI-CS)15,16,17 but poorly investigated in surgically treated CS patients. This is the first report to address early complications and long-term survival in patients undergoing heart surgery for CS with respect to pre-existing atrial fibrillation.
Methods
Data were collected in a retrospective fashion from the KROK (Polish National Registry of Cardiac Surgery Procedures) registry (available at: www.krok.csioz.gov.pl). The registry is an ongoing, nationwide, multi-institutional registry of heart surgery procedures in Poland; the details on registry conception and design were described previously18,19,20. Centers enrolling patients in the KROK registry are required to transfer the data concerning every cardiac surgery to the central database in the National Centre for Healthcare Information Systems at the Ministry of Health and are financially liable for data integrity and completeness. Follow-up data regarding mortality were obtained from the National Health Fund—the nationwide, obligatory, public health insurance institution in Poland and incorporated to the registry. The study was approved by the Institutional Board of Central Clinical Hospital of the Ministry of Interior, Centre of Postgraduate Medical Education, Warsaw, Poland and adheres to Helsinki Declaration as revised in 2013. Due to the anonymization of registry data, patient informed consent was waived by the Institutional Board of Central Clinical Hospital of the Ministry of Interior, Centre of Postgraduate Medical Education, Warsaw, Poland.
Study population
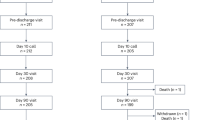
The registry included all adult patients undergoing heart surgery between and 1st Jan 2012 and 31st Dec 2021 and presenting with cardiogenic shock due to all causes. Only patients undergoing heart surgery for isolated pericardial effusion were excluded. Cardiogenic shock in the KROK registry was defined as per SHOCK trial criteria21 until 2016; from then on, European Society of Cardiology Heart Failure guidelines22 criteria were imposed (both available in the Supplementary Methods). Diagnosis of cardiogenic shock was left to discretion of treating physician. We divided the study cohort into patients with documented history of AF (prior-to-admission ICD-9/ICD-10 documentation codes, or on-admission ECG) before the index surgery, and patients without documentation of AF. Post-operative AF was not recorded and therefore not considered. The study flow chart of the present analysis is shown in Fig. 1.
Clinical variables and endpoints
For patients undergoing heart surgery, we considered and reported 3 categories of variables: (1) baseline demographics: age, gender, EuroSCORE II23 and its single components; (2) extent of coronary artery disease (CAD) and/or valvular and/or aortic disease and (3) surgical variables: urgency, operative technique (e.g. on-pump vs. off-pump coronary artery bypass grafting [CABG] surgery). The primary endpoint was death from any cause reported at 30 days and longest available follow-up for the comparison of AF and non-AF patients. In-hospital outcomes and lengths of stays in the intensive care unit (ICU) and hospital (HLoS) are reported and compared as well. Baseline clinical-, procedural- and outcome data at follow-up were entered into prespecified electronic case report forms. Follow-up status with respect to all-cause mortality is validated by Polish National Health Fund and incorporated into the KROK registry.
Statistical analysis
Registry records with > 5% of missing data were not considered; in those with < 5%, missing data were input by artificial neural networks24. Continuous variables were summarized as mean ± standard deviation if normally distributed; non-normal distributions were summarized as median and interquartile range (IQR) and compared with the Mann–Whitney U test or standard t test as appropriate. Categorical variables (number [%]) were compared with the Fisher’s exact test. Risk ratios (RRs) were used primarily for 30-day/in-hospital outcomes. Univariable and multivariable analyses to determine predictors of mortality were conducted. Similarly, we carried out univariable and multivariable analyses to identify the factors associated with the prevalence of AF. We built a non-parsimonious model including variables identified in multivariable analyses25 for propensity score matching (PSM); a 1 to 1 nearest neighbor matching was performed with replacement (caliper 0.2); the overall long-term mortality was assessed with Kaplan–Meier curves fitted before (unadjusted model) and after propensity score matching. Inverse probability weighting (IPW) was performed as sensitivity analysis in order not to exclude from adjusted analysis potentially substantial proportion of participants. Cox regression was used to determine long-term hazard ratio (HR) for all-cause mortality as stratified by AF and non-AF patients. As a further sensitivity analysis to assess the survival in AF and non-AF subsets, we further stratified patients according to pre-defined subgroups. STATA MP v13.0 software (StataCorp, College Station, TX USA) and the packages “psmatch2”, “robust”, “optmatch”, “matchIt” and “CRTgeeDR" in R Core Team 2013 were used.
Results
Baseline demographics
Within the investigated time-frames, 332,109 patients underwent cardiac surgery; Of those 4852 (1.5%) patients presented with CS and their registry records provided data relevant for the analyses. Preoperative AF was documented in 624 of 4852 (12.8%) patients, the mean age was 62 years and 3297 (68%) patients were men. Baseline characteristics of unadjusted group of patients are further available as Supplementary Table 1. Presence of underlying atrial fibrillation was associated with age (P < 0.001), repeat surgery (P < 0.001); diabetes (P < 0.001); hypertension (P = 0.002); chronic kidney and pulmonary disease (P = 0.026 and 0.005 respectively) as well as mitral valve disease (P < 0.001); patients presenting with coronary disease (P = 0.005) and acute aortic dissection (P = 0.021) less frequently had underlying AF in multivariable analysis (Supplementary Table 2). The origin of CS included acute myocardial infarction (36%), acute aortic dissection (22%), and valvular dysfunction (13%). Other etiologies of CS are shown in Supplementary Fig. 1 and Supplementary Table 3. Acute MI mechanical complications (free wall rupture, papillary muscle rupture, ventricular septal defect and left ventricle aneurysm) constituted 334 (6.9%) of cardiogenic shock causes (Supplementary Fig. 1).
Mechanical circulatory support was used preoperatively in 920 (21.4%) patients, and most commonly included intra-aortic balloon pump in 876 (18.1%) patients; followed by ECMO in 78 (3.0%) patients and VAD in 97 (2.0%).
Coronary artery bypass grafting was most commonly performed procedure [1594 pts (32.9%)] followed by aortic dissection repair in 957 (19.7%); aortic, mitral and tricuspid valve repair or replacement surgery was performed in 727 (15.0%), 551 (11.4%) and 140 (2.9%) cases respectively. Fifty-eight (1.2%) patients underwent orthotopic heart transplantation while 101 (2.1%) underwent VAD implantation. Median ICU length of stay was 101.5 h [Interquartile range (IQR) 47.3–213.6] and HLoS among those who survived to discharge 9.2 days (IQR 5.7–16.6). Surgical data are reported in Supplementary Table 4.
Thirty-day mortality was 35.6%. In-hospital complications are available as Supplementary Table 5. In multivariable analysis, age (P < 0.001); repeat surgery (P = 0.012); hypertension (P = 0.001); chronic kidney disease (P < 0.001); peripheral artery disease (P < 0.001); mechanical ventilation (P < 0.001) and surgical urgency (P < 0.001) were associated with long-term mortality (Supplementary Table 6). In an unadjusted analysis, patients with AF had almost 20% higher mortality risk (HR 1.19, 95% CIs 1.06–1.34; P = 0.004) (Supplementary Fig. 2).
PS-matched analysis
We performed a propensity score analysis after the exclusion of orthotopic heart transplantation patients. After the PS-matching 597 pairs were identified (Fig. 1). Baseline characteristics of the study cohort are summarized in Table 1. Patients with AF had more previous percutaneous coronary artery intervention (13.7% vs. 18.9%; P = 0.01), whereas no other significant differences regarding the prevalence of cardiovascular risk factors and comorbidities were seen (Table 1, Supplementary Fig. 3—SMD figure Love plot, Supplementary Fig. 4—PS distribution plot). Principal causes of cardiogenic shock are listed in Supplementary Table 7. We observed no marked differences between AF and no AF patients in terms of CS origin. Around 30% of patients in both groups were operated on shortly after MI (6.5% had mechanical AMI complications). In 15% of patients in both groups acute aortic dissection was the indication for emergent surgery, while pulmonary embolism and infective endocarditis accounted for around 10% in each group.
Surgical data are listed in Table 2. There was a trend towards higher prevelance of hemodynamic instability, defined as the use of iv inotropes (65.3% vs. 70.4%; P = 0.072) in the AF group. Coronary artery bypass grafting (23.5%) and mitral valve procedure (21.6%) were the most commonly performed procedures without significant differences between AF and no-AF patients. In the AF group, the tricuspid valve procedures (4% vs. 7.2%; P = 0.023) and surgical pulmonary embolectomy rates (1% vs. 3%; P = 0.021) were higher. Among patients with AF concomitant cardiac ablation was performed in 6 patients (1%) and left atrial appendage (LAA) closure in 12 (2%).
The use of mechanical circulatory support (pre-operative ventricular assist device (2% vs. 2%; P = 1.000) and extracorporeal membrane oxygenation (1.7% vs. 3.2%; P = 0.131) was similar in both groups.
In hospital outcomes are reported in Supplementary Table 8. The major postoperative outcomes: severe bleeding requiring re-thoracotomy, respiratory failure, neurological and gastrointestinal complications; superficial and deep sternal wound infection and the use of ECMO and intra-aortic balloon pump was similar in both groups. In the PS-matched analysis, total 30-day mortality was 33.6% and was numerically higher in AF group (34.7 vs. 32.5%; P = 0.462) with incidence rates varying across type of surgical procedures; AAD repair had highest (41.8%), followed by AVR/r (38.9%), CABG + valve (38.6%), TVR/r 36.8%, multivalve surgery 36.2% and mitral valve procedures (35.3%), without significant differences between AF and No AF groups but CABG group (42.7 vs. 26.9%; P = 0.005) in favor of no AF (Fig. 2). Median follow-up was 4.6 years (max.10.0 years) and it was 100% complete for the mortality outcome; AF remained associated with worse survival (HR 1.19, 95% CI 1.00–1.40; P = 0.045) (Fig. 3) at long term.
Proportional hazard assumption was not violated (P = 0.439) as also graphically assessed (Supplementary Fig. 5 and 6). In the IPTW analysis, AF was still associated with worse long-term survival with mortality increased in the AF cohort by 3.51% each year (95% CI 0.03–6.74%, P = 0.033). In the subgroup analysis, the harmful effect of AF on long-term mortality was seen in patients initially presenting with unstable coronary artery disease (P = 0.024) and valvular disease (P = 0.030), in particular IE (P = 0.007) (Fig. 4).
Discussion
To the best of our knowledge, this is the first study from a large national inpatient database to analyze the prognostic impact of underlying AF in various setting of CS requiring heart surgery. As major findings, history of AF strongly impacts (1) the survival at 30 days driven by reduction of mortality in patients undergoing CABG surgery and (2) and is associated with higher long-term mortality regardless of the etiology of CS. (3) postoperative complications were similar in patients with and without documented AF during index hospital stay; furthermore, (4) concomitant ablation of AF and closure of left atrial appendage are rarely performed during cardiac procedure for CS.
Atrial fibrillation is the most common cardiac arrhythmia in the general population and a lifetime risk of > 20% after the age of 5511,26. Its prevalence is estimated to at least double with ageing population11. Stroke is the most feared complication in patients with AF, however it also impacts on clinical outcome in specific clinical conditions such as AMI and heart failure or following cardiac surgery procedure13,14,15,16,17,27. As many as 28% of the patients admitted for heart surgery procedure present with AF with increasing rates depending on the presence of valvular dysfunction and extent of the cardiac disease28. AF is a well-known marker of high-risk patients and a predictor of postoperative complications including mortality, postoperative stroke, renal failure, prolonged ventilation, reoperation, and deep sternal wound infection13. Patients with preoperative diagnosis of AF also experience a higher adjusted long-term risk of all-cause death and of a cumulative risk of stroke and systemic embolism compared to those without13.
AF and CS post AMI
The prognostic impact of AF in the setting of CS complicating AMI has mostly been reported after percutaneous procedures. From the IABP-SHOCK II trial (600 patients enrolled, 169 with AF versus 431 without), there were no significant differences with respect to mortality at 30 days and 12 months between patients with and without AF29. Similarly, the rates of recurrent MI, repeat revascularization, and stroke did not differ between groups. The authors did not observe any interaction between the impact of IABP on clinical outcome and the prevalence of AF. Reflecting the above were the findings reported in a sub-analysis of the Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock trial15. The study included 686 patients (142 with AF history on admission, or newly detected AF during index hospitalization) and AF was not a significant predictor of 30-day and 1-year all-cause mortality. However, patients with AF already on admission (90 of 142), showed higher all-cause mortality at 30 days (58% vs. 37%; P = 0.02) and 1 year (63% vs. 39%; P = 0.004) compared with patients with newly detected AF during hospital stay. Furthermore, AF was associated with longer time to hemodynamic stabilization (4 vs. 3 days; P = 0.04) at 30 days. In another PS-matched study from NIS registry including 840 patients (420 with AF) who underwent PCI while on percutaneous VAD (Impella®) because of CS complicating AMI, all-cause in-hospital mortality rates between the two groups were similar (40.5% vs. 36.7%, P = 0.245). However, the AF group experienced a significantly higher rate of postprocedural respiratory complications (9.5% vs. 4.8%; P = 0.007), fewer routine discharges (13.1% vs. 30.2%; P < 0.001) and more frequent transfers to other healthcare facilities (27.3% vs. 17.8%; P < 0.001). The mean LOS (12 vs. 9 days; P < 0.001) and hospital charges ($308,478 vs. $277,982; P = 0.008 ) were higher in the AF group16.
AF and CS—surgical strategy
The impact of preoperative AF in patients requiring heart surgery for CS is poorly investigated and reported. In our study, CAD remains the major cause of CS and CABG remains the most common surgical treatment, respectively. The current guidelines do not exclude a role for emergency CABG that is usually regarded as the last resource and only in a very limited percentage of patients30. Patients undergoing isolated CABG for CS suffer up to 20% higher mortality rates comparing to those without and this occurs also with milder degrees of CS31,32. In one recent analysis from the STS database, of the 5259 patients with AMI complicated by CS who underwent CABG during the study period, 665 (12.6%) patients had AF which in a multivariable logistic regression analysis was associated with increased operative mortality (HR 1.44, 95% CI [1.18–1.77]; P < 0.001)33.
Recent reports on surgery for mitral papillary muscle rupture and CS from the Japan cardiovascular surgery database (196 patients, 140 CS) and STS database (1342 patients, 759 CS) do not address this issue (the former) or describe no impact of preoperative arrhythmias as predictors of operative mortality in multivariable logistic regression model (the latter)34,35. Sagakuchi et al. identified 1397 patients undergoing surgical repair of post-MI VSD (61.5% CS) from the national Japanese database and concluded that preoperative AF was not a significant prognostic factor (HR 0.79, 95% CI [0.50–1.23]; P = 0.29 in the multivariable analysis)36. Similarly, no relationship was observed between the prevalence of the AF and survival in the UK National Adult Cardiac Surgery Audit of post‐infarct ventricular septal defect repair (5.0% among survivors, 5.9% among non-survivors; P = 0.6)37. Accordingly, in our study, we did not observe differences in survival in the MI mechanical complications subgroup. However, we noted a significant relationship among patients with different CS etiology, particularly CAD and valvular decease.
One interesting finding of the current analysis is the low utilization rate of MCS devices in patients with CS in anticipation of or following the surgical treatment. In the setting of CS, temporary MCS can help to stabilize patients and grant time for decision-making about the definitive management31. In a recent STS report, AF occurrence in patients with AMI and CS undergoing CABG was higher in the MCS group suggesting a further negative hemodynamic impact of this arrhythmia31. In our analysis only 19.0% patients received MCS, and these most commonly included IABP (18.1%); followed by ECMO in 78 (3.0%) patients and VAD in 97 (2.0%). What is reflected in the present analysis is the approach to rush the patient to the OR and stabilize the condition with CPB in most cases rather than stabilize the patient first in the ICU.
“Anti-AF” approaches
This study shows that ablation of AF or LAAO during heart surgery for patients in CS is very seldom performed. From the 2020 STS report, only 18 patients among 1342 (1.3%) that underwent mitral valve surgery for ischemic papillary muscle rupture received ablation and in three major randomized trial on LAA closure (LAOS I–III) non-elective surgical cases were excluded by the study design38,39,40. Conditions related to CS requiring surgery are demanding and challenging operations and it is perfectly understandable that management of the cause of CS should be the priority. The current analysis could not address AF surgical management; yet, because of lower mortality in the no-AF matched, it may suggest there is a potential to reduce both early and long-term mortality when AF is addressed as well. Indeed, previous observational studies suggested similarly lower risk of long-term mortality in patients undergoing surgical ablation concomitant to CABG w/wo valvular procedure in patients in critical condition, with pre-op IABP and on pharmacological inotropic support20.
Limitations
There are certain limitations to the current retrospective study that need to be acknowledged; firstly, the registry did not collect, at the time of conception, the data regarding long-term outcomes other than all-cause mortality e.g. long-term stroke, rehospitalization for heart failure, repeat revascularization, re-do surgery and other procedures e.g. catheter ablation or PCI; these could further enhance the registry and might have influenced the remote outcome as well. Secondly, the registry does not collect the data regarding medical therapy especially regarding oral anticoagulation (OAC) therapy. Information regarding OAC in both pre-existing AF and postoperative AF, should one occur, would shed light to evolving thromboembolic risk in shock patients given the lack of unanimous recommendations regarding OAC institution in POAF and OAC postoperative reinstitution in pre-existing AF. Thirdly, certain detailed baseline and operative data such as AF type and duration were not collected by the registry; information on the timing of interventions, delay to surgery, duration of pre-op IABP, doses of inotropes and certain characteristics of mechanical ventilation and other ICU variables are missing. Finally, while PSM accounted for the variables included in the EuroSCORE II and other surgically relevant characteristics minimizing selection bias in an attempt to even baseline patients’ characteristics, unmeasured biases and confounders may remain, in particular in the setting of cardiogenic shock, making the association between AF and higher mortality in cardiogenic shock valid only to the extent an analysis of a non-RCTs study allows. On the other hand, multivariable analyses fully support the concept of AF as a hallmark of worse baseline condition and higher risk independently associated with worse prognosis both at early and long-term follow-up. The optimal timing of surgical intervention in patients with CS that could benefit of preoperative MCS is a matter of further debate not addressed by this study.
Conclusions
Among patients with CS referred for cardiac surgery, history of AF was a significant risk factor for long-term mortality. Thirty-day mortality was 36% with a significant difference between AF and no-AF in favour of the latter in subgroup of patients undergoing CABG. Addressing AF by concomitant ablation and/or left atrial appendage closure at the time of surgery may be considered to reduce thromboembolic risk and worsening of heart failure even in these highest risk patients. However, additional and dedicated studies investigating patients in CS and affected by preoperative AF should be undertaken to carefully analyze the actual impact and related therapeutic treatment to abolish such a cardiac arrhythmia in this peculiar hemodynamic setting.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hollenberg, S. M., Kavinsky, C. J. & Parrillo, J. E. Cardiogenic shock. Ann. Intern. Med. 131, 47–59. https://doi.org/10.7326/0003-4819-131-1-199907060-00010 (1999).
Gong, F. F., Vaitenas, I., Malaisrie, S. C. & Maganti, K. Mechanical complications of acute myocardial infarction: A review. JAMA Cardiol. 6, 341–349. https://doi.org/10.1001/jamacardio.2020.3690 (2021).
Khorsandi, M. et al. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: A systematic review and meta-analysis. J. Cardiothorac. Surg. 12, 55. https://doi.org/10.1186/s13019-017-0618-0 (2017).
Desch, S. Revascularization strategies in cardiogenic shock after acute myocardial infarction. Curr. Opin. Crit. Care 25, 379–383. https://doi.org/10.1097/MCC.0000000000000623 (2019).
Ibrahim, M. et al. Coronary artery bypass grafting in cardiogenic shock: Decision-making, management options, and outcomes. J. Cardiothorac. Vasc. Anesth. 35, 2144–2154. https://doi.org/10.1053/j.jvca.2020.09.108 (2021).
Akodad, M., Schurtz, G., Adda, J., Leclercq, F. & Roubille, F. Management of valvulopathies with acute severe heart failure and cardiogenic shock. Arch. Cardiovasc. Dis. 112, 773–780. https://doi.org/10.1016/j.acvd.2019.06.009 (2019).
Samsky, M. D. et al. Cardiogenic shock after acute myocardial infarction: A review. JAMA 326, 1840–1850. https://doi.org/10.1001/jama.2021.18323 (2021).
Pöss, J. et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J. Am. Coll. Cardiol. 69, 1913–1920. https://doi.org/10.1016/j.jacc.2017.02.027 (2017).
Beer, B. N. et al. Early risk stratification in patients with cardiogenic shock irrespective of the underlying cause—The cardiogenic shock score. Eur. J. Heart Fail. 24, 657–667. https://doi.org/10.1002/ejhf.2449 (2022).
Rao, P., Khalpey, Z., Smith, R., Burkhoff, D. & Kociol, R. D. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ. Heart Fail. 11, e004905. https://doi.org/10.1161/CIRCHEARTFAILURE.118.004905 (2018).
Morin, D. P. et al. The state of the art: Atrial fibrillation epidemiology, prevention, and treatment. Mayo Clin. Proc. 91, 1778–1810. https://doi.org/10.1016/j.mayocp.2016.08.022 (2016).
Gammie, J. S. et al. Atrial fibrillation correction surgery: Lessons from the society of thoracic surgeons national cardiac database. Ann. Thorac. Surg. 85, 909–914. https://doi.org/10.1016/j.athoracsur.2007.10.097 (2008).
Malaisrie, S. C. et al. Burden of preoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 155, 2358–2367. https://doi.org/10.1016/j.jtcvs.2018.01.069 (2018).
Saxena, A., Virk, S. A., Bowman, S. & Bannon, P. G. Systematic review and meta-analysis on the impact of preoperative atrial fibrillation on short- and long-term outcomes after aortic valve replacement. J. Cardiovasc. Surg. (Torino) 58, 943–950. https://doi.org/10.23736/S0021-9509.17.09814-7 (2017).
Feistritzer, H. J. et al. Prognostic impact of atrial fibrillation in acute myocardial infarction and cardiogenic shock. Circ. Cardiovasc. Interv. 12, e007661. https://doi.org/10.1161/CIRCINTERVENTIONS.118.007661 (2019).
Sonu, G. et al. The impact of atrial fibrillation on in-hospital outcomes in patients with acute myocardial infarction complicated by cardiogenic shock undergoing coronary revascularization with percutaneous ventricular assist device support. J. Atr. Fibrillation 12, 2179. https://doi.org/10.4022/jafib.2179 (2020).
Vallabhajosyula, S. et al. Burden of arrhythmias in acute myocardial infarction complicated by cardiogenic shock. Am. J. Cardiol. 125, 1774–1781. https://doi.org/10.1016/j.amjcard.2020.03.015 (2020).
Kowalewski, M., Jasiński, M., Staromłyński, J., Zembala, M., Widenka, K., & Brykczyński M et al. KROK Investigators. On-Pump versus Off-Pump coronary artery bypass surgery in atrial fibrillation. Analysis from the polish national registry of cardiac surgery procedures (KROK). PLoS One 15, e0231950. https://doi.org/10.1371/journal.pone.0231950 (2020).
Suwalski, P., Kowalewski, M., Jasiński, M., Staromłyński, J., Zembala, M. & Widenka K et al. KROK Investigators. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: Analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J. Thorac. Cardiovasc. Surg. 157, 1007–1018.e4. https://doi.org/10.1016/j.jtcvs.2018.07.099 (2019)
Suwalski, P. et al. Surgical ablation for atrial fibrillation during isolated coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 57, 691–700. https://doi.org/10.1093/ejcts/ezz298 (2020).
Hochman, J. S. et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl. J. Med. 341, 625–634. https://doi.org/10.1056/NEJM199908263410901 (1999).
Ponikowski, P. et al. ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200. https://doi.org/10.1093/eurheartj/ehw128 (2016).
Nashef, S. A. et al. EuroSCORE II. Eur. J. Cardiothorac. Surg. 41, 734–744. https://doi.org/10.1093/ejcts/ezs043 (2012).
Spinelli, I., Scardapane, S. & Uncini, A. Missing data imputation with adversarially-trained graph convolutional networks. Neural Netw. 129, 249–260. https://doi.org/10.1016/j.neunet.2020.06.005 (2020).
Okoli, G. N., Sanders, R. D. & Myles, P. Demystifying propensity scores. Br. J. Anaesth. 112, 13–15. https://doi.org/10.1093/bja/aet290 (2014).
Heeringa, J. et al. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur. Heart J. 27, 949–953. https://doi.org/10.1093/eurheartj/ehi825 (2006).
Ruddox, V. et al. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 24, 1555–1566. https://doi.org/10.1177/2047487317715769 (2017).
McCarthy, P. M. et al. Prevalence of atrial fibrillation before cardiac surgery and factors associated with concomitant ablation. J. Thorac. Cardiovasc. Surg. 159, 2245-2253.e15. https://doi.org/10.1016/j.jtcvs.2019.06.062 (2020).
de Waha, S. et al. Prognostic impact of atrial fibrillation in cardiogenic shock complicating acute myocardial infarction: A substudy of the IABP-SHOCK II trial. Clin. Res. Cardiol. 107, 233–240. https://doi.org/10.1007/s00392-017-1175-1 (2018).
Gaudino, M. et al. Long-term survival and quality of life of patients undergoing emergency coronary artery bypass grafting for postinfarction cardiogenic shock. Ann. Thorac. Surg. 101, 960–966. https://doi.org/10.1016/j.athoracsur.2015.08.066 (2016).
Acharya, D. et al. clinical characteristics and outcomes of patients with myocardial infarction and cardiogenic shock undergoing coronary artery bypass surgery: Data from the society of thoracic surgeons national database. Ann. Thorac. Surg. 101, 558–566. https://doi.org/10.1016/j.athoracsur.2015.10.051 (2016).
Khaladj, N. et al. Immediate surgical coronary revascularisation in patients presenting with acute myocardial infarction. J. Cardiothorac. Surg. 8, 167. https://doi.org/10.1186/1749-8090-8-167 (2013).
Cox, M. L. et al. Outcomes after coronary artery bypass grafting in patients with myocardial infarction, cardiogenic shock and unresponsive neurological state: Analysis of the Society of Thoracic Surgeons Database. Eur. J. Cardiothorac. Surg. 54, 710–716. https://doi.org/10.1093/ejcts/ezy114 (2018).
Fujita, T. et al. Mitral valve surgery for ischemic papillary muscle rupture: Outcomes from the Japan cardiovascular surgery database. Gen. Thorac. Cardiovasc. Surg. 68, 1439–1446. https://doi.org/10.1007/s11748-020-01418-y (2020).
Kilic, A., Sultan, I., Chu, D., Wang, Y. & Gleason, T. G. Mitral valve surgery for papillary muscle rupture: Outcomes in 1342 patients from the society of thoracic surgeons database. Ann. Thorac. Surg. 110, 1975–1981. https://doi.org/10.1016/j.athoracsur.2020.03.097 (2020).
Sakaguchi, G. et al. Surgical repair of post-infarction ventricular septal defect—Findings from a Japanese National Database. Circ. J. 83, 2229–2235. https://doi.org/10.1253/circj.CJ-19-0593 (2019).
Dimagli, A. et al. Surgical outcomes of post-infarct ventricular septal defect repair: Insights from the UK national adult cardiac surgery audit database. J. Card. Surg. 37, 843–852. https://doi.org/10.1111/jocs.16178 (2022).
Healey, J. S. et al. Left atrial appendage occlusion study (LAAOS): Results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am. Heart J. 150, 288–293. https://doi.org/10.1016/j.ahj.2004.09.054 (2005).
Whitlock, R. P. et al. Left atrial appendage occlusion study II (LAAOS II). Can. J. Cardiol. 29, 1443–1447. https://doi.org/10.1016/j.cjca.2013.06.015 (2013).
Whitlock, R. P. et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N. Engl. J. Med. 384, 2081–2091. https://doi.org/10.1056/NEJMoa2101897 (2021).
Acknowledgements
KROK registry is funded and run by the Polish Ministry of Health; the current analysis evolved in scientific collaboration with ISMETT, Palermo, Italy. The publication open access charge was granted by the Italian Ministry of Health. We acknowledge the contribution of late Professor Doctor Marian Zembala (1950-2022), pioneer in cardiac surgery, Head of Silesian Centre for Heart Diseases in Zabrze and the Minister of Health, in the conception and design of the KROK registry. KROK investigators are listed in the supplement. Work by Thoracic Research Centre (www.trc.org.pl).
Funding
The research received no additional funding.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: M.K., G.M.R.; data curation: M.K., G.M.R., M.P.; formal analysis: M.K., M.P.; funding acquisition: N.A.; investigation: all authors; methodology: all authors; project administration: M.K., P.S.; resources: M.K., P.S.; software: M.K.; supervision: M.K., P.S.; validation: all authors; visualization: M.K., M.P.; writing-original draft: all authors; writing-review and editing: all authors. Other: registry administration: Z.T..
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kowalewski, M., Raffa, G.M., Pasierski, M. et al. Prognostic impact of preoperative atrial fibrillation in patients undergoing heart surgery in cardiogenic shock. Sci Rep 13, 21818 (2023). https://doi.org/10.1038/s41598-023-47642-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47642-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.