Abstract
Aluminum (Al) toxicity limits crop production worldwide. Although studies have identified genes associated with Al tolerance in crops, a large amount of data remains unexplored using other strategies. Here, we searched for single substitutions and InDels across differentially expressed genes (DEGs), linked DEGs to Al-tolerance QTLs reported in the literature for common maize, and investigated the alternative splicing regulated by Al3+ toxicity. We found 929 substitutions between DEGs in Al-tolerant and 464 in Al-sensitive inbred lines, of which 165 and 80 were non-synonymous, respectively. Only 12 NS variants had deleterious predicted effect on protein function in Al-tolerant and 13 in Al-sensitive. Moreover, 378 DEGs were mapped in Al-QTL regions for the Al-tolerant and 213 for the Al-sensitive. Furthermore, Al stress is primarily regulated at the transcriptional level in popcorn. Important genes identified, such as HDT1, SWEET4a, GSTs, SAD9, PIP2-2, CASP-like 5, and AGP, may benefit molecular assisted popcorn breeding or be useful in biotechnological approaches. These findings offer insights into the mechanisms of Al tolerance in popcorn and provide a ‘hypothesis-free’ strategy for identifying and prioritizing candidate genes that could be used to develop molecular markers or cultivars resilient to acidic soils.
Similar content being viewed by others
Introduction
Aluminum (Al) is one of the most abundant elements in the Earth’s crust. In acid soils, with pH values < 5, the phytotoxic species Al3+ is solubilized in soil solution and becomes one of the major problems of crop cultivation by interfering with root function and reducing plant development1,2. Al toxicity inhibits root growth and biomass, causes nutrient imbalance, changes in photosynthetic activities, increased oxidative stress and disturbances in carbon dioxide assimilation rates3.
At molecular level, Al-mediated signaling pathways involve various processes, including the perception and triggering of stress responses and resistance to Al in roots. These processes result from induced changes in cellular homeostasis, direct interaction between Al3+ and plasma membrane Al-receptors, and Al-induced inhibition of membrane transport and/or involvement of plasma membrane signal transducers. These alterations form the basis for signal transduction cascades that activate Al-resistance pathways: the transcriptional activation of biosynthetic enzymes and membrane transporters, which are central to exclusion and tolerance mechanisms; and the post-translational modifications activating and regulating protein functions2.
The well-known documented tolerance mechanisms of Al stress in higher plants includes exclusion and detoxification mechanisms. The exclusion mechanism consists of organic acid (OA) exudation, preventing the ion Al3+ from entering the root apex, forming the non-toxic complex Al-OA. On the other hand, the detoxification mechanism consists of neutralizing the toxic Al3+ absorbed through sequestration and detoxification in subcellular compartments and/or translocating it away from the root tip2.
Al tolerance is considered a quantitative trait in maize. Quantitative trait loci (QTL) mapping studies for Al tolerance in common maize identified genomic regions associated with this trait across several chromosomes4,5,6,7,8. Despite its agronomic importance, ZmMATE17 and ZmALMT1 and ZmALMT29,10 have been characterized in common maize, but other genes and mechanisms involved in the tolerance to the phytotoxic Al ion are currently unknown.
Popcorn (Zea mays L. var. everta) has an important role in United States economy and also has a high demand in Brazil11. Like traditional maize, popcorn is extensively cultivated through tropics regions in Brazil, thus attracting the attention of breeders to obtain cultivars adapted to Brazilian conditions12. Al toxicity affects root development and causes damage and cell disorganization in the apical region in popcorn seedlings, ultimately compromising their growth and nutrient uptake13. The tolerance mechanisms in popcorn seedlings are involved with the increasing of sucrose content in the roots and shoots, starch decreasing in roots, and induced secretion of malate and fumarate14.
Gene expression is highly influenced by different environmental conditions and can trigger various point mutations across the genome. Plants exhibit diverse transcriptional, physiological, and fitness responses to abiotic stress15,16,17, and understanding how these variations affects gene expression as well as plant fitness and adaptation is crucial to providing solutions for crop breeding in the context of climate change. Beyond point mutations, alternative splicing is a dynamic post-transcriptional regulatory mechanism that produces multiple protein variants and regulates many physiological processes essential for plant growth and development, especially in response to stress conditions18. In this way, RNA-seq studies allow obtaining a large amount of data and provide new perspectives to uncover transcript dynamics and altered patterns under adverse environment conditions.
Recently, the gene expression profile of two popcorn inbred lines contrasting in Al-tolerance were tracked after 72 h of stress, revealing the mechanisms involved in a long-term Al-exposure19. This study used the RNA-seq approach and detected genes already known to be related to Al-tolerance, as well as others not previously described in hydroponic or soil experiments. Transcriptomic data analysis can provide valuable information on expressed genes in QTL regions, alternative splicing and SNPs associated with stress tolerance in plants. However, strategies to reduce the list of candidates are necessary when selecting candidates to use in breeding programs.
In this study, we performed comprehensive and extensive analyses to investigate potential candidates for Al-tolerance in popcorn. We assessed single substitutions and InDels across the differentially expressed genes (DEGs), linking them to Al-tolerance QTLs previously reported in the literature for common maize, and investigated the post-transcriptional regulation of popcorn under Al-stress. Our findings provide novel insights into the roles of different molecular processes underlying Al-tolerance in popcorn. They are useful to be explored in genetic engineering tools and/or as biomarkers in popcorn breeding programs.
Results and discussion
Al stress triggers polymorphisms in expressed genes
A total of 929 substitutions were identified among 128 DEGs in the Al-tolerant inbred line (11–133), and 464 substitutions were found among 67 DEGs in the Al-sensitive inbred line (11–60) (Supplementary Table 1). In both inbred lines, most of the substitutions were synonymous, and variations were also found in untranslated regions (UTRs) (Fig. 1).
From the non-synonymous variants, a PROVEAN analysis showed that 12 of them presented deleterious predicted effect on protein function in the 11–133 (Table 1), and 13 in the 11–60 inbred line (Table 2). DEGs presenting deleterious predicted effects on protein function, either in 11–133 or in 11–60, are related to abiotic stress response, i.e. Glutathione transferase 7 (GST7, Zm00001d042104), Phenylalanine ammonia-lyase 1 (PAL1, Zm00001d033286), Histone deacetylase (HDT1, Zm00001d012092), and SNF1-related protein kinase regulatory subunit beta-1 (SnRKβ1, Zm00001d010662). Deleterious mutations can disable the biosynthesis of metabolites, altering plant responses to environmental stimuli20. However, under certain conditions, they can play a crucial role in adaptive evolution21. Otherwise, we detected 67 substitutions with neutral predicted effect (PROVEAN score < -2.5) on biological protein function in 11–133 (Supplementary Table 2) and 36 in 11–60 (Supplementary Table 3).
Glutathione transferases are the main cellular detoxification enzymes that act against oxidative damage in plants22. The increased expression and activity of GST have been linked to aluminum tolerance in maize23. The predicted deleterious effect in GST7 function was by a substitution of the basic Arg with Gly within the conserved N-terminal domain (Table 1). In GSTs, the N-terminal domain forms a thioredoxin-like fold, and changes in this domain have been correlated with high levels of GST expression in maize24. Additionally, a missense substitution with neutral predicted effect was detected in the C-terminal domain in GST7 (Supplementary Table 2).
In addition to GSTs and the total glutathione accumulated in plant tissues under Al3+ toxicity, phenolic compounds are also regulated in response to this adverse condition, acting as potential defensive compounds. Phenylalanine ammonia-lyase is a key biosynthetic enzyme that produces intermediaries for polyphenolic compounds, and its activity increases in roots of Al-tolerant lettuce when exposed to Al toxicity25. In sorghum, the increasing of phytochelatins in roots can be related to Al3+ chelation26. Our findings suggest that this substitution in GST7 and PAL1 may confer an advantage in the detoxification of reactive oxygen species (ROS) and restriction of Al3+ in root cells of the Al-tolerant inbred line.
A single substitution in the polar residue region of HDT1 resulted in the change of a Pro to a Thr (Table 1). Additionally, five substitutions with neutral predicted effects were found in HDT1 (Supplementary Table 2). Histone acetylation and deacetylation play important roles in gene expression in eukaryotic cells. Maize exposed to different heavy metal stress conditions showed different acetylation levels due to different regulation of histone deacetylases27. These substitutions found in HDT1 may be important for the adaptation of popcorn roots to Al toxicity, however, epigenetic regulation in maize under Al-stress is poorly understood and needs further investigation.
In the Al-sensitive inbred line, the down-regulated kinase SnRKβ1 had a deleterious substitution in the AMPK1_CBM domain (Table 2). SnRK1 plays several roles in abiotic stress resistance, and the decrease of its expression is associated with stress sensitivity28. This gene also regulates organic acid metabolism and other signaling pathways. SnRK1 knockdown lines of Arabidopsis thaliana under dark conditions altered the content of organic acids, revealing the importance of this gene in metabolic adaptation to stress29. Based on this, this amino acid change may cause unfavorable effects on protein function, increasing sensitiveness of 11–60 to Al3+.
Although this study focused on non-synonymous variations within protein-coding regions, silent substitutions and variations in intragenic regions among the DEGs may also have benefits for post-transcriptional mechanisms in response to Al-stress. While synonymous substitutions do not alter the amino acid sequence, they can still affect gene expression, protein folding, and the fitness of the organism30. Intragenic substitutions/InDels within 3′-UTR and 5′-UTR regions can alter gene expression by changing binding sites for transcription factors and miRNAs20.
A total of 164 variations were found in 3′-UTR and 5′-UTR regions in Al-tolerant inbred line, while 109 in the Al-sensitive, and none of these genes were shared in both inbred lines (Supplementary Table S1). The same transcript of HDT1 that presented a NS variation (Zm00001d012092_T001) also presented two 3′-UTR variations, reinforcing its significance to Al-tolerance in popcorn. Furthermore, important up-regulated genes associated with abiotic stress presented variations in UTR regions. Were detected three variations in the 3′-UTR and 5′-UTR regions in SWEET4a (Zm00001d015905_T001), one in 3′-UTR in a Heavy metal transport/detoxification superfamily protein (Zm00001d027454_T001), one in 5′-UTR in GST12 (Zm00001d027540_T002) and six in both UTR regions for GST6 (Zm00001d027541_T001 and Zm00001d027541_T002). Curiously, GST6 was detected as a central hub in in silico protein–protein network interacting with several Al tolerance related genes19.
These finds shed light for the importance of substitutions in UTR regions as an adaptative force to the plant deal with Al stress, enhancing the expression and impacting in the mRNA stability and translation efficiency of Al tolerance related genes.
Linking RNA-seq data with QTLs previously described reduces the number of potential candidates for Al-tolerance
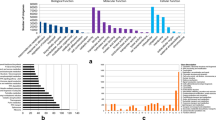
A total of 378 DEGs were identified within previous Al-QTL regions in 11–133 (Supplementary Table 4), and 213 genes in 11–60 (Supplementary Table 5). Gene ontology enrichment analysis showed that the majority of these DEGs were categorized under oxidoreductase activity and oxidation–reduction process, moreover, terms related to stress response and defense were enriched only in the Al-tolerant inbred line (Fig. 2). Out of this total, 56 DEGs were shared between both inbred lines (Fig. 3).
Gene ontology (GO) enrichment of top 10 GO terms of differentially expressed genes using PlantRegMap (http://plantregmap.gao-lab.org/) Zea mays database (p-value < 0.05).
Al-related genes found in common maize, like ZmMATE1, ZmALMT1 and ZmALMT2, were not differentially expressed in popcorn. This may be attributed to the timeframe of our study, which aimed to investigate the long-term molecular response to Al exposure, and to the genetic background of these popcorn inbred lines with the common maize genotypes tested in previous studies.
In 11–133, HDT1 and Stearoyl-acyl-carrier-protein desaturase 9 (SAD9, Zm00001d012221) were mapped in QTL75, and both showed changes with predicted deleterious effect on protein function, and the first transcript also presented variations in 3′-UTR region (Table 3, Supplementary Tables 1 and 4). SAD is a key enzyme in determining the global content of unsaturated fatty acids (UFA)31. Its expression decreased, and the predicted deleterious effect may lead to the reduction in the UFA content of lipids cells, changing lipid fluidity. Moreover, two Δ12-fatty-acid desaturases (Zm00001d023768 and Zm00001d023769) were down-regulated in 11–133 but up-regulated in 11–60 (Fig. 3, Supplementary Table 4), and mapped in QTL2. A high degree of fatty acid desaturation allows for high capability to stabilize the membrane fluidity, alleviating membrane damage from Al stress and limiting the entry of Al3+ into roots32.
Two aquaporins PIP2-2 (Zm00001d005410 and Zm00001d014285) were detected in QTL3, QTL14, and QTL175,7,8, and an ABC transporter (Zm00001d024600) in QTL24 for the Al-tolerant inbred line (Supplementary Table 4). Aquaporins and ABC transporters play an essential role in the vacuolar sequestration of Al3+, promoting Al tolerance in plants33. Aquaporins PIP1-1 and PIP2 were recently identified in the maintenance of hydration in Citrus limonia L. plants when exposed to Al stress34. On the same way, ABC transporters facilitate vacuolar sequestration of Al, playing an important role in the tolerance mechanism35. Both are involved in diverse processes in plant growth and development under abiotic stress36.
The SWEET3b (Zm00001d023673) and SWEET4a (Zm00001d015905) were identified in QTL24 and QTL147 in 11–133 (Supplementary Table 4). In addition, SWEET4a presented variations in UTR regions (Supplementary Table 1). To deal with environmental stress conditions, plants need to maintain a strict regulation in the storage and transport of vacuolar sugar37. The SWEET genes play an important role in tolerance to osmotic stress, cold, high salinity, and drought38. The role of SWEET transporters is poorly understood in plants exposed to Al3+ toxicity, but these genes may be related to the Al tolerant phenotype in popcorn.
We identified DEGs involved in cell wall modification in 11–133 inbred line that were mapped across several Al tolerance QTL (Supplementary Table 4). These genes are mainly involved in primary cell wall and xyloglucan metabolism. 39,40 previously reported some of the same genes in Arabidopsis that are involved in Al tolerance. 41 detected a xyloglucan endotransglycosylase protein 8 precursor in the Al tolerance QTL Alm1. These findings suggest that the process of structural organization of cell wall is crucial for root growth and development under Al3+ toxicity conditions in the Al-tolerant inbred line.
CASP-like 5 (Zm00001d010038) and Arabinogalactan peptide 20 (AGP, Zm00001d010434) were both mapped in QTL65 and found to be up-regulated in 11–133 but down-regulated in 11–60. CASP protein is involved in stress resistance and nutrient uptake42, and in Tamba black soybean under Al-stress, the expression of CASP protein is associated with root growth43. On the other hand, AGP plays multiple roles in plant growth and development, and under abiotic stress, it is involved in the thickening of cell wall by oxidative crosslinking mediating the stress signaling response44. The presence of these genes within an Al tolerance QTL may be responsive for the root and plant growth in acid soils.
In addition to reducing the number of candidates useful for developing molecular markers for marker-assisted selection or genomic selection for Al-tolerance, the identification of DEGs in QTL regions can provide insights into the molecular mechanisms underlying the traits controlled by these regions.
Response to Al stress is primarily regulated at the transcriptional level
For the Al-tolerant inbred line, we observed a total of 25,026 RI; 15,357 A3SS; 11,782 A5SS; 10,786 SE; and 626 MXE events, respectively. A single gene (Zm00001d002141), which encodes a UBP1-associated protein 2A, underwent differential SE events, with three exons whose inclusions increased upon treatment. A similar frequency of AS events was observed for the Al-sensitive inbred line, which had 25,039 RI; 15,345 A3SS; 11,791 A5SS; 10,743 SE; and 618 MXE events, respectively. Three genes (Zm00001d047479, Zm00001d036136, Zm00001d044494) underwent differential SE events in the Al-sensitive inbred line. These genes encode a superoxide dismutase (SOD), CYSTM domain-containing protein, and pectin acetylesterase (PAE), respectively.
The small number of genes undergoing differential AS (DAS) reveals that post-transcriptional regulation does not play a significant role in popcorn response under long-term Al exposure. In 11–60, the three genes that underwent differential SE events are involved in abiotic stress response. The SOD is already known to play a role in the detoxification mechanism in maize under Al stress45. In Arabidopsis, CYSTM is involved in several developmental processes under abiotic stresses conditions46. Silencing PAE and annexin in hairy roots of Medicago truncatula increased the sensitivity to Al stress, suggesting that these gene may be involved in Al resistance response47. In rice, a significant change of AS profile was observed in the tolerant cultivar under Al stress48. Also in rice, the response to cadmium stress is highly controlled at the post-transcriptional level, as several differentials AS events were detected49. Similarly, rice response to alkalinity stress also triggered many differentials AS events50.
While a substantial number of DAS events were not associated with Al-tolerance in popcorn, we propose that post-transcriptional regulation plays a role in establishing timely patterns of downstream gene expression in response to stress. The interplay between transcriptional and post-transcriptional regulation, including AS, is highly complex and context-dependent, for these reasons we believe that post-transcriptional regulation may act during the growth and development of popcorn plants under Al-stress. Therefore, conducting additional analyses to explore the involvement of AS during a time course exposure of popcorn seedlings to Al may enhance our understanding of the complexity response to Al stress.
In this study, we have explored the transcriptomic data beyond gene expression and demonstrated the potential that this tool can offer to researchers in reducing targets and saving time for further analysis. This strategy has allowed us to identify several genes with a wide range of functions correlated with Al stress response, such transporters, enzymes involved in cell wall modification, fatty acid biosynthesis, and genes involved in stress response and plant development. Although searching for DEGs in Al tolerance QTL regions can significantly reduce the number of candidates to be explored, the genetic background differences between the genotypes used in QTL studies and our popcorn inbred lines may allow new regions that control Al-tolerance on unmapped chromosomes. Furthermore, as the Al stress response is majorly regulated at the transcriptional level, the genetic variations found in this study can be validated and functionally characterized to understand their roles in Al stress tolerance. This information can be used to develop molecular markers for Al tolerance, which can be utilized in breeding programs to develop cultivars that are resilient to the challenges posed by changing environmental conditions.
Methods
Plant material and selection of differentially expressed genes
The first step was the selection of the genes from our previous work19. The transcriptome was generated from seven years old seedlings of two contrasting inbred popcorn lines developed by the Popcorn Breeding Program of the Universidade Federal de Viçosa (Viçosa, Brazil): 11–133 (Al-resistant) and 11–60 (Al-sensitive). These genotypes were selected based on a previous study13 to screen inbred popcorn lines with different Al sensitivity. Seedlings with uniform growth were picked randomly and transferred to a nutritive solution51,52 with constant aeration to acclimate for 24 h. Then, the treatment group was subjected to aluminum stress with 540 µM of AlCl3 (160 μM Al3+) for 72 h. To assess only the Al effect on seedlings growth, the pH was adjusted to 4.5 for both control and treatment conditions and the seedlings maintained in a growth chamber at 25 °C with a 12/12 h light/dark cycle. RNA from roots was extracted and sequenced follow by gene expression analysis as described by Pinto et al. (2021)19.
In silico mapping
To verify the localization of the DEGs within the previously described Al tolerance QTLs4,5,6,7,8, the positions of each delimiting QTL marker identified in different segregating populations in common maize (Zea mays) were examined using the MaizeGDB database (Table 3). To identify the DEGs of interest identified in our transcriptomic analysis inside the QTLs, we performed a simple comparison between the positions of the genes and the markers on the chromosome, as described in Mattiello et al.41.
Polymorphism discovery and protein sequence variation prediction
The maize reference genome (B73.RefGen_v4) was downloaded from Phytozome database (https://phytozome.jgi.doe.gov). The reads were mapped to the reference genome using BWA-MEM algorithm of BWA version 0.7.17 (http://bio-bwa.sourceforge.net/)53. A flag was added to identify the respective popcorn sample in each mapping file. The mapping files were processed using SortSam, MarkDuplicates and BuildBamIndex tools of Picard version 2.18.27 (https://github.com/broadinstitute/picard/). Variants were called using FreeBayes version 1.2.0 (https://github.com/ekg/freebayes)54 with a minimum mapping quality of 20, minimum base quality of 20, and minimum coverage of 20 reads at every position in the reference genome. After variant calling, SNPs were filtered using vcftools version 0.16.15 (https://vcftools.github.io/index.html) and annotated using Variant Effect Predictor55 available on Ensembl Plants web server (http://plants.ensembl.org/Tools/VEP). The translated protein sequences were the analyzed using the PROVEAN (Protein Variation Effect Analyzer) software tool (http://provean.jcvi.org/index.php) to predict whether an amino acid substitution or InDel has an impact on the biological function of a protein. A PROVEAN score less than or equal to -2.5 was considered to have a significant biological impact on the protein function.
Differential splicing analysis
The filtered reads were aligned to the maize reference genome (B73.RefGen_v4) using STAR56 with default settings. Replicate Multivariate Analysis of Transcript Splicing (rMATS)57 was employed to identify differential splicing events between the treatment and control conditions for both inbred lines. rMATS classifies alternative splicing (AS) into five categories, including skipped exon (SE), retained intron (RI), mutually exclusive exons (MXE), alternative 5′ splice sites (A5SS), and alternative 3′ splice sites (A3SS). To be considered for analysis, splicing events had to have at least 10 reads supporting exon inclusion (IJC + SJC) in all replicates. Differential splicing was considered statistically significant if the false discovery rate (FDR) was less than 0.05.
Consent to participate
All authors consented to participate of this research.
Declaration of use of plant material
The popcorn seeds used in this article followed the national standards required by Ministry of Agriculture, Livestock and Supply (MAPA), agency that regulates production, processing, repackaging, storage, analysis or seed trading activities in Brazil, according to Decree Nº. 10.586, of December 18, 2020, which regulates Law Nº. 10.711, of August 5, 2003. We emphasize that none of the seeds were collected for this work, once they belong to the Germplasm Bank of UFV and come from several cycles of interpopulation recurrent selection and more recently have been evaluated for some abiotic stresses by the Popcorn Breeding Program of UFV and that all works have the institution's full consent for its realization.
Data availability
The data sets supporting the results of this article are available in the NCBI SRA repository, http://www.ncbi.nlm.nih.gov/bioproject/508768.
References
Kochian, L. V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46, 237–260 (1995).
Kochian, L. V., Piñeros, M. A., Liu, J. & Magalhaes, J. V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66, 571–598 (2015).
Sade, H. et al. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. Biometals 29, 187–210 (2016).
Sibov, S. T. et al. Two genes control aluminum tolerance in maize: Genetic and molecular mapping analyses. Genome 42, 475–482 (1999).
Ninamango-Cárdenas, F. E. et al. Mapping QTLs for aluminum tolerance in maize. Euphytica 130, 223–232 (2003).
Conceição, L. D. H. C. S., Tessele, C. & Barbosa-Neto, J. F. Diallel analysis and mapping of aluminum tolerance in corn inbred lines. Maydica 54, 55–61 (2009).
Maron, L. G. et al. Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J 61, 728–740 (2010).
Guimaraes, C. T. et al. Genetic dissection of Al tolerance QTLs in the maize genome by high density SNP scan. BMC Genomics 15, 153 (2014).
Piñeros, M. A. et al. Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: The case of ZmALMT1 - an anion-selective transporter. Plant Journal 53, 352–367 (2008).
Ligaba, A., Maron, L., Shaff, J., Kochian, L. & Piñeros, M. Maize ZmALMT2 is a root anion transporter that mediates constitutive root malate efflux. Plant Cell Environ 35, 1185–1200 (2012).
Carvalho, C., Kist, B. B. & Beling, R. R. Anuário Brasileiro do Milho 2020. Preprint at (2020).
Moterle, L. M. et al. Combining ability of popcorn lines for seed quality and agronomic traits. Euphytica 185, 337–347 (2012).
Rahim, F. et al. Identification of contrasting tropical popcorn inbreds for studying aluminum toxicity tolerance inheritance. Euphytica 215, 47 (2019).
Pinto, V. B. et al. Deciphering the major metabolic pathways associated with aluminum tolerance in popcorn roots using label-free quantitative proteomics. Planta 254, 132 (2021).
des Marais, D. L. et al. Physiological genomics of response to soil drying in diverse Arabidopsis accessions. Plant Cell 24, 893–914 (2012).
Richards, C. L., Rosas, U., Banta, J., Bhambhra, N. & Purugganan, M. D. Genome-wide patterns of Arabidopsis gene expression in nature. PLoS Genet 8, e1002662 (2012).
Lasky, J. R. et al. Natural variation in abiotic stress responsive gene expression and local adaptation to climate in Arabidopsis thaliana. Mol Biol Evol 31, 2283–2296 (2014).
Ganie, S. A. & Reddy, A. S. N. Stress-induced changes in alternative splicing landscape in rice: Functional significance of splice isoforms in stress tolerance. Biology 10, 309 (2021).
Pinto, V. B. et al. Uncovering the transcriptional response of popcorn (Zea mays L. var everta) under long-term aluminum toxicity. Sci Rep 11, 19644 (2021).
Hegde, N., Doddamani, D. & Kushalappa, A. C. Identification and functional characterisation of late blight resistance polymorphic genes in Russet Burbank potato cultivar. Funct Plant Biol 48, 88–102 (2020).
Covert, A. W., Lenski, R. E., Wilke, C. O. & Ofria, C. Experiments on the role of deleterious mutations as stepping stones in adaptive evolution. Proc Natl Acad Sci U S A 110, E-3171 (2013).
Labrou, N. E., Papageorgiou, A. C., Pavli, O. & Flemetakis, E. Plant GSTome: Structure and functional role in xenome network and plant stress response. Curr Opin Biotechnol 32, 186–194 (2015).
Cançado, G. M. A. et al. Glutathione S-transferase and aluminum toxicity in maize. Funct Plant Biol 32, 1045 (2005).
Dixon, D. P., McEwen, A. G., Lapthorn, A. J. & Edwards, R. Forced evolution of a herbicide detoxifying glutathione transferase. J Biol Chem 278, 23930–23935 (2003).
Chen, Y. et al. Enhancement of polyphenolic metabolism as an adaptive response of lettuce (Lactuca sativa) roots to aluminum stress. Environ Pollut 261, 114230 (2020).
Malmir, H. A. The relations between phenylalanine-ammonia lyase, glutathione-s-transferase activities and the concentrations of total tannins, phytochelatins, glutathione, and peroxidation in two cultivars of sorghum (Sorghum bicolor (L.) Moench) exposed to aluminum. Agric Res 1, 240–250 (2012).
Shafiq, S. et al. The interplay between toxic and essential metals for their uptake and translocation is likely governed by DNA methylation and histone deacetylation in maize. Int J Mol Sci 21, 1–20 (2020).
Margalha, L., Confraria, A. & Baena-González, E. SnRK1 and TOR: Modulating growth–defense trade-offs in plant stress responses. J Exp Bot 70, 2261–2274 (2019).
Pedrotti, L. et al. Snf1-RELATED KINASE1-controlled C/S1-bZIP signaling activates alternative mitochondrial metabolic pathways to ensure plant survival in extended darkness. Plant Cell 30, 495–509 (2018).
Zwart, M. P. et al. Unraveling the causes of adaptive benefits of synonymous mutations in TEM-1 β-lactamase. Heredity (Edinb) 121, 406–421 (2018).
Hernández, M. L., Sicardo, M. D., Alfonso, M. & Martínez-Rivas, J. M. Transcriptional regulation of stearoyl-acyl carrier protein desaturase genes in response to abiotic stresses leads to changes in the unsaturated fatty acids composition of olive mesocarp. Front Plant Sci 10, 251 (2019).
Liu, X. et al. Plant lipid remodeling in response to abiotic stresses. Environ Exp Bot 165, 174–184 (2019).
Wei, Y., Han, R., Xie, Y., Jiang, C. & Yu, Y. Recent advances in understanding mechanisms of plant tolerance and response to aluminum toxicity. Sustainability (Switzerland) 13, 1–22 (2021).
Cavalheiro, M. F. et al. Low root PIP1-1 and PIP2 aquaporins expression could be related to reduced hydration in ‘Rangpur’ lime plants exposed to aluminium. Funct Plant Biol 47, 112–121 (2020).
Kar, D., Pradhan, A. A. & Datta, S. The role of solute transporters in aluminum toxicity and tolerance. Physiol Plant 171, 638–652 (2021).
Kang, J. et al. Plant ABC transporters. Arabidopsis Book 9, e0153 (2011).
Chandran, D. Co-option of developmentally regulated plant SWEET transporters for pathogen nutrition and abiotic stress tolerance. IUBMB Life 67, 461–471 (2015).
Seo, P. J., Park, J. M., Kang, S. K., Kim, S. G. & Park, C. M. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta 233, 189–200 (2011).
Zheng, S. J. et al. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 24, 4731–4747 (2012).
Zhu, X. F. et al. Xyloglucan endotransglucosylase-hydrolase17 interacts with xyloglucan endotransglucosylase-hydrolase31 to confer xyloglucan endotransglucosylase action and affect aluminum sensitivity in Arabidopsis. Plant Physiol 165, 1566–1574 (2014).
Mattiello, L., da Silva, F. R. & Menossi, M. Linking microarray data to QTLs highlights new genes related to Al tolerance in maize. Plant Science 191–192, 8–15 (2012).
Roppolo, D. et al. A novel protein family mediates Casparian strip formation in the endodermis. Nature 473, 380–383 (2011).
Wei, Y. et al. Plasma membrane proteomic analysis by TMT-PRM provides insight into mechanisms of aluminum resistance in tamba black soybean roots tips. PeerJ 8, e9312 (2020).
Mareri, L., Romi, M. & Cai, G. Arabinogalactan proteins: actors or spectators during abiotic and biotic stress in plants?. Plant Biosyst 153, 173–185 (2019).
Boscolo, P. R. S., Menossi, M. & Jorge, R. A. Aluminum-induced oxidative stress in maize. Phytochemistry 62, 181–189 (2003).
Xu, Y. et al. CYSTM, a novel non-secreted cysteine-rich peptide family, involved in environmental stresses in Arabidopsis thaliana. Plant Cell Physiol 59, 423–438 (2018).
Chandran, D. et al. Transcriptome profiling identified novel genes associated with aluminum toxicity, resistance and tolerance in Medicago truncatula. Planta 228, 151–166 (2008).
Awasthi, J. P. et al. Comparative RNA-seq analysis of the root revealed transcriptional regulation system for aluminum tolerance in contrasting indica rice of North East India. Protoplasma 258, 517–528 (2021).
He, F. et al. RNA-seq analysis of rice roots reveals the involvement of post-transcriptional regulation in response to cadmium stress. Front Plant Sci 6, 1136 (2015).
Li, N. et al. Transcriptome analysis of two contrasting rice cultivars during alkaline stress. Sci Rep 8, 9586 (2018).
Famoso, A. N. et al. Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiol 153, 1678–1691 (2010).
Magnavaca, R., Gardner, C. O. & Clark, R. B. Inheritance of aluminum tolerance in maize. in Genetic Aspects of Plant Mineral Nutrition 201–212 (Springer , Netherlands, 1987). doi:https://doi.org/10.1007/978-94-009-3581-5_18.
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Garrison, E. & Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:1207, (2012).
McLaren, W. et al. The ensembl variant effect predictor. Genome Biol 17, 122 (2016).
Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Shen, S. et al. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci U S A 111, E5593–E5601 (2014).
Acknowledgements
We thank the National Council for Scientific and Technological Development (CNPq), the Brazilian Federal Agency for Support and Evaluation of Graduate Education (Capes; Finance Code 001), and the Foundation for Research Support of Minas Gerais State (Fapemig) for financial support. We are grateful to the Núcleo de Análises de Biomoléculas of the Universidade Federal de Viçosa for providing the facilities for the data analysis.
Author information
Authors and Affiliations
Contributions
VBP conceived the idea, designed the experiments, analyzed the results, wrote and finalized the manuscript. PMPV, FAS and TMV; analyzed the data. MDB; conceived the idea, supervised experiments, reviewed manuscript. JMSV; conceived the idea, supervised experiments, reviewed manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinto, V.B., Vidigal, P.M.P., Dal-Bianco, M. et al. Transcriptome-based strategies for identifying aluminum tolerance genes in popcorn (Zea mays L. var. everta). Sci Rep 13, 19400 (2023). https://doi.org/10.1038/s41598-023-46810-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46810-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.