Abstract
Environmental pollution due to the improper use of the chemical fungicides represents a vital ecological problem, which affects human and animal health, as well as the microbial biodiversity and abundance in the soil. In this study, an endophytic fungus Aspergillus oryzae YRA3, isolated from the wild plant Atractylis carduus (Forssk.) C.Chr, was tested for its biocontrol activity against Rhizoctonia root rot of sorghum. The antagonistic potential of A. oryzae YRA3 was tested against Rhizoctonia solani in vitro. A full inhibition in the growth of R. solani was recorded indicating a strong antagonistic potential for this endophyte. To investigate the chemical composition of its metabolites, GC/MS analysis was used and thirty-two compounds in its culture filtrate were identified. Among these metabolites, some compounds with an antifungal background were detected including palmitic acid, 2-heptanone, and 2,3-butanediol. To these antifungal metabolites the antagonistic activity of A. oryzae YRA3 can be attributed. In the greenhouse experiment, treating of the infected sorghum plants with A. oryzae YRA3 significantly reduced severity of the Rhizoctonia root rot by 73.4%. An upregulation of the defensive genes (JERF3), (POD) and (CHI II) was recorded in sorghum roots when were inoculated with A. oryzae YRA3. In addition, an increment in the activity of peroxidase and polyphenol oxidase, as well as the total phenolic content in the sorghum roots was also recorded. Furthermore, the results obtained from the greenhouse experiment revealed a growth-promoting effect for inoculating the sorghum plants with A. oryzae YRA3. It can be concluded that A. oryzae YRA3 can be a probable biological agent to control this disease in sorghum. However, its evaluation under field conditions is highly needed in the future studies.
Similar content being viewed by others
Introduction
After wheat, maize, rice, and barley, sorghum (Sorghum bicolor (L) Moench) is the world's fifth most important cereal grain1. In 2021, the global cultivated area under sorghum was estimated at 40,93 million ha, which produced 61,36 million tons2. The archaeological evidences suggest this crop was initially grown between 5,000 and 8,000 years ago in northeastern Africa, close to the Egyptian-Sudanese border, and it spread throughout the rest of the world via trade routes3. Sorghum has been cultivated as a food crop for humans and as animal feed, for construction materials, or industrial raw materials. In both Africa and Asia, sorghum is an important cereal crop4. However, sorghum production is threatened by various pathogenic fungi that negatively affect the crop yield and growth in spite of the availability of effective chemical fungicides5.
Rhizoctonia solani Kühn represents a high global devastating pathogen, which threats many crops including sorghum and results in a high yield loss. This pathogen causes damping off, root rot, and stem canker diseases in a wide range of crops6. In addition, R. solani has the ability to persist in soil for long periods and withstands various unsuitable conditions. Various anastomosis groups (AGs) of this pathogen are known to infect different plant species, at varying degrees7. The disease symptoms include stunting of the plant and appearing of brown to reddish lesions along the root and stem of the plant, which may extend to girdle the stem leading to the plant death8. Due to the wide host range, long-term persistence in soil, and genetic variability of this pathogen, it is considered one of the dangerous plant pathogens, which results in high yield losses9.
In spite of the availability of some efficient chemical fungicides to control this disease10, these fungicides are not desired due to some health concerns. One of the most crucial global environmental problems is the incorrect use of the chemical fungicides in agriculture11. Heavy application of these fungicides has resulted in an environmental pollution affecting human and animal health. Moreover, the microbial equilibrium and the biodiversity of microorganisms in the soil have also been affected12. Therefore, researches in the recent years have focused on finding safe alternatives for the plant disease management. Biological control using beneficial microorganisms represents an eco-safe and effective solution for this problem13,14. Biological control of R. solani using different fungi and bacteria has been investigated by many researchers15. Rashad et al.16 reported an overexpression in the polyphenol biosynthesis genes in the sunflower plants that were infected by R. solani when were treated with the mycorrhizal fungus Rhizophagus irregularis.
Endophytic fungi inhabit the plant for a period of their life without causing any injury or symptoms for the plant17. Endophytes are known to produce unique bioactive metabolites, which enhance growth and induce tolerance of their hosts against various stresses. Aspergillus oryzae (Ahlb.) Cohn, a non-pathogenic and non-aflatoxigenic fungus that has been widely used in many fermentation industries such as enzymes production for baking. Furthermore, this fungus and its enzymes are accepted by FAO/WHO as food additives (probiotics), as well as in poultry18. Due to its high secreting ability, A. oryzae has been reported as an efficient cell factory for organic acids, industrial enzymes, and secondary metabolites along the past few decades19. It has various roles in the fields of food, therapeutics, forage, and the ecosystems20. Few researches have investigated the biocontrol activity of A. oryzae against various plant pathogens. In this concern, Alshannaq et al.21 reported an inhibitory effect for A. oryzae M2040 against production of aflatoxin B1 and growth of the toxigenic fungus A. flavus in vitro and on peanuts. The study reported also the production of A. oryzae M2040 for some antifungal compounds as a suggested mode of action. Furthermore, the nematicidal activity against Meloidogyne incognita was also reported for A. oryzae22.
This work was aimed at (1) studying the antifungal activity of the endophyte A. oryzae against R. solani, (2) detecting its antifungal metabolites, and (3) evaluating its biocontrol activity against Rhizoctonia root rot of sorghum.
Results
Molecular identification of the endophytic fungus YRA3
The endophytic fungus YRA3 was molecularly identified using the ITS region of the DNA. Data obtained from the NCBI BLAST based on the ITS sequence revealed that the endophytic fungus YRA3 had 100% similarity with that of A. oryzae (MK714921) confirming the identity of the endophytic fungus YRA3 as A. oryzae YRA3. The nucleotide sequence of A. oryzae YRA3 (584 bp) was deposited in the GenBank under the accession No. (OQ916424). The phylogenetic relationship with the closest species in the genus Aspergillus is illustrated in Fig. 1. The phylogenetic tree showed that A. oryzae YRA3 was clustered with A. oryzae (MK714921) in a distinct subcluster with 89% bootstrap support. This subcluster was grouped with 89% bootstrap support with A. flavus (HQ285616). In the same time, A. niger (HM136829) and A. fumigatus (EU664466) were separately grouped in distinct subclusters, while A. sydowii (NR_131259) and A. nidulans (AY373888) were grouped in one subcluster. Aspergillus terreus (OP113794) was clustered as an outgroup taxon.
Antifungal assay of A. oryzae YRA3 against R. solani
The antagonistic potential of A. oryzae YRA3 was assessed against R. solani in vitro (Fig. 2). A fast growth rate for A. oryzae YRA3 was observed, compared to R. solani in the second day of incubation. Three days of incubation, a full inhibition in the mycelial growth of R. solani and a full growth of A. oryzae YRA3 were recorded in the dual culture plate, compared with the control. The obtained result indicated a strong antagonistic activity of A. oryzae YRA3 against R. solani.
Gas chromatography/mass spectrometry (GC/MS)
The culture filtrate of A. oryzae YRA3 was analyzed by a GC/MS system to identify its chemical composition. Thirty-two substances were detected at different percentages (Fig. 3 and Table 1). The major contents included palmitic acid (25.1%), 2-heptanone (19.35%), pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro (17.4%), and 2,3-butanediol (14.3%). In addition, other constituents were detected at intermediate percentages such as 3-methyl-5-propylnonane (3.3%), heneicosane (2.81%), glyceraldehyde (2.6%), and 3-deoxy-d-mannoic lactone (1.9%). While the other constituents were found at minor extents.
Effect of inoculating the sorghum plants with A. oryzae YRA3 on the disease severity
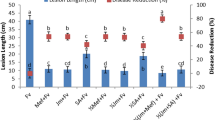
Biocontrol activity of A. oryzae YRA3 against Rhizoctonia root rot of sorghum was investigated under the greenhouse conditions. The obtained results showed that sorghum plants, which not received R. solani inoculum, did not show any disease symptoms. On the contrary, sorghum plants that were only inoculated with R. solani showed 73% severity. In the same time, the infected sorghum plants, which were inoculated with A. oryzae YRA3 showed 19.4% reduction in the disease severity than the infected plants, which were not inoculated with A. oryzae YRA3.
Effect of inoculating the sorghum plants with A. oryzae YRA3 on the transcriptional expression of the defensive genes
The transcriptional expression of jasmonate and ethylene-responsive factor 3 (JERF3), peroxidase (POD), and chitinase II (CHI II) in sorghum roots, which were infected with R. solani and/or treated with A. oryzae YRA3 is presented in Fig. 4. The obtained data showed that inoculation of the sorghum plants with R. solani (P) overexpressed JERF3, while no significant change in its expression level was observed in response to their inoculation with A. oryzae YRA3 (B), compared to the control plants (C). The highest expression level for this gene was recorded for sorghum plants, which were inoculated with R. solani and A. oryzae YRA3, recording 9.7-fold. The results indicated that inoculation of sorghum plants with R. solani and/or A. oryzae YRA3 upregulated POD, at varying extents, compared with the control plants. However, R. solani had more inducing effect than A. oryzae YRA3 in this concern. The highest expression level of POD was recorded for the sorghum plants, which were inoculated with R. solani and A. oryzae YRA3, recording 7-fold. For CHI II, the obtained results showed that inoculation of the sorghum plants with R. solani and/or A. oryzae YRA3 considerably triggered CHI II, at varying degrees, compared to the negative control treatment. However, R. solani was more effective than A. oryzae YRA3 in this regard. The highest expression level of CHI II was recorded for the sorghum plants, which were inoculated with R. solani and A. oryzae YRA3, recording 5.5-fold.
The expression level of jasmonate and ethylene-responsive factor 3 (JERF3), peroxidase (POD), and chitinase II (CHI II) genes in sorghum roots that were infected with Rhizoctonia root rot and/or inoculated with A. oryzae YRA3. Where, C = non-inoculated and non-infected sorghum plants (control), P: infected and non-inoculated with A. oryzae YRA3, B: uninfected and inoculated with A. oryzae YRA3, and PB: infected and inoculated with A. oryzae YRA3. In each subfigure, based on Tukey’s HSD test at p ≤ 0.05, no significant difference is recorded between columns that are subscripted with the same letter. Standard error of each value is illustrated by the error bar.
Effect of inoculating the sorghum plants with A. oryzae YRA3 on the plant growth
Table 2 presents the growth parameters of the sorghum plants, which were inoculated with R. solani and/or A. oryzae YRA3. Compared to the control plants, results from the greenhouse experiment indicated that inoculation with R. solani led to a significant reduction in most of the evaluated growth parameters (shoot and root lengths and shoot and root dry weights), but not on number of leaves. On the contrary, inoculating the sorghum plants with A. oryzae YRA3 significantly promoted most of the evaluated growth parameters, compared to the control, recording the highest values in this regard. In addition, inoculating of the infected sorghum plants with A. oryzae YRA3 significantly enhanced their growth parameters, except for the number of leaves, compared with that of the infected plants, which were not inoculated with A. oryzae YRA3.
Effect of inoculating the sorghum plants with A. oryzae YRA3 on the total phenols and activity of the antioxidant enzymes
Total phenols and activity of the antioxidant enzymes peroxidase (POD) and polyphenol oxidase (PPO) in the infected sorghum plants that were inoculated with A. oryzae YRA3 are presented in Table 3. Data revealed that inoculation of the sorghum plants with R. solani led to an increment in the total phenols content as well as the activity of POD and PPO. In the same time, inoculating of the sorghum plants with A. oryzae YRA3 led to an increment in these parameters. However, the priming effect due to inoculating with R. solani was higher than that due to A. oryzae YRA3. The highest values in these parametrs were recorded for sorghum plants, which were inoculated with R. solani and A. oryzae YRA3, recording 312.8 ± 3.1 mg.g-1 fresh weight for the total phenols content and 3.59 ± 0.2 ∆A470 min−1 g−1 fresh weight and 2.69 ± 0.1 ∆A420 min−1 g−1 fresh weight for activity of POD and PPO, respectively.
Discussion
Rhizoctonia root rot is a destructive worldwide disease, which affects a wide host range resulting in a high yield loss6,7. Researches in the recent years have indicated that many of the endophytic fungi and bacteria have been recognized as promising biocontrol agents against various phytopathogenic fungi23,24,25. In this study, the antagonistic activity of A. oryzae YRA3 was assessed against the mycelial growth of R. solani in vitro. The obtained results showed a very strong antagonistic activity resulting in a complete inhibition of R. solani growth. This result is in agreement with that of Houda Sara et al.26 who reported an antifungal activity of the culture extract of A. oryzae against Candida albicans as well as an antibacterial activity against a wide range of bacteria. This result can be explained in the light of two modes of action: the first is the competition of A. oryzae YRA3 for the space and/or nutrients. The fast growth rate of A. oryzae YRA3 compared to that of R. solani, which was recorded in this study supports this mechanism. The second probable mechanism is the antibiosis via production of hydrolytic enzymes and/or antifungal metabolites. Endophytic fungi are considered an important source of unique bioactive metabolites27. Around 20,000 natural and bioactive products have been described from different endophytic microorganisms28. This ability can be attributed to the variation in the ecological niches in which these endophytes inhabit and the biotic and/or abiotic stresses they face29. To prove this hypothesis, a GC/MS analysis of the culture filtrate of A. oryzae YRA3 was carried out. Among the detected 32 compounds, different bioactive metabolites with antifungal background were identified, to them the antifungal behavior of A. oryzae YRA3 can be attributed. Among them, 2-heptanone, palmitic acid, heneicosane, and 2,3-butanediol are known to have an antifungal activity against different fungi30,31. In this concern, Wu et al.32 reported the antifungal activity of 2-heptanone against Fusarium oxysporum, the causal of Fusarium wilt of watermelon. Different antifungal mechanisms have been discussed in this regard such as increasing electrolyte leakage of the pathogen cell via disturbance of the cell membrane permeability and suppression of protein synthesis33.
Under the greenhouse conditions, a significant suppression in the Rhizoctonia root rot severity was achieved in the infected sorghum plants that were inoculated with A. oryzae YRA3, indicating the probable biocontrol activity of this endophyte. To investigate effect of application of this endophyte on the plant immunity, the transcriptional expression of three defense-related genes (JERF3, POD, and CHI II), that represent different signaling pathways, was studied. qPCR results indicated that JERF3, POD, and CHI II were significantly upregulated due to inoculation with A. oryzae YRA3. This indicates the resistance-inducing effect of A. oryzae YRA3 on the sorghum plants, particularly in case of the infection with R. solani. JERF3 is a responsive factor, which regulates a set of the resistance genes through both jasmonic acid and ethylene signaling pathways resulting in an induction of the plant immunity against the fungal infection34. This means that the recorded overexpression of JERF3 in this study may led to triggering of other defensive genes in the inoculated sorghum plants. This information was supported by the recorded upregulation of POD, and CHI II in this study that was associated with the JERF3 upregulation. CHI II gene encodes for the antifungal chitinase enzyme. This enzyme plays a crucial role in degrading the chitin, the main structural unit in the fungal cell wall35. Degradation of the chitin units in the fungal cell wall leads to a disturbance in the cell permeability, leakage of the cytoplasmic electrolytes, and finally the cell death36. The recorded upregulation of CHI II in this study due to application of A. oryzae YRA3 revealed the inducing effect of this endophyte on the plant resistance against the penetration of R. solani to the sorghum roots. The antioxidant gene POD encodes the peroxidase enzyme, which has an antioxidant role against the free radicals and the reactive oxygen species (ROS) resulted due to the different biotic or a biotic stresses37. Upregulation of POD was supported also by the recorded increment in the levels of the activity of the antioxidant enzymes (POD and PPO) in the infected sorghum plants that were inoculated with A. oryzae YRA3, compared with the untreated-infected plants. These antioxidant enzymes act as scavengers for the destructive free radicals and ROS that severely destroy the infected plant cells38. The observed overexpression of POD due to application of A. oryzae YRA3 indicated the inducing effect of this endophyte on the resistance of sorghum plants against fungal infection. The recorded overexpression of these defense-related genes indicated another indirect biocontrol mechanism of the endophyte A. oryzae YRA3.
One of the most interesting results obtained in this study is the increment in the content of the phenolic compounds in the sorghum roots due to the inoculation with A. oryzae YRA3. The phenolic compounds are very fungi-toxic substances that naturally produced in the plants as a defense line against the fungal penetration and to prevent the transfer of the pathogen from cell to cell39. The increment in the phenolic content is usually used as a marker for the induction of the plant resistance40. In the line with this result, El-Sharkawy et al.41 reported an increment in the phenolic compounds in the wheat plants when were inoculated with the endophyte Epicoccum nigrum HE20 against stripe rust. Different mechanisms are known to be involved in priming plant resistance via endophytes such as thickening of the cell walls, phytoalexins secretion and synthesis of PR-proteins, which play a restricting role against the pathogen penetration and development42. Furthermore, a previous study reported that 2,3-butanediol, which detected in this study as a metabolite of A. oryzae YRA3, can induce the production of the root exudates which modulate the growth of the rhizospheric fungi and bacteria43. In addition, our results showed a growth-promoting effect for the application of A. oryzae YRA3 on the sorghum plants. The results showed a production of 2,3-butanediol in the culture filtrate of A. oryzae YRA3 that is known as a growth enhancer in many plant species43. This result is in agreement with that reported by Wu et al.32 who reported the growth-promoting effect of 2,3-butanediol on Arabidopsis thaliana plantlets. Production of this substance may explain the observed growth-promoting effect of A. oryzae YRA3.
Conclusion
These results demonstrated that A. oryzae YRA3, isolated from the wild plant Atractylis carduus, has an antagonistic potential against R. solani in vitro. In addition, this endophyte showed a biocontrol activity under the greenhouse conditions, which may qualify A. oryzae YRA3 as a promising candidate to control Rhizoctonia root rot of sorghum as well as a plant growth-promotor. However, further investigations are still needed to evaluate its efficacy under the field conditions.
Materials and methods
Sorghum cultivar and used fungi
Sorghum grains (cv. Shandaweel-1), obtained from Agricultural Research Center, Egypt, were used during the greenhouse evaluation. A virulent strain of R. solani (AG-2–2 IIIB) was kindly obtained from Agricultural Research Center, Egypt. The fungus was maintained on potato dextrose agar medium (PDA) at 4 °C. The fungal inoculum was prepared by culturing the phytopathogenic fungus R. solani on a sterilized maize/soil medium (1:3, v/v) for 15 days at 30 °C.
Isolation and identification of the endophytic fungus
The endophytic fungus was isolated from the wild plant Atractylis carduus (Forssk.) C.Chr., which was collected from a semi desert area in Borg EL-Arab, Alexandria, Egypt. Different pieces of the wild plant (stem, roots, and leaves) were surface sterilized by immersing in NaOCl (0.5%) for 2 min, followed by immersing in ethanol (70%) for 2 min, then washing in dist. water. The surface sterilized plant pieces were put on PDA plates (4 pieces per plate) and incubated at 30 °C for 72 h. The recovered fungus was purified using the hyphal tip technique and morphologically identified based on its microscopic and cultural properties according to Pitt et al.44. The wild plant was identified by Dr. Ahmed Abd El-gawad, and deposited at the Herbarium of Faculty of Agriculture, Mansoura University under the deposition number (AW 1318/22/102).
Molecular identification
The endophytic fungus YRA3 was molecularly identified by amplifying the internal transcribed spacer (ITS) part in the DNA. Extraction of the total DNA was performed using QiAamp DNA Kit (Qiagen, Germany). The universal primers ITS1 (5’TCC GTA GGT GAA CCT TGC GG3’) and ITS4 (5’TCC TCC GCT TAT TGA TAT GC3’) were used to amplify the ITS region (600 bp) according to Madbouly et al.23. To identify the endophytic fungus YRA3, the produced sequence was compared with various sequences in NCBI database. Sequence of the endophyte YRA3 and the closest ones from the GenBank database were aligned using the ClustalW algorithm and the phylogeny tree was constructed using the neighbor-joining method via MEGAX-10.2.445.
Assay of the antagonistic potential of A. oryzae YRA3 against R. solani in vitro
The antagonistic potential of A. oryzae YRA3 was tested against R. solani in vitro using the dual culture method. On a PDA plate, a 7 mm-diameter disc of A. oryzae YRA3, taken from a 5-days-old culture, and another disc from a 5-days-old culture of the pathogen were inoculated (7 cm) in opposite to each other in the plate. Plates singly inoculated with each fungus served as a control treatment. The PDA plates were incubated at 28 °C for a week. The mycelial growth of the growing fungi and the inhibition percentage were measured.
GC/MS analysis
The chemical composition of the cultural filtrate of A. oryzae YRA3 was analyzed using a GC/MS-QP2010 system (Shimadzu, Japan). The DB-5HT capillary column (30 m, 0.32 mm, and 0.10 µm,) was used. The programed oven temperature was as follows: 50 °C/1 min, 180 °C/1 min, 230 °C/2 min, and then 250 °C. All compounds were identified according to the NIST 11 Spectral Library (Gaithersburg, MD, USA).
The greenhouse experiment
Surface sterilized sorghum grains (using hypochlorite solution 5% and ethanol 70%, 1 min for each) were planted in plastic pots filled with sterilized sandy-clay soil (sand:clay 2:1 v/v) at 5 grains per pot. To prepare the inoculum of A. oryzae YRA3, the fungus was grown on PDA at 28 °C for a week. A conidial suspension was prepared in sterile water, mixed and adjusted at 106 conidia mL−1. Sorghum grains soaked in the conidial suspension of A. oryzae YRA3 for 2 h were planted in plastic pots at 5 grains per pot. Soil infestation was done 14 days post planting by mixing the pathogen inoculum with the upper layer of the soil at 2.5%. Four replicates of each treatment were applied. The pots were irrigated when necessary, and arranged in a complete randomized design in a greenhouse (31/20 °C day/night and 73% humidity). The treatments in this experiment were as follows: C = untreated control, P: infected with R. solani, B: uninfected-treated with A. oryzae YRA3, and PB: infected and treated with A. oryzae YRA3.
Quantification of the transcriptional expression level of the defense-related genes
Seven days after infection, sorghum roots of each treatment were sampled. Extraction of the total RNA was done using RNeasy Kit (Qiagen, Hilden, Germany). cDNA of the extracted RNA was synthesized using a SureCycler 8800 thermocycler (Agilent, Santa Clara, CA, USA). The reaction composed of 20 μL containing total RNA (3 μL), buffer (2.5 μL), primer (5 μL), reverse transcriptase (0.2 μL), dNTPs (2.5 μL), and RNase-free water (6.8 μL). The program was performed at 42 °C/1 h, and at 70 °C/10 min. The real-time PCR was done by a Rotor Gene system (Qiagene, USA). The reaction 20 μL composed of 3 μL cDNA, 12.5 μL SYBR Green Master Mix (Bioline, Germany), 1.5 μL of each primer, and 1.5 μL RNase-free water. The used primers are presented in Table 4. The reference gene utilized was β-actine. The qPCR program was as follows: 1 cycle (95 °C/3 min), 45 cycles (95 °C/15 s, 56 °C/30 s, and 72 °C/30 s).
Disease evaluation
To evaluate the disease severity, five sorghum roots of each treatment were collected 45 days post infection and rated according to a 5-degrees scale of Carling et al.46, where 0: no disease, 1: discoloration without necrosis, 2: discoloration with low necrosis, 3: discoloration with high necrosis, and 4: complete death. The following equation was used to calculate the disease severity47:
where; n1: the number of plants with score 1; n2: the number of plants with score 2; etc.; n5: the number of plants with score 5; and N: total number of plants used in the experiment.
Plant growth evaluation
For each treatment, five sorghum plants were uprooted, washed under tap water, and evaluated for the growth parameters (lengths and dry weights of plant shoot and root, and leaves number). The plant samples were dried at 85 °C for 72 h before be weighted.
Total phenols and enzymes activity
Total phenols and activity of POD and PPO enzymes were estimated 45 days post infection in the sorghum roots. For extraction, 0.5 g of the sorghum root was homogenized in 5 mL ethanol (80%), then centrifuged at 3000 rpm for 20 min. The supernatant was collected and evaporated to the dryness. The residue was dissolved in 5 mL distilled water and used for estimation of the total phenols according to Malik and Singh48. Activity of POD was determined according to Maxwell and Bateman49, while activity of PPO was determined as described by Galeazzi et al.50.
Statistical analyses
The data were statistically analyzed using the CoStat software51. Comparing of the means were done based on Tukey’s HSD test at p ≤ 0.05.
Ethical approval
Authors confirm that all the methods and experiments in this study, including the collection of plant material, complied with the institutional, national, and international guidelines and legislation.
Data availability
Nucleotide sequence of A. oryzae YRA3 (584 bp) was deposited in the NCBI database under the accession No. (OQ916424) https://www.ncbi.nlm.nih.gov/nuccore/OQ916424.
References
Rashwan, A. K., Yones, H. A., Karim, N., Taha, E. M. & Chen, W. Potential processing technologies for developing sorghum-based food products: An update and comprehensive review. Trends Food Sci. Technol. 110, 168–182 (2021).
FAOSTAT. Food and agriculture organization of the United Nations, 2023. http://www.fao.org/faostat/en/#data/QC (2023) doi:https://doi.org/10.1017/S0020818300006160.
Venkateswaran, K., Elangovan, M. & Sivaraj, N. Origin, domestication and diffusion of Sorghum bicolor. in Breeding Sorghum for Diverse End Uses (eds. Aruna, C., Visarada, K. B. R. S., Bhat, B. V. & Tonapi, V. A. B. T.-B. S. for D. E. U.) 15–31 (Woodhead Publishing, 2018). doi:https://doi.org/10.1016/B978-0-08-101879-8.00002-4.
Aruna, C. & Visarada, K. B. R. S. Sorghum grain in food and brewing industry. in Breeding Sorghum for Diverse End Uses (eds. Aruna, C., Visarada, K. B. R. S., Bhat, B. V. & Tonapi, V. A. B. T.-B. S. for D. E. U.) 209–228 (Woodhead Publishing, 2018). doi:https://doi.org/10.1016/B978-0-08-101879-8.00013-9.
Sharma, I., Kumari, N. & Sharma, V. Sorghum Fungal Diseases. in (eds. Lichtfouse, E. & Goyal, A.) 141–172 (Springer International Publishing, 2015). doi:https://doi.org/10.1007/978-3-319-16988-0_7.
Naseri, B. Epidemics of rhizoctonia root rot in association with biological and physicochemical properties of field soil in bean crops. J. Phytopathol. 161, 397–404 (2013).
Dubey, S. C., Tripathi, A., Upadhyay, B. K. & Deka, U. K. Diversity of Rhizoctonia solani associated with pulse crops in different agro-ecological regions of India. World J. Microbiol. Biotechnol. 30, 1699–1715 (2014).
Ajayi-Oyetunde, O. O. & Bradley, C. A. Rhizoctonia solani: taxonomy, population biology and management of rhizoctonia seedling disease of soybean. Plant Pathol. 67, 3–17 (2018).
Rashad, Y. M., Aseel, D. G. & Hafez, E. E. Antifungal potential and defense gene induction in maize against Rhizoctonia root rot by seed extract of Ammi visnaga (L.) Lam. Phytopathol. Mediterr. 57, 73–88 (2018).
Khan, M. F. R. & Carlson, A. L. Efficacy of fungicides for controlling Rhizoctonia solani on sugar beet. Powerpoint Present. J. Crop Prot. 5, 33–38 (2012).
Tian, Z., Wang, J. W., Li, J. & Han, B. Designing future crops: challenges and strategies for sustainable agriculture. Plant J. 105, 1165–1178 (2021).
Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P. & Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Heal. 4, 148 (2016).
Al-Askar, A. A. et al. Streptomyces griseorubens E44G: A Potent Antagonist Isolated from Soil in Saudi Arabia. J. Pure Appl. Microbiol. 8, 221–230 (2014).
Rashad, Y. M. & Moussa, T. A. A. Biocontrol agents for fungal plant diseases management. In Cottage Industry of Biocontrol Agents and Their Applications: Practical Aspects to Deal Biologically with Pests and Stresses Facing Strategic Crops (eds. El-Wakeil, N., Saleh, M. & Abu-hashim, M.) 337–363 (Springer International Publishing, 2019). doi:https://doi.org/10.1007/978-3-030-33161-0_11.
Abdel-Fattah, G. M., El-Haddad, S. A., Hafez, E. E. & Rashad, Y. M. Induction of defense responses in common bean plants by arbuscular mycorrhizal fungi. Microbiol. Res. 166, 268–281 (2011).
Rashad, Y., Aseel, D., Hammad, S. & Elkelish, A. Rhizophagus irregularis and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules 10, 1–20 (2020).
Nisa, H. et al. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 82, 50–59 (2015).
Zamanizadeh, A., Mirakzehi, M. T., Agah, M. J., Saleh, H. & Baranzehi, T. A comparison of two probiotics Aspergillus oryzae and Saccharomyces cerevisiae on productive performance, egg quality, small intestinal morphology, and gene expression in laying Japanese quail. Ital. J. Anim. Sci. 20, 232–242 (2021).
Li, Q. et al. Recent advances in the development of Aspergillus for protein production. Bioresour. Technol. 348, 126768 (2022).
Rousta, N. et al. Filamentous fungus Aspergillus oryzae for food: From submerged cultivation to fungal burgers and their sensory evaluation—A pilot study. Foods 10, 10112704 (2021).
Alshannaq, A. F. et al. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Sci. Rep. 8, 16871 (2018).
Kim, T. Y. et al. Nematicidal activity of kojic acid produced by Aspergillus oryzae against Meloidogyne incognita. J. Microbiol. Biotechnol. 26, 1383–1391 (2016).
Madbouly, A. K., Rashad, Y. M., Ibrahim, M. I. M. & Elazab, N. T. Biodegradation of aflatoxin B1 in maize grains and suppression of its biosynthesis-related genes using endophytic Trichoderma harzianum AYM3. J. Fungi 9, 20209. https://doi.org/10.3390/jof9020209 (2023).
Rashad, Y. M., Abdalla, S. A. & Shehata, A. S. Aspergillus flavus YRB2 from Thymelaea hirsuta (L.) Endl, a non-aflatoxigenic endophyte with ability to overexpress defense-related genes against Fusarium root rot of maize. BMC Microbiol. 22, 229 (2022).
Rashad, Y. M., Abdalla, S. A. & Sleem, M. M. Endophytic Bacillus subtilis SR22 triggers defense responses in tomato against rhizoctonia root rot. Plants 11, 2051 (2022).
Houda Sara, N., Rachid, B., Julio, G., Jesus, R.-S.M. & Teresa, V. In Vitro Antimicrobial, Antiviral and Cytotoxicity Activities of Aspergillus oryzae Isolated From El-Baida Marsh in Algeria. J. Drug Deliv. Ther. 10, 191–195 (2020).
Wei, F. et al. Evaluation of the biocontrol potential of endophytic fungus Fusarium solani CEF559 against Verticillium dahliae in cotton plant. Biomed. Res. Int. 2019, 3187943 (2019).
Ownley, B. H., Gwinn, K. D. & Vega, F. E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. BioControl 55, 113–128 (2010).
Strobel, G. & Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 67, 491–502 (2003).
Prasath, K. G., Tharani, H., Kumar, M. S. & Pandian, S. K. Palmitic acid inhibits the virulence factors of Candida tropicalis: Biofilms, cell surface hydrophobicity, ergosterol biosynthesis, and enzymatic activity. Front. Microbiol. 11, 864. https://doi.org/10.3389/fmicb.2020.00864 (2020).
Vanitha, V. et al. Heneicosane—A novel microbicidal bioactive alkane identified from Plumbago zeylanica L.. Ind. Crops Prod. 154, 112748 (2020).
Wu, Y., Zhou, J., Li, C. & Ma, Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. Microbiol. Open 8, e00813 (2019).
Bhattacharyya, A. et al. Mechanistic insight into the antifungal effects of a fatty acid derivative against drug-resistant fungal infections. Front. Microbiol. 11, 2116. https://doi.org/10.3389/fmicb.2020.02116 (2020).
Rashad, Y. M., Fekry, W. M. E., Sleem, M. M. & Elazab, N. T. Effects of mycorrhizal colonization on transcriptional expression of the responsive factor JERF3 and stress-responsive genes in banana plantlets in response to combined biotic and abiotic stresses. Front. Plant Sci. 12, 742628. https://doi.org/10.3389/fpls.2021.742628 (2021).
Loc, N. H., Huy, N. D., Quang, H. T., Lan, T. T. & Thu Ha, T. T. Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus. Trichoderma asperellum PQ34. Mycology 11, 38–48 (2020).
Rashad, Y. M., Al-Askar, A. A., Ghoneem, K. M., Saber, W. I. A. & Hafez, E. E. Chitinolytic Streptomyces griseorubens E44G enhances the biocontrol efficacy against Fusarium wilt disease of tomato. Phytoparasitica 45, 227–237 (2017).
Zandi, P. & Schnug, E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology 11, 201554. https://doi.org/10.3390/biology11020155 (2022).
Kidwai, M., Ahmad, I. Z. & Chakrabarty, D. Class III peroxidase: an indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 39, 1381–1393 (2020).
Rashad, Y., Aseel, D. & Hammad, S. Phenolic Compounds Against Fungal and Viral Plant Diseases. In Plant Phenolics in Sustainable Agriculture (eds. Lone, R., Shuab, R. & Kamili, A. N.) 201–219 (Springer, Singapore, 2020). DOI:https://doi.org/10.1007/978-981-15-4890-1_9.
Chowdhary, V., Alooparampil, S., V. Pandya, R. & G. Tank, J. Physiological Function of Phenolic Compounds in Plant Defense System. in (ed. Badria, F. A.) Ch. 10 (IntechOpen, 2022). DOI:https://doi.org/10.5772/intechopen.101131.
El-Sharkawy, H. H. A., Rashad, Y. M. & Elazab, N. T. Biocontrol potential of the endophytic Epicoccum nigrum HE20 against stripe rust of wheat. Pestic. Biochem. Physiol. 194, 105517 (2023).
Fontana, D. C. et al. Endophytic fungi: Biological control and induced resistance to phytopathogens and abiotic stresses. Pathogens 10, 505257. https://doi.org/10.3390/pathogens10050570 (2021).
Yi, H. S. et al. Impact of a bacterial volatile 2,3-butanediol on Bacillus subtilis rhizosphere robustness. Front. Microbiol. 7, 993. https://doi.org/10.3389/fmicb.2016.00993 (2016).
Pitt, J. I. & Hocking, A. D. Aspergillus and Related Teleomorphs BT - Fungi and Food Spoilage. in (eds. Pitt, J. I. & Hocking, A. D.) 275–337 (Springer US, 2009). DOI:https://doi.org/10.1007/978-0-387-92207-2_8.
Tamura, K., Dudley, J., Nei, M. & Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 (2007).
Carling, D. E., Pope, E. J., Brainard, K. A. & Carter, D. A. Characterization of mycorrhizal isolates of Rhizoctonia solani from an orchid, including AG-12, a new anastomosis group. Phytopathology 89, 942–946 (1999).
Taheri, P. & Tarighi, S. Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J. Plant Physiol. 167, 201–208 (2010).
Malik, C. P. & Singh, M. B. Estimation of total phenols. In Plant Enzymology and Histo-Enzymology (ed. Malik, C. P.) (Kalyani Publishers, 1980).
Maxwell, D. P. & Bateman, D. F. Changes in the activities of some oxidases in extracts of Rhizoctonia-infected bean hypocotyls in relation to lesion maturation. Phytopathology 57, 132–136 (1967).
Galeazzi, M. A. M., Sgarbieri, V. C. & Constantinides, S. M. Isolation, purification and physicochemical characterization of polyphenol oxidase (PPO) from a dwarf variety of banana (Musa cavendishii, L). J. Food Sci. 46, 150–155 (1981).
CoStat. Cohort Software. (798 Lighthouse Ave., PMB 320 Monterey, USA, 2005).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study did not receive any external funding.
Author information
Authors and Affiliations
Contributions
Y.R. contributed to the idea and designing of the study, greenhouse experiment, GC/MS analysis, and editing of the manuscript. S.A. contributed to the molecular investigations, and M.T. contributed to the statistical analyses of the data and the manuscript editing and revision. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rashad, Y.M., Al Tami, M.S. & Abdalla, S.A. Eliciting transcriptomic and antioxidant defensive responses against Rhizoctonia root rot of sorghum using the endophyte Aspergillus oryzae YRA3. Sci Rep 13, 19823 (2023). https://doi.org/10.1038/s41598-023-46696-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46696-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.