Abstract
Cryopreservation is an important technique used in the conservation of various plant tissues. This study proposes a cryopreservation method for the long-term conservation of eastern bracken fern gametophytes (Pteridium aquilinum var. latiusculum). Encapsulation–dehydration of the gametophytes was performed, and the exogenous sucrose and abscisic acid (ABA) preculture conditions were investigated. Gametophytes are sensitive to dehydration and drying, and the following treatment conditions were applied: encapsulation by alginate containing 0.75 M sucrose, 18-h loading treatment with 0.75 M sucrose, and 6-h drying treatment. The survival rate following cryopreservation was determined. The water content of < 27.5% in the alginate beads after dehydration and drying was found to be appropriate for ensuring survival. Additionally, performing an exogenous sucrose and ABA preculture was essential before encapsulation to achieve a survival of ≥ 90%. The high stress induced by cryopreservation and exogenous preculture regulated the expression of PaSuSy, PaLEA14, and PaABI1b and the endogenous ABA content. In eastern bracken gametophytes, ABI1 appears to be a negative regulator of ABA signaling. These results indicate that the encapsulation–dehydration method is effective for the long-term conservation of eastern bracken fern gametophytes, and exogenous preculture alleviates abiotic stress and increases the survival rate.
Similar content being viewed by others
Introduction
Gene banks favor cryopreservation over other techniques as it enables the safe preservation of various plant tissues (such as seeds, spores, shoot tips, somatic embryos, and calluses) in a small space. Although well-established cryopreservation protocols exist for the safe preservation of many vegetatively propagated crops1 such as potatoes2, apples, pears3, and ornamental horticulture plants4,5, it can also be applied for the preservation of a wide range of flora including ferns6,7,8,9,10.
An important criterion for successful cryopreservation is the prevention of intracellular ice crystal formation. This is achieved using plant vitrification solutions (PVS). Various PVSs such as PVS211 and PVS312 have been developed, and they primarily comprise sucrose, glycerol, and ethylene glycol in a liquid basal medium. These components improve freezing- and desiccation tolerance, which improves survival after cryopreservation. Additionally, various cryopreservation methods such as encapsulation–dehydration13, cryo-plates14 and cryo-mesh15 have been developed to improve the survival ratio16. The encapsulation–dehydration method is based on a new vitrification-based protocol that was developed in the 1990s13,17. Briefly, samples are precultured in media containing high sucrose concentrations, gelled with an insoluble calcium alginate layer, and dehydrated prior to cryopreservation. This technique improves freezing- and desiccation tolerance and significantly lowers the water content in the samples18. Particularly, sucrose and abscisic acid (ABA) are primarily used for fern preculture. The effect of using 10 μM of ABA during fern preculture has been investigated for several fern species such as Davallia fejeensis, Drynaria quercifolia, Cibotium glaucum, Adiantum trapeziforme, Adiantum tenerum, Polypodium aureum19, and Selaginella uncinata20. A preculture medium containing 0.25 M sucrose and 10 μM ABA was found to enhance the survival rate of Asplenium cuneifolium Viv21 and Cyathea australis22 following cryopreservation.
Desiccation and freezing during cryopreservation can adversely affect tissue survival and regrowth. Plants counteract freezing-induced damage and protect the cells by accumulating sugars in response to low temperatures23. However, high levels of exogenous sugars can significantly increase the freezing tolerance of cells24. ABA is a phytohormone that controls tolerance to drying and protects plant cells from dehydration25. Furthermore, sucrose and ABA regulate the expression of key genes associated with freezing and desiccation stresses26,27,28,29. Sucrose synthase (SuSy) and sucrose phosphate synthase (SPS) are enzymes that play key roles in plant sugar metabolic pathways30. SuSy converts sucrose to glucose or fructose, which increases soluble carbohydrate accumulation, possibly resulting in improved stress tolerance31. Moreover, the expression of sucrose-related genes such as SuSy and SPS change in response to abiotic stresses (e.g. salt, drought, and cold stress)32,33. Abscisic acid insensitive (ABI) is a key transcription factor in the ABA signaling pathway that is directly involved in dehydration tolerance34. ABA-induced protein kinase (serine/threonine-protein kinase) and ABI1 also play major roles in the ABA signaling pathway35,36. Furthermore, plant seeds inherently possess a strong drying tolerance, which enables them to withstand late maturation stages that are characterized by the rapid decrease in water content owing to the expression of late embryogenesis abundant (LEA) proteins37. These proteins exert osmoprotectant effects or desiccation tolerance38. They have been studied in moss and ferns24. For example, Khandelwal et al.39 reported that freezing and desiccation tolerance is enhanced by exogenous ABA in Physcomitrella patens protonemata. Additionally, gene expression levels of LEA and ABI increased under these conditions.
Other previous studies primarily focused on enhancing the regeneration and survival of fern gametophytes by enhancing preculture conditions. However, this study goes further by examining the relationship between the expression of genes involved in freezing and desiccation tolerance and endogenous ABA levels while using sucrose and ABA during preculture. We believe that these results would fill the knowledge gap in the research on storage physiology for the better long-term conservation of ferns. Therefore, we propose an efficient cryopreservation method for eastern bracken (Pteridium aquilinum var. latiusculum) gametophytes. We also tried to determine the conditions that would enhance survival through the use of exogenous sucrose and ABA preculture. Additionally, this study explores the accumulation of endogenous ABA in gametophytes as a direct result of the preculture conditions and reports on the expression of the related genes.
Methods
Plant materials
Sporophylls of P. aquilinum var. latiusculum (eastern bracken) were collected in a greenhouse at Chungbuk National University, Cheongju, Korea, in September 2018 (36°37′29.1″N, 127°27′17.1″E). No approval or permission was required for the collection of these samples (sporophylls). Spore disinfection and germination were performed according to the methods described by Jang et al.40. Briefly, a spore solution (1 mg·mL−1) was centrifuged (3 min, 1811×g), and the supernatant was removed. The spore was sterilized with 1.4% (v/v) sodium hypochlorite (Yuhanclorox Co., Ltd., Hwaseong, Korea) for 13 min and washed thrice with sterilized water. Thereafter, the spores were inoculated in Knop medium and germinated at 25 °C under a 16/8-h photoperiod with a light intensity of 30 μmol·m−2·s−1. Subsequently, the gametophytes obtained from the spores were subcultured on double-strength MS basal medium at 8-week intervals. These were used for further experiment.
Encapsulation, loading, and drying
Encapsulation–dehydration methods were used to assess the effect of the encapsulation treatment on survival during cryopreservation. The encapsulation–dehydration process was performed according to the procedures described by Fabre and Dereuddre13 and Kulus et al.41 with necessary minor modification. Unencapsulated (untreated) and encapsulated samples were prepared, and their survival ratio was compared after the loading and drying processes. The survival of the gametophytes was analyzed with respect to the loading (0, 12, 18, and 24 h) and drying (0, 2, 4, and 6 h) time durations. For the encapsulation–dehydration process, 1 g of gametophytes was mechanically fragmented using a scalpel and mixed with 40 mL of calcium-free liquid MS medium containing 0.75 M (255 g·L−1) sucrose and 30 g·L−1 sodium alginate (CAS 9005-38-3; Duksan Company, Ansan, Korea). Then, the mixture was taken in a 100-mL plastic syringe (Zhejiang Huafu Medical Equipment Co., Ltd., Jiaxing, China); encapsulated gametophyte beads were obtained by dripping the mixture as droplets into 100 mM sterilized CaCl2 solution (CAS 10035-04-8; Daejung Chemicals & Metals Co., Ltd., Siheung, Korea). Next, the alginate beads were immersed in an Erlenmeyer flask containing 100 mL of sterilized loading solution (calcium-free liquid MS medium with 0.75 M sucrose) and cultured with shaking (125 rpm). Later, the alginate beads were removed from the loading solution, placed on two sheets of filter paper, and dried in a laminar flow on a clean bench (wind velocity, 0.3–0.45 m·s−1). The dried alginate beads were placed into a 2-mL cryotube and exposed to liquid nitrogen (LN) for 1 h. The LN-exposed cryotubes were thawed at 25 °C for 30 min and the gametophytes were subsequently cultured for 4 weeks in MS medium. Finally, the recovered gametophytes were cultured at 25 °C under a fluorescent lamp (30 μmol·m−2·s−1) and a 16/8-h photoperiod. The method described here was used to perform all encapsulation–dehydration experiments in this study.
Determination of water content in encapsulated gametophytes at various loading and drying time durations
The water content of the encapsulated beads was measured before exposure to LN. Measurements were performed for 15 alginate beads per treatment in three replicates (n = 45). The weight of the alginate beads containing fragmented gametophytes was determined first. Next, the weight of the alginate beads that were dried for 72 h in a hot air dryer (60 °C) was calculated. These two values were used to determine the water content of the encapsulated gametophytes.
Preculture with sucrose and ABA
Preculture was performed to enhance survival during cryopreservation. Gametophytes were subcultured in 2MS medium containing sucrose (0, 0.4, 0.7 and 1.0 M) or ABA (10 or 100 μM) and precultured for 72 h. These gametophytes were evaluated for survival under the previously described encapsulation–dehydration conditions (with encapsulation, 18 h loading, and 6 h drying). All survival ratios were evaluated by observing 10 encapsulated gametophytes after cryopreservation, and five replicates of the examination were performed. Furthermore, the surviving gametophytes were subjected to 4 weeks of recovery culture and then subcultured in 2MS growth medium for 4 weeks. The increase in fresh weight was determined for the gametophytes. The normal proliferation of gametophytes was evaluated by measuring the weight per alginate bead, which included the weight of the surviving gametophytes. The gametophyte weight determination in the samples was performed for ten replicates. The weight of fresh alginate beads, which includes the weight of fragmented gametophytes before treatment, was 74 ± 7 mg.
RNA extraction, reverse transcription PCR and PaSuSy, PaABI1b, and PaLEA14 gene expression analysis
Non-treated fresh gametophytes (control) and precultured samples were immersed immediately in LN. The collected samples were finely ground using a mortar and pestle with LN. At least 3 g of gametophytes were used for each extraction. RNA extraction was performed as per the protocols reported by Chang et al.42 and Gasic et al.43 Assessment of RNA integrity and concentration was performed using a DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE, USA). cDNA was synthesized from the extracted RNA samples using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) by following the manufacturer’s protocols. The target genes (PaSuSy, PaSPS, PaABIb1, and PaLEA14) were selected from the Blast2GO data provided by Der et al.44 The primer sequences for performing RT-qPCR were designed using Primer3 version 0.4.0 (https://bioinfo.ut.ee/primer3-0.4.0/) (Table 1). Actin (PaAct1, F_5′-AGCTCTTGCTCGAAGTCCAA-3′, R_5′-CCACATGCTATCCTCCGTCT-3′, product size 168 bp, Tm 60 °C) was used as the reference gene. Reverse transcription PCR was performed using 300 ng/uL of cDNA and primer pairs for the four target genes and reference gene. PCR amplicons were confirmed using 1.5% agarose gel electrophoresis for 1 h. The agarose gel images were cropped, and uncropped images are provided in Supplementary Information. Real-time quantitative PCR (RT-qPCR) was performed as described by Oh et al.45. The relative gene expression for each treatment sample was calculated based on to the expression level of the control. The relative gene expression of each sample was examined in three replicates.
Endogenous ABA content in precultured encapsulated gametophytes
ABA analysis was performed by following the protocols described by Pan et al.46. The gametophytes that were used to analyze the endogenous ABA content were obtained after 24 h of recovery culture and washing with distilled water. To extract the gametophyte samples from each treatment, 50 mg of the ground sample that was obtained after freeze-drying was placed in a 2-mL tube. To this, 500 μL of the extraction solvent [2-propanol/H2O/concentrated HCl (2:1:0.002, v/v/v, %)] was added. Then, the sample was homogenized at 100 rpm and 4 °C for 30 min. To each homogenized sample, 1 mL of dichloromethane was added and shaken further for 30 min (100 rpm, 4 °C) and centrifuged for 5 min (13,000×g, 4 °C) (Smart R17 Plus; Hanil Scientific Inc., Gimpo, Korea). After the separation of the layers, 900 μL of the supernatant was transferred into a new 2-mL tube. The supernatant was evaporated using vacuum concentrators, and the recovered sample was dissolved in 100 μL methanol for further use. The standard solution used was 2-cis,4-trans-abscisic acid, which was dissolved in 1 mL methanol and diluted to a concentration of 1 mg·mL−1.
High-performance liquid chromatography (HPLC) was performed using an Agilent 1260 series system (Agilent Technologies, Palo Alto, CA, USA). Chromatographic separation was performed on an Agilent Eclipse Plus C18 column (4.6 × 50 mm, 3.5 μm particle size; Agilent Technologies, Palo Alto, CA, USA). The HPLC mobile phase was 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B). The gradient was first started at 5% (B), increased to 95% (B) for 1 min, and maintained at 95% (B) for 4 min before being rapidly changed to 5% (B) for 0.1 min and maintained for 0.9 min. The flow rate, column oven temperature, and injection volume were 500 μL·min−1, 30 °C, and 10 μL, respectively.
An API-4000 (SCIEX, Framingham, MA, USA) mass spectrometer instrument equipped with an electrospray ionization source was used in the negative and MRM modes. BioAnalyst version 1.6.1 and Analyst software version 1.6.1 were used for equipment operation and data analysis, respectively. During ionization, high-purity nitrogen gas was used as the spray and drying gas at a gas pressure of 60 psi. The ion spray voltage and an ionization source temperature were − 4.5 kV and 600 °C, respectively. Q1 and Q3 were analyzed using LC–MS/MS multiple reaction monitoring using unit resolution. Analysis of the standard solution and each sample was performed in triplicate.
Data collection and statistical analysis
Gametophyte survival was observed using a microscope (SZ61; Olympus Corporation, Tokyo, Japan). Gametophyte images were captured using a CMOS camera (eXcope F630; Dixi Sci., Daejeon, Korea) and eXcope 3.7.12277 software. Data are presented as the mean ± standard error for each treatment. Factorial analysis was performed using Duncan’s multiple range test using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and significance was set at P < 0.05. Survival data (encapsulation, loading time, and drying time) and water content (loading and drying times) were analyzed using three- and two-way ANOVA using SAS version 9.4; significance was set at P < 0.05.
Ethics approval and consent to participate
Plant sample collection was performed in accordance with the relevant guidelines and regulations such as “The IUCN Policy Statement on Research Involving Species at Risk of Extinction” and “The Convention on the Trade in Endangered Species of Wild Fauna and Flora”.
Results
Survival of thawed cultured gametophytes after cryopreservation
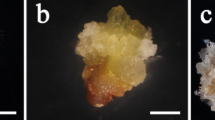
Unencapsulated gametophytes did not survive the exposure to LN regardless of the loading and drying time durations (Table 2). Alginate bead-encapsulated gametophytes showed a tendency to survive only after drying for ≥ 4 h regardless of the loading time. After 4 weeks of recovery culture, unencapsulated gametophyte cells died, whereas the encapsulated gametophytes regenerated normally (Fig. 1). Particularly, alginate bead-encapsulated gametophytes showed 74.0% survival after an 18 h loading and 6 h drying period. This was the highest survival rate observed in this study. These results show that encapsulation, loading time, and drying time significantly affected gametophyte survival after LN exposure and showed a highly significant correlation.
Determination of water content of encapsulated gametophytes
The total water content in the alginate beads (which includes that of the gametophytes) was investigated differently depending on loading and drying times (Fig. 2). Untreated alginate beads initially contained 83.7% water, which reduced to 8.0% after 24 h of loading treatment. Similarly, drying treatment significantly reduced the water content to 68.4, 43.0, and 26.6% after drying for 2, 4, and 6 h, respectively. Survival was confirmed based on the drying treatment, which differed depending on the loading time; however, the water content in the beads was determined at 4 h (31.0−43.0%) and 6 h (20.1−27.5%). Drying was also visually observed as wrinkling on the surface of the alginate beads and size reduction (Fig. 3).
Changes in water content due to encapsulation-dehydration at varying loading and drying times in eastern bracken gametophytes. Vertical bars represent mean ± standard error (n = 3). zDifferent letters indicate a significant difference as per Duncan’s multiple range test at P < 0.05. NS not significant, ***significance at P < 0.001.
Enhanced survival and plant regeneration of gametophyte after preculture
Preculture with sucrose and ABA significantly improved gametophyte survival after cryopreservation (Fig. 4A). The survival ratio of gametophytes increased to 90.2 − 100% with preculture, whereas the survival rate without preculture was 64 ± 8.1%. Survival rates did not significantly differ based on the sucrose and ABA concentrations used in preculture. Meanwhile, alginate bead-encapsulated gametophytes that were subcultured in 2MS medium following recovery culture proliferated normally after 4 weeks (Fig. 4B,C). The fresh weight of the cultured gametophytes increased significantly compared with the initial fresh weight in the alginate beads (74 ± 7 mg). Although treatment without preculture resulted in a relatively low survival ratio, it showed the highest fresh weight per alginate bead at 992 mg. Moreover, as sucrose concentration (0.4, 0.7, 1.0 M) increased, the fresh weight (857, 686, 565 mg) decreased. In contrast, an increase in ABA concentration (10, 100 μM) increased the fresh weight (598, 918 mg). Subsequently, the gametophytes subjected to regeneration were sown in soil ex vitro, and the gametophytes that regenerated successfully after cryopreservation formed sporophytes normally (Fig. 5).
Cryopreservation of eastern bracken gametophytes enhanced by sucrose and ABA preculture. (A) survival ratio enhanced by preculture; (B) and (C) regenerated gametophytes encapsulated for 4 weeks after 4 weeks of recovery culture. Vertical bars represent mean ± standard error (a, n = 5; b, n = 10). zDifferent letters indicate a significant difference as per Duncan’s multiple range test at P < 0.05.
Gene expression of PaSuSy, PaABI1b, and PaLEA14 in gametophytes after preculture and cryopreservation
PaSuSy and PaABI1b were upregulated in all treatment groups compared with those in the control (non-treated fresh gametophytes) (Fig. 6). PaSuSy gene expression in the sucrose and ABA preculture groups was relatively higher than that in the non-preculture treatment group. Preculture treatment with 0.4 M sucrose induced the highest gene expression (Fig. 6A). In contrast, PaLEA14 and PaABI1b expression was downregulated in the sucrose and ABA preculture treatment groups compared with that of the non-preculture group (Fig. 6B,C). However, PaLEA14 expression level did not differ between the two preculture treatments, although higher PaABI1b expression was observed in the sucrose treatment group than in the exogenous ABA treatment group with respect to preculture. The reverse transcription PCR results (Fig. 6D and Supplementary Figs. S1–S4) showed that PaSPS expression was not observed in any of the treatment groups.
Relative expression of PaSuSy (A), PaABI1b (B), PaLEA14 (C), and reverse transcription PCR results (cropped) of four target genes and PaAct1 (D) in the cryopreserved eastern bracken gametophytes after preculture. Control, non-treated fresh gametophytes. Vertical bars represent mean ± standard deviation (n = 3). zDifferent letters indicate a significant difference as per Duncan’s multiple range test at P < 0.05. The original agarose gel electrophoresis is presented in Supplementary Figs. 1–4.
Endogenous ABA content after preculture and cryopreservation of gametophytes
Exogenous ABA preculture treatment induced high levels of endogenous ABA (Fig. 7). The ABA content in the control was 3.6 ng·g−1, whereas those of the 10 and 100 μM ABA preculture treatment samples were significantly higher at 66.9 and 375.0 ng·g−1, respectively. In contrast, the endogenous ABA contents of the non-preculture and sucrose preculture treatment samples were similar and ranged from 2.4–3.9 ng·g−1.
Endogenous ABA content of eastern bracken gametophytes enhanced by sucrose and ABA preculture. Control, fresh gametophytes that have not undergone encapsulation or cryopreservation. Vertical bars represent mean ± standard deviation (n = 3). zDifferent letters indicate a significant difference as per Duncan’s multiple range test at P < 0.05.
Discussion
The encapsulation–dehydration method of preservation has been applied to various plant species, and gametophytes of six fern species have been successfully cryopreserved by Pence19,47. In the present study, the unencapsulated gametophytes did not survive and the water content in the encapsulated gametophytes differed depending on the duration of dehydration and drying, which considerably affected survival. Thus, dehydration, drying, and water content are key factors that must be carefully monitored to achieve a high survival rate following cryopreservation. In fact, their effects have been demonstrated in the preservation of various ferns, including Osmunda regalis48, Lepisorus longifolius, Pteris adscensionis49, Cyathea dealbata, Dicksonia fibrosa, Phyllitis scolopendrium50 and Asplenium scolopendrium var. americanum51. If the cells are not sufficiently dehydrated, they would be damaged from cryoinjury due to intracellular ice crystal formation, whereas excessive dehydration causes damage by desiccation and osmotic stress52. Hence, although dehydration and drying are essential to achieve the highest survival rate in encapsulated samples, data suggest that an appropriate range exists for the preferred water content during cryopreservation. As the moisture content in seeds and spores before cryopreservation affects survival rates after cryopreservation, it was adjusted to an appropriate level to minimize losses due to cryopreservation in plants such as Pteris vittata (0.025 g H2O/g dry weight), Polystichum setiferum (0.039 g H2O/g dry weight)53, Prunus avium (9.0–16.9%), Prunus armeniacal (7%), and Paeonia emodi (18.33%)4,54,55. Additionally, maintaining a 5.0–5.4% moisture content in eastern bracken spores before cryopreservation was found to be effective7. In the present study, a sample with a high survival rate of 74.0% was found to have a water content of ≤ 27.5%, proving that the encapsulation–dehydration method is suitable for the long-term conservation of eastern bracken gametophytes.
Generally, abiotic stress increases sucrose levels in plants32 while simultaneously regulating the expression of genes involved in sucrose synthesis and metabolism30. In the present study, sucrose preculture enhanced survival, suggesting a protective role for sucrose in shielding cells from damage caused by dehydration, desiccation, and freezing, particularly in relation to PaSuSy expression. In fact, strong abiotic stress (dehydration, drought, and freezing) during cryopreservation upregulated PaSuSy expression as did exogenous sucrose and ABA preculture. Upregulation of SuSy by abiotic stress has been reported in various plant species including Arabidopsis thaliana56, Glycine max L.57, Gossypium hirsutum L.58 and Zea mays30 Meanwhile, PaSuSy, which plays a role in sucrose synthesis and cleavage in the sucrose degradation pathway, may have been upregulated to a higher degree than PaSPS because the cells had a sufficient supply of sucrose. Thus sucrose is a key factor for the successful cryopreservation and revival of eastern bracken gametophytes.
Various treatments in the cryopreservation process (osmosis, dehydration, ice crystal formation during freezing, and thawing) can act as strong plant cell stressors59. The stress of cryopreservation significantly increased the expression of PaLEA14 in the non-preculture samples than in the control. The activation of LEA or LEA-like proteins is upregulated by various abiotic stresses60 and it improves dehydration- and desiccation tolerance61,62. The proteins encoded by LEA and LEA-like gene groups, including LEA1, LEA2, LEA3, LEA4, LEA5, LEA6, LEA7, and LEA8, may exhibit different expression and gene regulation patterns63. ABA is involved in LEA-like protein accumulation and improves stress tolerance in the liverwort Marchantia polymorpha26. Exogenous ABA upregulated 14 LEA-like transcripts in Linderniaceae species64 and LEA expression in Solanum tuberosum61. However, in the present study, exogenous sucrose and ABA preculture decreased PaLEA14 expression. This trend of decreased expression level is contradictory to the high survival rate observed in the samples, suggesting that preculture offsets a considerable amount of stress exerted by the cryopreservation process. Stress alleviation through preculture has been reported in various plant species. Preculture with exogenous ABA had a positive effect on osmotic stress and the activities of caspase-3-like and caspase-9-like enzymes that characterize programmed cell death. This resulted in improved survival of Lilium pumilum shoot tips after cryopreservation65. Additionally, Htwe et al.59 analyzed transcriptome data obtained from banana shoot meristem subjected to high sucrose pretreatment before cryopreservation and reported differential expression in genes related to osmotic and oxidative stress.
Similar to the effect on PaLEA14, stress due to the cryopreservation process significantly increased PaABI1b expression in non-preculture samples. ABI1 encodes protein serine/threonine phosphatases (PP2C), which is a monomeric enzyme that is involved in ABA signaling66. The PP2C group A in Arabidopsis, including ABI1, ABI2, and AtPP2CA, has been studied extensively and is recognized as a negative regulator of ABA signaling and responses66,67. Komatsu et al.68 reported two PP2C groups similar to ABI1 in Physcomitrella patens, and the freezing tolerance of the Arabidopsis abi1-1 mutant decreased significantly at higher expression levels of this gene. In the present study, the expression level of PaABI1b was higher in non-preculture samples than in the sucrose and ABA preculture samples. This result implies that preculture enhances dehydration- and freezing tolerance and survival and that the ABI1 gene acts as a negative regulator of ABA signaling in the eastern bracken gametophyte. Furthermore, PaABI1b exhibited a relatively low expression level in exogenous ABA preculture, which was opposite to the effect observed for endogenous ABA content. Verslues and Bary69 reported that ABI1 elevates ABA content in response to exogenous ABA. Furthermore, Lu et al.70 reported that ABI1 serves as a crucial negative regulator of ABA signaling, impeding ABI1 function in the presence of ABA. These results indicate that negative regulators of ABA signaling induce ABA accumulation and may explain the role of negative regulators of ABA signaling.
Conclusions
In conclusion, the results of this study indicate that long-term conservation can be achieved in eastern bracken gametophytes through cryopreservation. On one hand, the encapsulation–dehydration method acts as a strong abiotic stress on the gametophyte cells. On the other hand, it shields the cells against dehydration, desiccation, and freezing (Fig. 6). Exogenous sucrose and ABA preculture increased survival and regulated the expression of genes related to stress and endogenous ABA content. The expression of PaSuSy, which is involved in sucrose synthesis and metabolism, was upregulated. The considerable stress due to the cryopreservation process significantly increased PaLEA14 and PaABI1b expression levels in non-preculture samples. However, PaABI1b was relatively downregulated in exogenous ABA preculture and showed an opposite trend to that of endogenous ABA accumulation. These results indicate that the PaABI1b gene augments the role of the negative regulators of ABA signaling in eastern bracken gametophytes. The results of this study can potentially contribute to better cryopreservation protocols for additional fern species.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wang, M.-R. et al. Advances in cryopreservation of in vitro-derived propagules: Technologies and explant resources. Plant Cell Tissue Organ Cult. 144, 7–20. https://doi.org/10.1007/s11240-020-01770-0 (2021).
Vollmer, R. et al. Cryopreservation of potato shoot tips for long-term storage. In Solanum tuberosum. Methods in Molecular Biology Vol. 2354 (eds Dobnik, D. et al.) 21–54 (Humana, 2021). https://doi.org/10.1007/978-1-0716-1609-3_2.
Zidova, P., Paprstein, F. & Sedlak, J. Cryopreservation of alginate-coated in vitro-grown apices of apple, pear and sweet cherry. Acta Hortic. 1234, 175–179. https://doi.org/10.17660/ActaHortic.2019.1234.23 (2019).
Ren, R. et al. ROS-induced PCD affects the viability of seeds with different moisture content after cryopreservation. Plant Cell Tissue Organ Cult. 148, 623–633. https://doi.org/10.1007/s11240-021-02219-8 (2022).
Pathirana, R., Mathew, L., Jibran, R., Hunter, D. A. & Morgan, E. R. Cryopreservation and cryotherapy research on horticultural crops in New Zealand. Acta Hortic. 1234, 29–35. https://doi.org/10.17660/ActaHortic.2019.1234.4 (2019).
Ballesteros, D. & Pence, V. C. Fern conservation: Spore, gametophyte, and sporophyte ex situ storage, in vitro culture, and cryopreservation. In Current Advances in Fern Research (ed. Fernández, H.) 227–249 (Springer, 2021). https://doi.org/10.1007/978-3-319-75103-0_11.
Jang, B. K. & Lee, C. H. Effect of temperature and relative humidity on the viability and longevity of eastern bracken (Pteridium aquilinum var. latiusculum) spores for long-term storage. Sci. Hortic. 288, 110362. https://doi.org/10.1016/j.scienta.2021.110362 (2021).
Nebot, A., Philpott, V. J., Pajdo, A. & Ballesteros, D. Cryopreservation of fern spores and pollen. In Cryopreservation and Freeze-Drying Protocols. Methods in Molecular Biology Vol. 2180 (eds Wolkers, W. F. & Oldenhof, H.) 623–637 (Humana, 2021). https://doi.org/10.1007/978-1-0716-0783-1_33.
Pence, V. C. & Bruns, E. B. The tip of the iceberg: Cryopreservation needs for meeting the challenge of exceptional plant conservation. Plants 11, 1528. https://doi.org/10.3390/plants11121528 (2022).
Pence, V. C. et al. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 250, 108736. https://doi.org/10.1016/j.biocon.2020.108736 (2020).
Sakai, A., Kobayashi, S. & Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell. Rep. 9, 30–33. https://doi.org/10.1007/BF00232130 (1990).
Nishizawa, S., Sakai, A., Amano, Y. & Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 91, 67–73. https://doi.org/10.1016/0168-9452(93)90189-7 (1993).
Fabre, J. & Dereuddre, J. Encapsulation–dehydration: A new approach to cryopreservation of Solanum shoot-tips. CryoLetters 11, 413–426 (1990).
Yamamoto, S. et al. Development of a cryopreservation procedure using aluminum cryo-plates. CryoLetters 32, 256–265 (2011).
Funnekotter, B., Bunn, E. & Mancera, R. L. Cryo-mesh: A simple alternative cryopreservation protocol. CryoLetters 38, 155–159 (2017).
Deepa, A. V. & Thomas, T. D. In vitro strategies for the conservation of Indian medicinal climbers. In Vitro Cell. Dev. Biol. Plant 56, 784–802. https://doi.org/10.1007/s11627-020-10084-x (2020).
Palanyandy, S. R., Gantait, S., Subramaniam, S. & Sinniah, U. R. Cryopreservation of oil palm (Elaeis guineensis Jacq.) polyembryoids via encapsulation–desiccation. 3 Biotech 10, 9. https://doi.org/10.1007/s13205-019-1997-9 (2020).
Engelmann, F., Arnao, M.-T.G., Wu, Y. J. & Escobar, R. Development of encapsulation dehydration. In Plant Cryopreservation: A Practical Guide (ed. Reed, B. M.) 59–68 (Springer, 2008). https://doi.org/10.1007/978-0-387-72276-4_4.
Pence, V. C. Cryopreservation of in vitro grown fern gametophyte. Am. Fern J. 90, 16–23. https://doi.org/10.2307/1547258 (2000).
Pence, V. C. Cryopreservation of shoot tips of Selaginella uncinate. Am. Fern J. 91, 37–40 (2001).
Tomiczak, K., Makowski, D., Sliwinska, E. & Mikuła, A. The development of an in vitro propagation and conservation system for the endangered serpentine fern Asplenium cuneifolium Viv. Plant Cell Tissue Organ Cult. 154, 161–175. https://doi.org/10.1007/s11240-023-02524-4 (2023).
Mikuła, A., Jata, K. & Rybczyński, J. J. Cryopreservation strategies for Cyathea australis (R. Br.) Domin. CryoLetters 30, 429–439 (2009).
Wanner, L. A. & Junttila, O. Cold-induced freezing tolerance in Arabidopsis. Plant Physiol. 120, 391–400. https://doi.org/10.1104/pp.120.2.391 (1999).
Thomashow, M. F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 50, 571–599. https://doi.org/10.1146/annurev.arplant.50.1.571 (1999).
Zeevaart, J. A. D. & Creelman, R. A. Metabolism and physiology of abscisic acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 439–473. https://doi.org/10.1146/annurev.pp.39.060188.002255 (1988).
Akter, K., Kato, M., Sato, Y., Kaneko, Y. & Takezawa, D. Abscisic acid-induced rearrangement of intracellular structures associated with freezing and desiccation stress tolerance in the liverwort Marchantia polymorpha. J. Plant Physiol. 171, 1334–1343. https://doi.org/10.1016/j.jplph.2014.05.004 (2014).
Nagao, M. et al. Accumulation of theanderose in association with development of freezing tolerance in the moss Physcomitrella patens. Phytochemistry 67, 702–709. https://doi.org/10.1016/j.phytochem.2006.01.031 (2006).
Takezawa, D., Komatsu, K. & Sakata, Y. ABA in bryophytes: How a universal growth regulator in life became a plant hormone?. J. Plant Res. 124, 437–453. https://doi.org/10.1007/s10265-011-0410-5 (2011).
Tan, T. et al. Abscisic acid INSENSITIVE3 is involved in cold response and freezing tolerance regulation in Physcomitrella patens. Front. Plant Sci. 8, 1599. https://doi.org/10.3389/fpls.2017.01599 (2017).
Bilska-Kos, A. et al. Sucrose phosphate synthase (SPS), sucrose synthase (SUS) and their products in the leaves of Miscanthus × giganteus and Zea mays at low temperature. Planta 252, 23. https://doi.org/10.1007/s00425-020-03421-2 (2020).
Ozturk, M. et al. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant 172, 1321–1335. https://doi.org/10.1111/ppl.13297 (2021).
Gong, X. et al. Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol. Plant 153, 119–136. https://doi.org/10.1111/ppl.12225 (2015).
Sami, F., Yusuf, M., Faizan, M., Faraz, A. & Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 109, 54–61. https://doi.org/10.1016/j.plaphy.2016.09.005 (2016).
Zhang, Y., Liu, X., Zhang, K., Zhang, D. & Guan, K. An abscisic acid INSENSITIVE3-like gene from the desert moss Syntrichia caninervis confers abiotic stress tolerance and reduces ABA sensitivity. Plant Cell Tissue Organ Cult. 133, 417–435. https://doi.org/10.1007/s11240-018-1394-9 (2018).
Gómez-Cadenas, A. et al. An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc. Natl. Acad. Sci. 96, 1767–1772. https://doi.org/10.1073/pnas.96.4.1767 (1999).
Sah, S. K., Reddy, K. R. & Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7, 571. https://doi.org/10.3389/fpls.2016.00571 (2016).
Shih, M. D., Hoekstra, F. A. & Hsing, Y. C. Late embryogenesis abundant proteins. Adv. Bot. Res. 48, 211–255. https://doi.org/10.1016/S0065-2296(08)00404-7 (2008).
Delseny, M. et al. Late embryogenesis abundant (LEA) protein gene regulation during Arabidopsis seed maturation. J. Plant Physiol. 158, 419–427. https://doi.org/10.1078/0176-1617-00353 (2001).
Khandelwal, A. et al. Role of ABA and ABI3 in desiccation tolerance. Science 327, 546. https://doi.org/10.1126/science.1183672 (2010).
Jang, B. K., Cho, J. S., Kwon, H. J. & Lee, C. H. Optimal conditions for spore germination and gametophyte and sporophyte production in the autumn fern Dryopteris erythrosora. Hortic. Environ. Biotechnol. 60, 115–123. https://doi.org/10.1007/s13580-018-0097-9 (2019).
Kulus, D., Rewers, M., Serocka, M. & Mikuła, A. Cryopreservation by encapsulation-dehydration affects the vegetative growth of chrysanthemum but does not disturb its chimeric structure. Plant Cell Tissue Organ Cult. 138, 153–166. https://doi.org/10.1007/s11240-019-01614-6 (2019).
Chang, S., Puryear, J. & Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11, 113–116. https://doi.org/10.1007/BF02670468 (1993).
Gasic, K., Hernandez, A. & Korban, S. S. RNA extraction from different apple tissues rich in polyphenols and polysaccharides for cDNA library construction. Plant Mol. Biol. Rep. 22, 437–438. https://doi.org/10.1007/BF02772687 (2004).
Der, J. P., Barker, M. S., Wickett, N. J., dePamphilis, C. W. & Wolf, P. G. D. Novo characterization of the gametophyte transcriptome in bracken fern, Pteridium aquilinum. BMC Genom. 12, 1–14. https://doi.org/10.1186/1471-2164-12-99 (2011).
Oh, S., Shin, H., Arora, R., Kim, K. & Kim, D. Proline accumulation and related gene expression during spring regrowth in three Rosaceae species. Hortic. Environ. Biotechnol. 58, 21–26. https://doi.org/10.1007/s13580-017-0101-9 (2017).
Pan, X., Welti, R. & Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 5, 986–992. https://doi.org/10.1038/nprot.2010.37 (2010).
Pence, V. C. Growth of fern gametophytes after 20 years of storage in liquid nitrogen. Fern Gaz. 20, 337–346 (2018).
Makowski, D., Tomiczak, K., Rybczyński, J. J. & Mikula, A. Integration of tissue culture and cryopreservation methods for propagation and conservation of the fern Osmunda regalis L. Acta Physiol. Plant. 38, 1–12. https://doi.org/10.1007/s11738-015-2037-y (2016).
Barnicoat, H., Cripps, R., Kendon, J. & Sarasan, V. Conservation in vitro of rare and threatened ferns—Case studies of biodiversity hotspot and island species. In Vitro Cell. Dev. Biol. Plant 47, 37–45. https://doi.org/10.1007/s11627-010-9303-x (2011).
Mikuła, A., Makowski, D., Walters, C. & Rybczyński, J. J. Exploration of cryo-methods to preserve tree and herbaceous fern gametophytes. In Working with Ferns: Issues and Applications (eds Fernández, H. et al.) 173–192 (Springer, 2011). https://doi.org/10.1007/978-1-4419-7162-3_13.
Pence, V. C. Propagation and cryopreservation of Asplenium scolopendrium var. americanum, the American hart’s-tongue fern. Am. Fern J. 105, 211–225. https://doi.org/10.1640/0002-8444-105.3.211 (2015).
Mohanty, P., Das, M. C., Kumaria, S. & Tandon, P. High-efficiency cryopreservation of the medicinal orchid Dendrobium nobile Lindl. Plant Cell Tissue Organ Cult. 109, 297–305. https://doi.org/10.1007/s11240-011-0095-4 (2012).
Ballesteros, D. & Walters, C. Water properties in fern spores: Sorption characteristics relating to water affinity, glassy states and storage stability. J. Exp. Bot. 58, 1185–1196 (2007).
Chmielarz, P. Cryopreservation of dormant orthodox seeds of forest trees: Mazzard cherry (Prunus avium L.). Ann. For. Sci. 66, 405. https://doi.org/10.1051/forest/2009020 (2009).
Kovalchuk, I., Turdiev, T., Mukhitdinova, Z., Frolov, S. & Reed, B. M. Cryopreservation of native Kazakhstan apricot (Prunus armeniaca L) seeds and embryonic axes. CryoLetters 35, 83–89 (2014).
Déjardin, A., Sokolov, L. N. & Kleczkowski, L. A. Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem. J. 344, 503–509. https://doi.org/10.1042/bj3440503 (1993).
Du, Y. et al. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 146, 1–12. https://doi.org/10.1016/j.plaphy.2019.11.003 (2020).
Loka, D. A., Oosterhuis, D. M., Baxevanos, D., Noulas, C. & Hu, W. Single and combined effects of heat and water stress and recovery on cotton (Gossypium hirsutum L.) leaf physiology and sucrose metabolism. Plant Physiol. Biochem. 148, 166–179. https://doi.org/10.1016/j.plaphy.2020.01.015 (2020).
Htwe, C. S. S., Rajkumar, S., Pathania, P. & Agrawal, A. Transcriptome profiling during sequential stages of cryopreservation in banana (Musa AAA cv Borjahaji) shoot meristem. Plants 12, 1165. https://doi.org/10.3390/plants12051165 (2023).
Zhang, Y. et al. Transcriptome analysis reveals genes commonly responding to multiple abiotic stresses in rapeseed. Mol. Breed. 39, 158. https://doi.org/10.1007/s11032-019-1052-x (2019).
Chen, Y. et al. The role of the late embryogenesis-abundant (LEA) protein family in development and the abiotic stress response: A comprehensive expression analysis of potato (Solanum tuberosum). Genes 10, 148. https://doi.org/10.3390/genes10020148 (2019).
Gao, J. & Lan, T. Functional characterization of the late embryogenesis abundant (LEA) protein gene family from Pinus tabuliformis (Pinaceae) in Escherichia coli. Sci. Rep. 6, 19467. https://doi.org/10.1038/srep19467 (2016).
Bies-Ethève, N. et al. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Mol. Biol. 67, 107–124. https://doi.org/10.1007/s11103-008-9304-x (2008).
van den Dries, N., Facchinelli, F., Giarola, V., Phillips, J. R. & Bartels, D. Comparative analysis of LEA-like 11–24 gene expression and regulation in related plant species within the Linderniaceae that differ in desiccation tolerance. New Phytol. 190, 75–88. https://doi.org/10.1111/j.1469-8137.2010.03595.x (2011).
Wang, Q., Zhu, M., Zhang, L. & Liu, Y. Abscisic acid increases the viability of cryopreserved shoot tips of Lilium pumilum by regulating the osmotic stress and programmed cell death. Plant Cell Tissue Organ Cult. https://doi.org/10.1007/s11240-023-02594-4 (2023).
Merlot, S., Gosti, F., Guerrier, D., Vavasseur, A. & Giraudat, J. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 25, 295–303. https://doi.org/10.1046/j.1365-313x.2001.00965.x (2001).
Singh, A., Jha, S. K., Bagri, J. & Pandey, G. K. ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in Arabidopsis. PLoS One 10, e0125168. https://doi.org/10.1371/journal.pone.0125168 (2015).
Komatsu, K. et al. Functional analyses of the ABI1-related protein phosphatase type 2C reveal evolutionarily conserved regulation of abscisic acid signaling between Arabidopsis and the moss Physcomitrella patens. Plant Mol. Biol. 70, 327–340. https://doi.org/10.1007/s11103-009-9476-z (2009).
Verslues, F. E. & Bray, E. A. Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. J. Exp. Bot. 57, 201–212. https://doi.org/10.1093/jxb/erj026 (2006).
Lu, Y. et al. ABI1 regulates carbon/nitrogen-nutrient signal transduction independent of ABA biosynthesis and canonical ABA signalling pathways in Arabidopsis. J. Exp. Bot. 66, 2763–2771. https://doi.org/10.1093/jxb/erv086 (2015).
Author information
Authors and Affiliations
Contributions
B.K.J. and C.H.L. Conceptualization; B.K.J. and S.O. Investigation; S.O., J.S.C. and D.K. Formal analysis; B.K.J. Writing—Original draft preparation; B.K.J., J.S.C. and C.H.L. Writing—Reviewing and Editing. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jang, BK., Oh, S., Kim, D. et al. Exogenous preculture with sucrose and abscisic acid improves post-cryopreservation survival of eastern bracken fern gametophytes. Sci Rep 13, 18518 (2023). https://doi.org/10.1038/s41598-023-45941-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45941-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.