Abstract
Environmental warming is associated with reductions in ectotherm body sizes, suggesting that larger individuals may be more vulnerable to climate change. The mechanisms driving size-specific vulnerability to temperature are unknown but are required to finetune predictions of fisheries productivity and size-structure community responses to climate change. We explored the potential metabolic and cardiac mechanisms underlying these body size vulnerability trends in a eurythermal fish, barred surfperch. We acutely exposed surfperch across a large size range (5–700 g) to four ecologically relevant temperatures (16 °C, 12 °C, 20 °C, and 22 °C) and subsequently, measured their metabolic capacity (absolute and factorial aerobic scopes, maximum and resting metabolic rates; AAS, FAS, MMR, RMR). Additionally, we estimated the fish’s cardiac thermal tolerance by measuring their maximum heart rates (fHmax) across acutely increasing temperatures. Barred surfperch had parallel hypoallometric scaling of MMR and RMR (exponent 0.81) and a weaker hypoallometric scaling of fHmax (exponent − 0.05) across all test temperatures. In contrast to our predictions, the fish’s aerobic capacity was maintained across sizes and acute temperatures, and larger fish had greater cardiac thermal tolerance than smaller fish. These results demonstrate that thermal performance may be limited by different physiological constraints depending on the size of the animal and species of interest.
Similar content being viewed by others
Introduction
Several ectothermic species are decreasing in body size directly in response to environmental warming1,2,3,4,5. This is especially concerning in fisheries species because of their importance in providing food security, sustaining economies, and supporting human well-being through recreational opportunities6. Within a given fish species, vulnerability to temperature change is often highest during early life stages and in spawning adults4,7,8,9. However, the physiological mechanisms underpinning these trends remain unresolved. One hypothesis suggests that there is a temperature- and size-specific mismatch between a fish’s rising metabolic demand (metabolic rate, MR) and its ability to supply oxygen to meet this demand (e.g., cardiorespiratory mechanisms; diffusion of O2 across the gills, heart rates, fH)10,11. Specifically, the loss of cardiac function is closely linked with the decline in metabolic capacity and thermal tolerance in numerous ectotherms12,13,14,15,16,17. The heart supports aerobic capacity in fishes by delivering oxygen, nutrients, and hormones to the working tissues and by removing metabolic waste14,18,19. Therefore, cardiac function may be the mechanism driving size-specific vulnerability to warming in fishes.
Scaling relationships describe how body size affects any trait, including MR and fH20. Metabolic rates commonly scale with body mass following a positive power function, MR = a*BMb, where b < 1 when estimated across-taxa21 and tend to range from b = 0.80 to b = 0.89 in fishes21,22,23,24,25 (BM = body mass, a = a context-specific coefficient, b = scaling slope or exponent; Fig. 1). However, scaling slopes and intercepts vary inter-specifically and intra-specifically24,25,26, with activity level11,22, across species lifestyles, and in response to temperature22,28,30,31. Many researchers have explored how these various factors alter the scaling of MR, including temperature11,23,27,32,33, but the mass-scaling of cardiac thermal tolerance and heart rates in ectotherms remains unclear. Unlike metabolism, heart rates tend to scale negatively with body mass34 (e.g., cockroaches35, cetaceans36, snakes37). Specifically, it is proposed that scaling of fH follows a reciprocal function to metabolism, fH = a*BM−b (e.g., b ≈ − 0.25 mammals and birds20,38,39,40), but in fishes, the relationship between fH and body mass can be bell-shaped35 and flat18,41 (b = 0).
Conceptual presentation of body size and temperature influence on aerobic metabolic rate, maximum heart rate, and cardiac thermal tolerance in ectotherms. (a,b) Scaling of maximum and resting metabolic rates (MMR and RMR, dark and light lines, respectively), and absolute aerobic scope (AAS = MMR–RMR; shaded) under optimal thermal conditions. (e,f) Scaling of factorial aerobic scope (FAS = MMR/RMR), a metric indicating metabolic constraint under optimal temperatures. (i,j) Scaling of maximum heart rates (fHmax). Individual or species-specific thermal performance curves without considering body size are depicted for MMR, RMR, AAS (c), FAS (g), and fHmax (k). (d,h,l) The hypothesized scaling relationships under temperatures above optimal for the animal. Directional change in scaling from optimal temperatures (greyscale, dashed lines) to warm are presented in panels (d,h). The equations for each column are provided on the bottom; BM = body mass, a = scaling intercept, b = scaling slope; * the quadratic fit = 2nd order polynomial fits can be used to describe TPC. Isometric scaling (b = 1 or −1) describes proportional change in performance with body mass. No scaling (b = 0) describes mass independence. The dots in (c,j,k) mark temperatures presented in panels (d,h,l) respectively. Cardiac thermal tolerance metrics are shown in (k): 1 = TAB, breakpoint temperature; 2 = TPEAK, the temperature at peak fHmax; 3 = PEAKfHmax corresponding to TPEAK; 4 = TARR, the temperature at first cardiac arrhythmia. All figures are for conceptual representations, only.
Besides body size, temperature is the most prominent factor governing physiological rates in ectotherms42,43. The temperature dependence of biological rates is described by thermal performance curves, TPC44, which are performance-specific and typically non-linear (Fig. 1)45,46,47. Resting metabolic rates (RMR) measured in resting, non-reproducing, non-digesting ectotherms rise exponentially with increasing temperature (Fig. 1c). In contrast, maximum metabolic rate (MMR) may rise continuously with increasing temperature, peak and plateau, or peak and decline44 (Fig. 1c). Factorial aerobic scope (FAS = MMR / RMR) generally declines with increasing temperatures48 (Fig. 1g), which indicates an increasing metabolic constraint with warming. Alternatively, absolute aerobic scope (AAS = MMR–RMR), which represents an individual’s aerobic capacity to thrive (e.g., move, digest, reproduce), peaks at optimal temperatures and may plummet towards both warm and cold temperatures (Fig. 1a). Similarly, maximum fH (fHmax) increases steadily with warming until it first begins to slow at a breakpoint temperature (TAB) and then fHmax reaches the peak (PEAKfHmax at the corresponding peak temperature, TPEAK) (Fig. 1k). The temperature after TPEAK when the heartbeat becomes irregular (arrhythmic) is TARR (°C) (Fig. 1)12. TAB, TPEAK and TARR provide key functional temperature tolerance indices derived from TPCs of fHmax and are directly linked with aerobic metabolic capacity12,49,50 (Fig. 1k). TPCs for metabolic rates can be life stage and thus body size specific51,52 suggesting that TPCs of fHmax and cardiac thermal tolerance could also change with body size. Therefore, the scaling of metabolic rates and cardiac thermal tolerance may differ across temperatures.
Here, we studied the metabolic rates and cardiac thermal tolerance of barred surfperch (Amphistichus argenteus), a temperate viviparous marine fish species from a thermally dynamic coastal habitat (surf zone). Coastal temperatures change seasonally (Fig. 2b) but are also characterized by high daily thermal variability (Fig. 2c). To thrive in the surf zone, barred surfperch must be able to respond to the acute temperature swings44,53,54,55,56 they frequently encounter. Additionally, barred surfperch are a good model for studying size and life stage-specific physiology because they give live birth to fully developed juveniles (< 3 g, lab-measured), reach an adult size of ~ 2.0 kg57, and live in the surf zone their entire lifetime, thus juveniles, subadults, and spawning adults experience the same thermal conditions. We measured each individual’s metabolic capacity across acute ecologically relevant temperatures (12, 16 (control), 20, 22 °C; Fig. 2), and fHmax during acute warming from 16 °C to the upper functional temperature limit, or where the heart became arrhythmic. We hypothesized that the perch’s MMR and RMR would differ in their scaling slopes and in response to acute temperature change23,58,59. Specifically, we predicted that (i) bMMR > bRMR under optimal temperature conditions59,60,61 (Fig. 1b), that (ii) bMMR would decrease with increasing temperatures because larger individuals may have compromised cardiovascular and oxygen supply capacity compared to smaller counterparts11,62,63 and (iii) bRMR would increase with increasing temperatures because larger mature individuals are more temperature sensitive and likely invest more energy than small individuals towards reproduction when food is not limited (Fig. 1d)64, thus (iv) bAAS < bMMR < bRMR and bFAS < 0 under warming (Fig. 1h). These trends would reveal a decline in aerobic performance in larger adult fish under warming11,65. Further, we hypothesized that fHmax would scale negatively4, bfHmax < 0, and that bfHmax would decrease with increasing temperature, the rationale being that PEAKfHmax would be lower in larger fish (Fig. 1l). Our study provides mechanistic insight into temperature-modulated mass scaling relationships of aerobic capacity, and cardiac thermal tolerance in fishes.
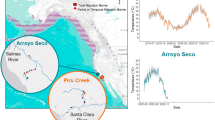
Habitat range and thermal conditions of barred surfperch. (a) Pacific coastline in North America with an inset of Santa Barbara and Ventura Counties (white) in California, U.S. Red diamond marks Naples Kelp Forest, a temperature monitoring site (data in b and c; Santa Barbara Coastal Long Term Ecological Research program107; coord: 34.42388, −119.95053), and fish collection site (Haskell’s Beach; 34.430767, −119.916717). Collection site was approximately 2 km from the temperature logger. (b) Recorded temperatures from May to September across years 2001–2021 with marked ecologically relevant temperatures (12, 16, 20, 22 °C). (c) Recorded temperatures across 8 h presenting examples for selected acute temperature change treatments (shaded grey: temperatures change from 16 °C at a green line to 20 °C (n = 9; pink line) and to 12 °C (n = 9; blue line) at an average rate of 2 °C h−1). Native range sourced from fishbase.org, referencing Global Biodiversity Information Facility (https://www.gbif.org/) and Ocean Biodiversity Information System (https://obis.org/).
Results
Scaling relationships
Aerobic metabolic performances (MMR, RMR, AAS) scaled hypoallometrically with body size, and the acute temperature change consistently impacted the scaling intercepts but not the slopes (bAAS > bMMR ≈ bRMR) (Fig. 3). This suggests that body size and temperature independently, not interactively, affect aerobic metabolism in barred surfperch (models with the interaction between temperature and mass were ∆BIC > 15; Supplementary Table S1 online). Specifically, MMR scaled with b = 0.810 {CI95%: 0.79, 0.83} with significantly increasing intercepts across increasing temperature (ANOVA: ln(a): χ2(3) = 463.88, P < 2.2e−16) (Fig. 3a). The scaling slope of RMR was consistent at bRMR = 0.809 {CI95%: 0.77, 0.85}, also with significantly increasing intercepts with increasing temperatures (ANOVA: ln(a): χ2(3) = 515.971, P < 2.2e−16) (Fig. 3b).
Consistent hypoallometric scaling of maximum and resting metabolic rates across acute temperatures in barred surfperch. (a) Maximum metabolic rates (MMR) and (b) resting metabolic rates (RMR) scaled similarly with b = 0.81 and consistently with acute temperature change from 16 °C to 12 °C (blue), 16 °C (green; control), 20 °C (light pink), and 22 °C (dark pink) (mixed model estimates). The squares are reproductively active females. Fish were repeat tested at each temperature; MMR: n = 83 (238); RMR: n = 81 (233), individuals (observations).
Further, the scaling of aerobic scope (both AAS and FAS) did not indicate that larger barred surfperch had a lower aerobic metabolic performance than the smaller perch. AAS scaled hypoallometrically and consistently across temperatures with bAAS = 0.883 {CI95%: 0.85, 0.92} (Table 1). Opposite to our prediction, FAS scaled weakly and positively across temperatures with bFAS = 0.020 {CI95%: − 0.01, 0.05} (Table 1). Additionally, all temperature-specific scaling relationships of AAS were bAAS ~ 0.88 (Supplementary Fig. S1, Table S2 online). Together, the resulting bAAS > bRMR ≈ bMMR suggests that larger fish did not lose aerobic capacity with acute warming. Similarly, the positive scaling of FAS suggests that larger individuals did not develop a greater aerobic constraint under acute warming compared to smaller individuals.
The fHmax scaled consistently negatively with body mass with bfHmax = − 0.052 {CI: − 0.07, − 0.03} (Fig. 4). Therefore, body mass and temperature independently influenced fHmax (models with T * ln(BM) were ∆BIC > 45) (Fig. 4b). At their acclimation temperature (16 °C), the fish with the lowest recorded fHmax (74.40 beats min−1) was 233.0 g, and the fish with the highest recorded fHmax (104.38 beats min−1) was 12.59 g (Fig. 4a). The scaling slope of bfHmax decreased at higher acute temperatures (b = − 0.068 at 16 °C to b = − 0.036 at 24 °C; Supplementary Fig. S2, Table 1 online).
Negative and consistent scaling of maximum heart rate (fHmax) in barred surfperch across acute temperatures. (a) Individual fHmax across acutely increased temperatures; the errors are SD of fH across 15 s. (b) Estimated common mass scaling relationships of fHmax across all temperatures (mixed model). In all panels: colored lines and symbols indicate 16, 20, 22 °C where metabolic rates were measured in the same fish, and mortality was observed following acute exposure to 24 °C (red markers). n = 30 all panels.
The TPCs of fHmax varied across individuals of different sizes (Fig. 4a). All cardiac thermal tolerance metrics, TARR, TAB, and TPEAK, similarly and positively scaled with body mass (b = 0.030 to 0.034; Fig. 5a,b,c; Supplementary Fig. S3 online). The TAB, TPEAK, and TARR were lower in juvenile perch (< 50 g) compared to the adults by an average of 1.62 °C, 1.79 °C, and 1.72 °C, respectively. Specifically, the smaller wild-caught fish (~ 10 g) had a ~ 1.5–2 °C lower TAB, TARR and TPEAK compared to the larger fish (> 100 g). The TPEAK ranged between 19.90–26.90 °C (n = 30, size range: 8.3–249 g), while the cardiac arrhythmias (TARR) ranged between 21.66 and 28.90 °C (n = 29). At an individual level, the measured values of two upper thermal tolerance metrics, TPEAK and TARR, were only apart by a mean of 1.75 °C (range: 0.47–3.70 °C; TARR minus TPEAK). The TAB had a broad range: 18.34 °C (14.90 g fish) and 24.49 °C (249 g fish), but was significantly lower in laboratory-born juveniles compared to wild-caught juveniles (ANOVA: F(1) = 5.28, P = 0.031) (Supplementary Table S3 online). The PEAKfHmax was not significantly associated with body mass (Fig. 5d, Supplementary Table S3 online) but did not exceed 168.9 beats min−1 across tested fish. Lastly, ventricle mass scaled with body mass with b = 0.855 {CI95%: 0.80, 0.91} (Table 1, Supplementary Fig. S4 online). Altogether, we found that larger fish had slightly higher cardiac thermal tolerance compared to juveniles.
Mass scaling of cardiac thermal tolerance indices. The TPEAK (a), TARR (b), and TAB (c) were weakly but significantly associated with body mass, but PEAKfHmax (d) was independent of mass. In all panels: open symbols show laboratory-born juveniles and closed symbols are wild-collected fish (origin). Size range: 8.3 to 249 g.
Mass-independent temperature effects
Mass-corrected and mass-specific MMR and RMR increased with increasing acute temperatures between 12 to 20 °C. The fish’s mean MMR increased from 4.61 mgO2 min−1 65 g−1 at 12 °C to 7.50 mgO2 min−1 65 g−1 at 22 °C, but significantly changed only up to 20 °C (wild-caught fish; post-hoc: 12 vs. 16 °C, 16 vs. 20 °C: P < 0.001, Supplementary Tables S3, S4 online) (Fig. 6a), and their RMR increased significantly across all acute temperatures (mean at 12 °C: 2.133 mgO2 min−1 65 g−1, at 22 °C: 4.45 mgO2 min−1 65 g−1; wild-caught fish) (Fig. 6b, Supplementary Tables S3, S4 online). Additionally, laboratory-born juveniles had significantly higher RMR than wild-caught juveniles (ANOVA: χ2(1) = 11.035, P = 8.94e−4).
Metabolic performance is maintained across a broad range of acute temperatures (12, 16, 20, 22 °C) in barred surfperch. Mass-specific and mass-normalized (65 g fish, mean size in the study) aerobic metabolic performance across temperatures: (a) Maximum metabolic rate (MMR), (b) Resting metabolic rates (RMR), (c) Absolute aerobic scope (AAS = MMR–RMR), and (d) Factorial aerobic scope (FAS = MMR /RMR) (d). Shaded symbols are individual fish, the large, solid symbols are estimated group means (± SE). Origin refers to lab-born versus wild-caught juveniles. Fish were repeat tested across temperatures; MMR: n = 83 (238); RMR: n = 81 (233), individuals (observations); Table 1.
The similar increase of MMR and RMR with acute warming resulted in minimal change in AAS across temperatures. The highest estimated mean was 2.90 mgO2 min−1 65 g−1 at 20 °C (mass-normalized to 65 g; Fig. 6c) (post-hoc: 16 vs. 20 °C P = 0.0076; 12 vs.16 °C and 20 vs. 22 °C, P = ns). These findings suggest that aerobic metabolic capacity in barred surfperch was maintained across acute ecologically relevant acute temperatures.
In contrast, FAS declined with increasing temperature. The FAS was the highest at 12 °C (mean = 2.08; range = 1.27–3.60), and it dropped to its lowest at 22 °C (mean = 1.57; range = 1.29–2.17) (Fig. 6d). Similarly to RMR, FAS was significantly lower in laboratory-born juveniles (ANOVA: χ2(1) = 8.013, P = 0.005; Supplementary Table S3 online). Therefore, the fish of all sizes were experiencing an aerobic metabolic constraint that increased with acute warming.
The temperature sensitivity of mass-independent aerobic and cardiac performances was generally low (Q10 ≤ 2) and decreased with increasing temperatures. The RMR was the most temperature-sensitive, followed by fHmax, and MMR (Fig. 7). MMR and RMR increased with increasing temperature, but above 20 °C, the rate of increase slowed (Figs. 6a,b, and 7d). The RMR increased most rapidly from 12 to 16 °C (Q10 (12–16 °C) = 2.43, Q10 (16–20 °C) = 1.89, and Q10 (20–22 °C) = 1.87, Fig. 7d). Meanwhile, the Q10 of MMR remained low across all test temperatures (Q10 < 1.75, Fig. 7d). The Q10 of fHmax decreased steadily across temperatures from ~ 2.0 to 1.35 and plateaued after ~ 23 °C (Q10 ~ 1.20) until a precipitous drop at 27 °C (Fig. 7d). The fish’s aerobic scopes (AAS and FAS) were the least temperature-sensitive. Specifically, the Q10 values for AAS were below 1.46, and Q10 for FAS were all < 1.0 (Fig. 7d). Lastly, considerable interindividual variation was common across all performances and temperatures (Supplementary Table S4 online).
Scaling relationships and acute thermal performance across physiological metrics in barred surfperch. (a,b) Scaling slopes of metabolic and cardiac performance were not consistently different across acute temperatures but varied across traits (white triangles; slope values noted above, error is a 95% CI; mixed model results). Colored circles are temperature-specific scaling slopes. Thermal performance curves (c) and Q10 (d) of mean mass-independent aerobic metabolic performances and fHmax. Red symbol and line mark 24 °C, a temperature at which 50% mortality was observed during a respirometry trial. MR = metabolic rate (RMR = resting; MMR = maximum), AAS = absolute aerobic scope, FAS = factorial aerobic scope, TARR = temperature at first cardiac arrhythmia, TAB = Arrhenius breakpoint temperature, TPEAK = temperature at highest recorded fHmax, VM = ventricular mass.
Discussion
Several fish species have been declining in body mass1,66, suggesting that larger individuals may be more vulnerable to warming than smaller ones2. However, the underlying mechanisms of this intraspecific trend are unclear2,53,64. If warming causes metabolic scaling slopes to shift such that bMMR < bRMR (Fig. 1), then it would indicate a proportionally greater loss of aerobic capacity with increasing temperatures in larger individuals11. Therefore, measuring species-specific scaling relationships across ecologically-relevant temperatures and thermal exposure times can help us identify how vulnerability may change across a fish’s lifetime.
Here, the consistent and parallel bMMR and bRMR (b = 0.81) across a 10 °C temperature range (12–22 °C) suggests that aerobic capacity was not suffering under acute warming in larger barred surfperch. This was reaffirmed by the consistent, slightly positive bFAS = 0.02 (bFAS ≈ 0.05 temperature-specific) and the consistent bAAS = 0.88 (Supplementary Table S2 online). The temperature-insensitive bMMR ≈ bRMR may be explained by the distinctive lifestyles and life history of barred surfperch. The temperature sensitivity of metabolic scaling is known to be species-specific23,27,32,33. For example, after a 2–4 week temperature-acclimation, bRMR decreased with increasing temperature in round stingray67 and coregonids33, did not change in cyprinids33,68,69, and varied in response to changing temperatures in Atlantic cod70, European perch71, and yellow perch72. However, interspecific level meta-analyses on fish suggest that scaling slopes decrease with increasing temperatures11. Interspecific variation in how temperature affects RMR and MMR scaling relationships could be caused by various factors, including species ecology22, acclimation71, phylogeny33, and intraspecific reproductive trade-offs31,62, and possibly the methodology used to measure metabolic rate (i.e., especially MMR,73,74,75,76). For perch, they spend their entire lifetime in the surf zone, where they routinely maneuver into the swash zone to feed on hard-shelled sand crabs77. They also experience rapid, acute (hourly), as well as longer-term (seasonal) temperature variations (Fig. 2), which likely accounts for the low acute thermal sensitivity of metabolic rate across a broad range of temperatures. Therefore, their ecology and generally non-athletic lifestyles (minimal difference between MMR and RMR) could partly explain why scaling of RMR and MMR was similar and insensitive to acute temperature change (e.g., metabolic level boundary hypothesis59,60). Barred surfperch may be living close to their full aerobic capacity investing most of their available energy in foraging, digestion, growth, and reproduction, though prioritization of these different functions will vary across ontogeny. Possibly, the scaling of these vital performances show different thermal sensitivities compared to MMR and RMR78. Here, MMR was measured using a standard chase protocol limiting our interpretation—possibly MMR induced following prolonged swimming or maximum feeding could lead to different temperature effects on bMMR, bAAS, and bFAS. Still, the consistent bMMR ≈ bRMR suggest that larger barred surfperch did not have reduced aerobic capacity during acute warming.
If the scaling slope for FAS was negative, this would indicate that larger surfperch experience a greater aerobic constraint than smaller ones48. However, we found a slightly positive scaling of FAS across acute temperatures. In another perch species (European perch), FAS scaled negatively (bFAS = − 0.033) when acclimated to an optimal temperature (15 °C) for three weeks, and decreased further to bFAS = − 0.067 at a suboptimal temperature (28 °C)71. In contrast, bFAS ≈ 0.01 and did not change in leopard coral grouper that were acclimated to 28.5 °C and 33 °C for 3–5 days79. This discrepancy between studies might be due to acclimation versus the acute nature of temperature change or indicate species-specific responses to temperature change.
The aerobic metabolic capacity of fishes depends on the function of their heart, which plays a key role to ensure sufficient O2 supply to meet demand at the tissues. The heart is also often the first organ system that fails in fish under thermally challenging conditions49,50,80. Thus, we predicted that maximum heart rates would scale negatively with mass, and specifically that increasing temperature would have a negative effect on fHmax scaling slopes. Here, we found that larger individuals had lower fHmax than smaller ones, although the scaling relationship was consistent across temperatures and weak (b = − 0.05). In contrast, various meta-analysis and modelling studies have demonstrated that heart rates scale with more negative slopes in mammals at rest39,40 (bfH = − 0.25) and during exercise81 (bfH = − 0.187), birds at rest39 (bfH = − 0.28) and during flight81 (bfH = − 0.187), lizards82 at 30 °C (bfH = − 0.15), and terrestrial snakes37 at 25 °C (bfH = − 0.229). A recent experimental study on five cetaceans species reported heart rate scaling slopes between bfH = − 0.16 and − 0.3436. However, the mild scaling of fHmax in our study aligns with previous findings in fishes34,41,83. A study using the same experimental protocol reported bfHmax = − 0.1 in redband trout83. Additionally, a statistically significant body size effect on fHmax was found in adult Arctic char84, adult brown trout84, Baltic herring embryos9, and on the field fH in adult Chinook salmon85. A study on Atlantic salmon parr (~ 11 g) and post-smolts (~ 300 g) at 20 °C measured fHmax of 157 beats min−1 and 130 beats min−1, respectively86. Assuming these mean values comply with the fHmax mass scaling relationship, it would result in bfHmax ~ − 0.06, a very similar slope to ours (bfHmax = − 0.05). In comparison to Atlantic salmon, we measured lower fHmax at 20 °C, specifically ~ 123 beats min−1 in fish < 15 g and ~ 96 beats min−1 in > 200 g fish, which may be attributed to the lower athleticism of perch22. However, our PEAKfHmax values for ~ 200 g fish (~ 122 beats min−1) compare well to 115 beats min−1 in ~ 450 g European perch56 (note the difference in methods). It is worth noting that in our study PEAKfHmax did not significantly relate to body mass, but it could be explained by the fact that PEAKfHmax was achieved at different temperatures across individuals. The biological significance of the negative scaling of fH and its temperature dependence is not clear yet, but certainly important to consider within and across species14.
The temperature at which the steady increase of fHmax with acute warming begins to slow (TAB), the fHmax peaks (TPEAK), and the heartbeat becomes arrhythmic (TARR) all indicate functional thermal limits in fish16. Surprisingly, larger barred surfperch (~ 200 to 250 g) had higher cardiac thermal tolerance than young-of-the-year (~ 10 to 15 g). Similarly, a life-stage specific difference in TAB and TPEAK has been found in Atlantic salmon86. Specifically, the parr (~ 11 g) had lower TAB by ~ 2.5 to 5 °C and higher TPEAK by ~ 2.5 to 3 °C, but no differences in TARR compared to post-smolts (~ 300 g)86. In our study, TAB, TPEAK, and TARR were all lower in juveniles (< 50 g)77 compared to adults by an average of 1.62 °C, 1.79 °C, and 1.72 °C, respectively. Both studies suggest that intra-specifically, the optimal temperatures for cardiac performance are lower in smaller fish. In contrast, a study on cardiorespiratory capacity in adult Chinook salmon tested within 13 to 25 °C found cardiac arrhythmias at 25 °C only in the largest individuals (2.1–5.4 kg size range)62, and thus their results would suggest that the largest adults had lower thermal tolerance. The discrepancy between studies could be due to species- and life-stage-specific physiologies or methods used to measure fH. Still, the empirical evidence suggests that acute TPCs of fHmax is a plastic trait54,55,87,88,89 that varies across species14, life stage86, and body size.
While outside of the scope of this study, other physiological and morphological factors could help identify the aerobic limitations associated with body mass and temperature. In fish under warming, increasing heart rates is the dominant mechanism to achieve higher cardiac output, CO14. However, fH is not the only performance contributing to CO (CO = fH * VS; mL min−1; VS = cardiac stroke volume). Aerobic metabolism and cardiac output are directly linked by Fick’s equation, which states that oxygen uptake rate (i.e., MR) is a product of VS, fH, and the difference between the O2 content in arterial and venous blood: MR = fH * Vs * (CaO2—CvO2); MR = CO * (CaO2—CvO2), where CaO2 and CvO2 are oxygen content (ml dl−1) in arterial and venous blood, respectively14. Scaling of oxygen content in blood, CO and VS are not well established in fishes62. However, White and Kearney (2014) outlined that the discrepancy between the scaling of MR and fH can be explained by the scaling of VS because increased MR can be achieved by increasing either (or both) fH and VS, e.g., assuming reciprocal relationships bMR = 0.75 and bfH = − 0.25, then bVs ≈ 1 [bMR (0.75) = bVs (1) + bfHmax (− 0.25)]20,90. Thus, our estimated bMR = 0.81 and bfH = − 0.05 would suggest a scaling slope of ~ 0.86 for VS [bMR (0.81) = bVs (0.86) + bfHmax (− 0.05)]. Further, in fishes, the cardiac stroke volume is positively correlated with relative ventricular mass91, which in our species scaled with bVM = 0.85. Therefore, it would not be entirely surprising that bVs ≈ 0.86 matching bVM = 0.85. Larger hearts also allow for greater cardiac power output supporting higher MMR92,93, and so the strong hypoallometric scaling heart mass could partly explain why bMMR ≠ 1, and bMMR ≈ bRMR in barred surfperch. For comparison, the ventricular mass scaled with bVM = 0.939 in freshwater perches94, bVM ~ 1 in salmonids95, and bVM ~ 0.9 to 0.95 broadly across endotherms and ectotherms (mammals, birds, fishes, amphibians, and reptiles)81,96. These near isometric scaling slopes of VM across taxa could mechanistically support the trend that bMMR ~ 1 > bRMR. Lastly, to our knowledge, only one study97 has estimated (and reported) mass effects on CO in fish species (rainbow trout) and found isometric scaling (bQ = 1), while in mammals, CO can scale hypoallometrically and depend on activity (b < 0.90)81. It is critical to note that these cautiously outlined ideas are untested. Further, the contributions of fH and VS to CO and the scaling CO, VS, CaO2, CvO2 and their change with changing temperature in barred surfperch are unknown and warrant further study.
The concept of temperature-sensitive metabolic scaling has been incorporated in several hypotheses, which mainly focus on acclimation, maternal, and evolutionary effects of temperature on metabolic scaling11,65,98,99. It has been hypothesized that larger water-breathing ectotherms are more aerobically constrained under O2 demanding conditions, like warming and exercise, because of limited O2 supply11,99. An interspecific meta-analysis by Rubalcaba et al. (2020) developed a framework of scaling models that included biological and environmental factors that limit O2 supply (e.g., scaling of gill surface area, O2 partial pressures driving diffusion rates, ventilation rates), and showed that increasing activity (MMR) and increasing temperatures (species habitat temperature) interactively lead to decreased metabolic scaling slopes. Alternatively, the Gill Oxygen Limitation hypothesis predicts that relative gill surface area decreases with increasing body size, thus limiting sufficient O2 supply as fish grow larger99. In addition to gill surface area, other physiological mechanisms (e.g., ventilation frequency) have been suggested to interactively limit oxygen supply in larger fish, thus directly affecting scaling of resting metabolic rates69,100. Under warming, limitations in O2 supply are thought to disproportionally negatively impact larger fish, especially their MMR. At an intraspecific level, the bMMR has been shown to increase61 or decrease70,71 following a non-monotonic pattern with increasing acclimation temperatures, thus providing mixed evidence. Oxygen supply mechanisms were not tested in our study and thus their contributions driving metabolic scaling relationships are unknown. Any discrepancies comparing inter-specific frameworks and intraspecific studies, including ours, may be due to the species examined, type of temperature exposure, and experimental approaches (e.g., MMR measurement methods). Even still, in our study, oxygen supply was unlikely to be a limiting factor irrespective of individual size and acute temperature exposure because bMMR and bRMR were consistent, the bAAS > bMMR ≈ bRMR and bFAS > 0 across acute temperatures. Studies examining the scaling of other pieces in the oxygen cascade101, will be useful for better understanding warming-associated constraints in fish across sizes.
Independent of fish’s body size, aerobic performance was maintained across an acute 10 °C range (12 to 22 °C) and had low thermal sensitivity (Q10 < 2). This is characteristic of eurythermal species like opaleye45, which share a similar coastal range to our study species, and other perch species (European perch71,89, yellow perch72). Notably, in barred surfperch, the RMR and MMR were somewhat low (mean 3.93 and 7.06 mgO2 min−1 65 g−1 at 20 °C in wild-caught fish, respectively), leading to a low AAS (mean 2.90 mgO2 min−1 65 g−1 at 20 °C), and FAS (≤ 2.27). These values are similar to those observed in a close relative, the striped surfperch (Embiotoca lateralis), where metabolic rate was ~ 1.3 mgO2 min−1 kg−1 swimming at 0.5 body lengths s−1 and ~ 3 mgO2 min−1 kg−1 swimming maximally in 11 °C102. Additionally, European perch had a similar AAS at 20 °C (~ < 3 mgO2 min−1 kg−1)71,89. Perch species may be generally classified as non-athletic due to their lifestyle and ecology, thus possessing a lower aerobic capacity22.
Aerobic and cardiac thermal performance of barred surfperch showed signs of decline above ~ 20 °C. Even though the barred surfperch’s AAS was maintained across the 10 °C range, their MMR did not continue to increase significantly from 20 °C to 22 °C, TAB was ~ 20 °C, FAS was < 2 at 22 °C, TPEAK was ~ 24 °C, and their hearts became arrhythmic at ~ 26 °C. Furthermore, we unexpectedly observed 50% mortality in lab-born juveniles under an acute 24 °C exposure (during a discontinued respirometry trial). Our results agree with those studies where TAB and optimal temperature for aerobic metabolism overlap in fishes12,49,103. Altogether, the measured cardiac and metabolic performances indicate that aerobic capacity declined > 20 °C, the functional thermal limit was ~ 22 °C, and the acute upper thermal limits were likely between ~ 24 and ~ 26 °C in barred surfperch. South of our study location, in Baja California, the temperature in the surf zone can reach 24 °C lasting up to 16 h104. Thus, if the observed 50% mortality of juveniles at 24 °C is representative of barred surfperch, a northward shift in this species could be possible as their current suitable habitat from Northern California, USA, to Baja California, Mexico, constricts with coastal warming and temperature extremes105. Alternatively, perch from northern or southern habitats, or lab-born versus wild fish, may possess different functional and absolute thermal limits. Though, the population genetic structure, and developmental plasticity of barred surfperch are unknown.
Barred surfperch, a viviparous species, is a great study model for body size studies because fish of any life stage occupy the same thermally dynamic surf zone ecosystems with distinct seasonal, diurnal (acute, hourly), and anomalous (heatwaves and upwelling) temperature changes (Fig. 2). For instance, the juveniles that have lower acute cardiac thermal tolerance may be particularly vulnerable under acutely increasing temperature (≥ 20 °C) in the wild. Additionally, the geographic range of barred surfperch intersects 52 Marine Protected Areas106 with common temperature conditions from ~ 12 °C to ~ 23 °C77,107 (Fig. 2), their economic and recreational value is continuously increasing, and they fulfill key ecological roles by connecting aquatic and terrestrial fauna and flora communities. Therefore, this study system also provides the opportunity to integrate ecophysiology into management and conservation.
This study specifically explored the effects of ecologically relevant acute temperatures as opposed to the effects of a multi-week thermal acclimation44,89. Although barred perch must respond to both acute (hourly) and seasonal temperature changes, acute timescales (2 °C h−1) are particularly relevant for coastal California fishes. They experience high daily thermal fluctuations during the summer and early fall and are exposed to rapid temperature changes associated with coastal upwelling in the spring and fall108. When encountering acute temperature change, fish predominantly modulate their heart rate to ensure adequate oxygen delivery to the tissues with changing metabolic needs, and thus fHmax is a highly relevant target mechanism in studying thermal physiology in fish19. Further, in fishes, fHmax can acclimate rapidly, within the first 48 to 72 h after temperature change55, while metabolic rates can take from 72 h to ~ 5 days to stabilize109,110. Our results suggest that barred surfperch must be able to physiologically respond to acute temperature change within hours in the wild, are physiologically able to maintain their AAS across a 10 °C range up to ~ 22 °C. Possibly, the phenotypic changes following full muti-week physiological thermal acclimation44,53,89 could underscore different scaling relationships compared to acutely exposed fish. Further, this study suggests that thermal history and developmental plasticity could play an important role in aerobic capacity and thermal tolerance. Specifically, lab-born juveniles had lower cardiac thermal tolerance, and thus aerobic capacity compared to wild-caught juveniles which may have been due to different thermal history during development. Lab born-juveniles were acclimated to static 16 °C throughout their development in the lab which could have reduced their physiological capacity and plasticity in response to acute temperature change, unlike their wild counterparts that experience a wide breath of acute and seasonal temperatures (Fig. 2).The next step may be to explore the scaling of time to acclimation and acclimation capacity of metabolism, cardiac function, and other physiological performances across organ systems and species52,53.
In conclusion, some studies with ectotherms have found that larger individuals are more vulnerable to warming than smaller ones2,11,79. However, this study did not find any warming-associated constraints in large fishes. Consistent mass scaling of metabolic performance (bRMR = bMMR 0.81; O2 demand) together with negative and weak scaling of maximum heart rates (bfHamx = − 0.05; O2 supply mechanism) suggested that inadequate oxygen supply is an unlikely constraint on cardiac performance and metabolic rates in barred surfperch under warming. In fact, larger barred surfperch had superior cardiac thermal tolerance compared to smaller counterparts, as indicated by positive scaling of TAB, TPEAK, and TARR (b ~ 0.03). Barred surfperch currently experience temperatures close to their acute functional thermal limit. Together, this study suggests that body size vulnerability to warming is nuanced and not a universal trait.
Materials and methods
All data and statistical analyses were done in R v. 4.2.0 (2022). All animal handling and holding procedures were compliant with Protocol # 945 approved by the University of California, Santa Barbara Institutional Animal Care and Use Committee, and fish were collected under approved California Department of Fish and Wildlife collection permits. All methods were performed in accordance with the relevant guidelines and regulations. The study is reported in accordance with ARRIVE guidelines.
Animals
Barred surfperch, Amphistichus argenteus (N = 61; ~ 5 to 700 g) were caught in the beach zone in Santa Barbara County using a seine net (50 ft with catch bag, 30 ft no catch bag) or hook and line in April through May in 2021 (Spring experiments), and July 2021 (Summer experiments). Fish were transported to the University of California, Santa Barbara, in aerated filtered ambient flow-through seawater (> 80% air saturation). Wild-caught fish were kept in various size tanks (409 L, 303 L, and 94.6 L tanks; 2–13 fish per tank). Fish were grouped by size to avoid social stress between differently sized individuals. Barred surfperch are livebearers giving birth to fully developed juveniles in spring and early summer77. Five females were collected gravid during spring experiments (confirmed during dissections), giving birth to 79 juveniles (~ 2–3 g) in the laboratory (parent females to each offspring could not be assured; > 1 gravid female per tank). Laboratory-born juveniles were transferred to 37.9 L tanks at 16 °C (N = 6 to 12 fish per tank). Fish were kept at 16 °C (± 1.0 °C) using mixed chilled or heated filtered ambient seawater at > 90% air saturation under a 10D:14L light cycle. Water quality was tested weekly using commercial test kits (NO2− < 0.25 ppm, NO3− < 20 ppm, NH3 < 0.25 ppm, pH = 7.7 to 8.0, all matching ambient ocean seawater). Fish were fed daily to satiation with a diverse carnivorous diet (fresh or thawed mussels, thawed shrimp, squid, scallops, frozen brine shrimp, fresh sand crabs, Emerita analoga). Feeding was discontinued ~ 36 h before the respirometry trial. Fish were tagged with a visible fluorescent Elastomer tag (Northwest Marine Technology, Inc) and provided at least a 3-day recovery between trials.
Aquatic intermittent-flow respirometry
Methods reported following published guidelines for intermittent respirometry111. Oxygen consumption rates (MO2), a proxy for metabolic rates, were measured using intermittent flow respirometry across four acute temperatures in a repeated measurement design. Fish were first tested at 16 °C (acclimation temperature) and then after an acute temperature change (2 °C h−1) at 20 °C, 12 °C, and 22 °C (one round of trials was done in shuffled order, confirming it did not affect results). The ramp rate was selected to mimic ecologically relevant acute thermal events in kelp forests and nearshore environments along the Pacific coastline where barred surfperch live (Fig. 2a,c). The temperature was changed directly in housing tanks by adjusting incoming flow rates of chilled (10 °C) and warm (~ 20 to 22 °C) filtered seawater and by using submersible heaters with control unit; the same approach was used to maintain temperatures at their target level during respirometry. Fish were kept at their treatment temperature for at least 30 min before chasing (± 1 °C). Because only two fish could be chased at the time, the time that fish spent in acutely changed temperature before the chase varied between 30 to approx. 90 min. A higher, 24 °C acute temperature treatment was initially considered but led to 50% mortality (n = 4/8) in a group of laboratory-born juveniles. This treatment was discontinued. After an overnight respirometry trial, fish were returned to their housing tanks, and the temperatures were brought back to 16 °C at the same rates (i.e., 2 °C h−1).
Respirometry setup consisted of custom-built plastic chambers of various sizes (minimum 0.272 L, maximum 32.120 L), allowing for 19.2 to 93.9 net respirometer volume to fish body mass ratio112. Each chamber had one recirculating water loop and one flush loop, both connected to flow-controlled pumps (Ehaim compactON, Eheim universal; EHEIM GmbH & Co. KG. Deizisau, Germany). A robust fiberoptic oxygen sensor (PyroScience GmbH, Aachen, Germany) was placed in the recirculating loop. The temperature was controlled using submersible heaters and monitored using a Pt100 temperature probe (PyroScience GmbH, Aachen, Germany). Oxygen sensors and a temperature probe were connected to FireSting Optical Oxygen Meter (PyroScience GmbH, Aachen, Germany). All respirometry trials were performed in an environmental chamber, minimizing disturbance during the trial.
MMR was elicited following a standard chase and air exposure protocol (3-min chase, 1-min air exposure)73. Chase tanks were selectively sized to allow bursting in all fish. Immediately after air exposure, fish were placed in the respirometry chamber, and their metabolic rates were recorded (MMRCHASE). Fish recovered in respirometers overnight on an automated 15-min cycle of flush: measure (11:4, 10:5, 9:6, or 8:7 min, according to fish mass to chamber volumes ratio and temperature), yielding > 60 MO2 measurements. During trials, oxygen levels were at > 70% air saturation and within ± 1 °C of the experimental temperature. After the respirometry trial, fish were weighed to the nearest 0.01 g (fish < 60 g) or nearest 0.1 g (fish > 60 g), measured for length (cm), sexed when possible, and returned to their housing tanks. Chases were performed between 0900 and 1300 h, and fish were removed from the chambers between 0700 and 0900 h. Background respiration by microorganisms was measured in empty respirometry chambers before and after each trial. The background respiration levels were mean of 10% (median = 6.6%) of individuals respiration.
Arrhenius breakpoint temperature
Arrhenius breakpoint temperature (ABT) tests were set up and carried out following established methods12 previously used on marine fish45,87. We used a custom-built ABT test tank (33L × 20.5W x 22H cm, Igloo Playmate Elite Cooler 16 qt, filled to 12 L) that contained i) an elevated sling with silicone fish beds (n = 1 to 2; each with plastic straps to secure fish), ii) a circulation loop with flow control valve and soft plastic tubing to irrigate the gills of fish during the trial, iii) two air stones to keep oxygen levels at > 90% air saturation, and iv) heating coil connected to a Polystat recirculating heater/chiller unit (Cole-Palmer, Vernon Hills, IL, USA) to regulate the water temperature. The test tank was filled with seawater with a maintenance dose of anesthetic (65 mg MS-222 1 g L−1 buffered with NaHCO3− at 1:1 or higher ratio). Flow rates across the gills were kept between 25 ml s−1 and 55 ml s−1, depending on fish size.
Individual fish selected for the ABT test were anesthetized in 80 mg MS-222 1 g L−1 buffered with NaHCO3−, weighed to the nearest 0.01 g, and securely placed on the fish bed (laying on the side slightly tilted down and flow passing the gills). A stainless-steel Needle Tip Electrode (ADInstruments INC, Colorado Springs, CO, USA) was placed just under the skin on the ventral surface by the pericardium to detect an ECG signal. The ECG signal was amplified and filtered using Dual Bio Amp and Powerlab data acquisition system (ADInstruments INC, Colorado Springs, CO, USA) at the following settings: 60 Hz Notch filter; Mains filter; Low-Pass: 2Kz; High Pass: 10 Hz; Range: 2 mV. No more than four individuals were tested at the time.
Once all fish were positioned in the test tank, they were left undisturbed for a 30-min equilibration period at 16 °C. Atropine sulfate (1.2 mg kg−1 in 0.9% NaCl) was then injected intraperitoneally to block vagal tone, which was followed by a 15-min equilibration period. Then, isoproterenol (4 μg kg−1 in 0.9% NaCl) was injected intraperitoneally to maximally stimulate β-adrenoreceptors, which was followed by the final 15-min equilibration period. The temperature was then increased by 1 °C every 6 min (ramp rate 1 °C 5 min−1) and held steady for 1 min. Temperature and maximum heart rate (fHmax) for data analysis were recorded within the last 30 s of each 1 °C increment (i.e., minutes 5:30 to 6:00 of each temperature ramp interval). Incremental temperature ramp was continued until the heart became arrhythmic (TARR), defined by a clear transition from rhythmic to arrhythmic beating, or until missed QRS peak underlying a precipitous decrease in heart rate12. This was an endpoint of the ABT test, and the fish was immediately removed from the anesthetic, euthanized, and their ventricle was excised and weighed (nearest 0.001 g). All fish were also weighed to the nearest 0.01 g (fish < 60 g) or nearest 0.1 g (fish > 60 g), measured for length (cm), and sexed when possible.
Data analysis
Aerobic metabolism measurements were analyzed, and metabolic capacity metrics were estimated using custom-written functions in R (https://github.com/kraskura/AnalyzeResp_0). The decreasing dissolved O2 content (mgO2 L−1) collected during respirometry trials during each measurement cycle was plotted over time (min) and fitted with simple linear regression (‘lm’ in R). All linear regressions were visually assessed for quality and linearity. Only regressions with R2 > 0.96 were used for analysis. The selected regression slopes were used to calculate individuals’ oxygen uptake (MO2, mgO2 min−1), a proxy for metabolic rate following: MO2 = [(mfish*V) − (mbackground*V)], where slope (m) is the decline of O2 content (mgO2 L−1) over time (min), and V is the volume of the respirometer (L).
Maximum metabolic rate (MMR) is often elicited after a strenuous swim, chase, or during digestion74,75,76, resting, or minimum metabolic rate is measured in post-absorptive, non-reproductively active, resting individuals113. Barred surfperch behaviorally respond to various types of exercise by laying down on their side (personal observation in field and laboratory). This was observed in the respirometers immediately after the chase, likely contributing to why 74% of MMR values were observed during spontaneous overnight activity (lights turning on or off71); still, the values were comparable with those after the chase (Supplementary Fig. S5 online). Similarly, studies on a similar fish species, European perch, noted that MMR was observed via turning on a light-source and gently tapping the respirometer71,114. Additionally, five adult females from spring experiments were reproductively active. Males are reproductively active in the fall, but mature gonads were not observed during dissections. Acknowledging these constraints, the AAS was calculated using RMR as a baseline of AAS, and MMR was the highest MO2 recorded across ≥ 3 min at any time point during the trial. RMR was calculated as the mean of the 10 lowest estimated MO2 values after excluding the five lowest values from the entire trial113. We excluded the first 60 s of each measurement (mixing or wait period) but ensured that all MO2 measurements were at least 180 s long. The factorial aerobic scope (FAS) was calculated as FAS = MMR / RMR.
The electrocardiogram data from Arrhenius breakpoint temperature trials were analyzed directly in LabChart 8 (ADInstruments INC, Colorado Springs, CO, USA). Maximum heart rate (ƒHmax) was calculated for each 1 °C increment during 15 s (± 2 s) visually assessed measurements. The heart rate (beats min−1) was calculated by automated ECG analysis tools available in LabChart 8, and each fit was confirmed visually. The ƒHmax values recorded before TARR were used to establish acute TPC of maximum heart rate for each fish, which was then used to calculate several cardiac performance metrics. The breakpoint at which the incremental increase in individual fish fHmax changed rates was estimated on regression ln(ƒHmax) ~ 1000/Temperature (in Kelvin) using the segmented function in R (package ‘segmented’115) with parametric bootstrap (n = 100 boot samples). The breakpoint estimate was not included when: (i) the confidence interval of the estimated breakpoint exceeded ± 1.5 °C (n = 2), (ii) the breakpoint was not statistically identified (n = 1). The temperature ( °C) corresponding to the breakpoint was calculated and is referred to as TAB. The PEAKfHmax refers to the highest ƒHmax recorded across all temperatures (i.e., the peak of the acute TPC of maximum heart rate), and the temperature at PEAKfHmax is referred to as TPEAK.
Morphometrics, sample sizes, and sex, when available, are provided in Supplementary Table S5 online. Barred surfperch are sexually dimorphic, but the sex-specific characteristics are not fully developed until fish reach approximately > 7 g in size.
Statistical analysis
Mass scaling relationships were estimated for metabolic performance metrics (RMR, MMR, AAS, FAS), for cardiac physiology performances (ƒHmax, TAB, TARR, TPEAK, and PEAKfHmax ), and between ventricle mass and body mass. All performance metrics and body mass (BM, kg) were natural log-transformed to comply with the linear homoscedastic form of the scaling law (Fig. 1; ln(performance) = ln(a) + b*ln(BM)) where b = scaling exponent defining scaling slope, and ln(a) = scaling coefficient, or the intercept.
Consistency of scaling relationships across temperature treatments for RMR, MMR, AAS, FAS, and ƒHmax were determined using mixed-effect linear models (‘lmer’ in ‘lme4’ package116). We included a random intercept effect of individual fish to account for repeated measures. The independent explanatory variables were body mass (lnBM; continuous), temperature (°C; categorical), origin (laboratory-born, wild-collected fish; categorical), and sex (when available; categorical). Including temperature as a categorical variable (fixed effect) allowed us to test for differences in scaling relationships (slope and intercept) across temperature treatments, while still detecting the shape of thermal performance curves (n = 4 temperatures are insufficient to robustly estimate TPC on continuous scales). We considered size class (< 50 g “juvenile”, > 50 g “adult”) as an explanatory variable. Since it had no significant effect, it was not further considered (data-deficient sets). Complementary models were compared using BIC, where the model with the lowest BIC score was accepted as the best fit24 (Supplementary Table S1 online). Cardiac physiology measures (TAB, TARR, TPEAK, and PEAKfHmax) and ventricle mass (VM) were independent and modeled using simple, generalized linear models (‘lm’, ‘glm’). Supplementary to these analyses, we estimated temperature-specific (12, 16, 20, and 22 °C) scaling relationships for RMR, MMR, AAS, FAS, and fHmax; refer to supplementary material online for models and results. All model residuals were normally distributed, and fits were visually assessed.
The significance of body mass, temperature, sex, or origin was tested using Type II ANOVA (‘car’ package117). The significance of performance between different temperatures was tested using the Tukey post hoc test with the Kenward-Roger degrees of freedom method (‘emmeans’118). To evaluate only temperature effects on metabolic performances (RMR, MMR, AAS, and FAS) and fHmax, we mass-normalized individual values to represent performance of a mean size fish, 65 g (i.e., removed hypoallometric scaling effects90) using scaling relationships from best mixed effect models. Metabolic performances are expressed in mass-specific units (mgO2 min−1 65 g−1). The mass-independent mean values were used to calculate the temperature sensitivity coefficient, Q10, following Q10 = R2 / R1(10/ (T2—T1), where R1 and R2 are the average performance values at their corresponding temperatures, T1 and T2. Lastly, we used a 95% confidence interval (CI) and standard error (SE) to report the error of mean estimates. All reported values are maximum likelihood estimates. The significance was accepted at P < 0.05.
Data and code availability
The original data files, and all analysis and statistics scripts are available on GitHub: https://github.com/kraskura/KK_etal_perch_scaling_temp. The respirometry data were analyzed using custom functions documented on Github: https://github.com/kraskura/AnalyzeResp_0.
References
Audzijonyte, A. et al. Fish body sizes change with temperature but not all species shrink with warming. Nat. Ecol. Evol. 4, 809–814 (2020).
Dahlke, F. T., Wohlrab, S., Butzin, M. & Pörtner, H.-O. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369, 65–70 (2020).
Oke, K. B. et al. Recent declines in salmon body size impact ecosystems and fisheries. Nat. Commun. 11, 4155 (2020).
Pörtner, H. O. & Farrell, A. P. Physiology and climate change. Science 322, 690–692 (2008).
Pottier, P., Burke, S., Drobniak, S. M. & Nakagawa, S. Methodological inconsistencies define thermal bottlenecks in fish life cycle: A comment on Dahlke et al. 2020. Evol Ecol 36, 287–292 (2022).
Intergovernmental Panel on Climate Change. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (2022).
Auer, S. K., Agreda, E., Chen, A. H., Irshad, M. & Solowey, J. Late-stage pregnancy reduces upper thermal tolerance in a live-bearing fish. J. Therm. Biol. 99, 103022 (2021).
Kingsolver, J. G. & Buckley, L. B. Ontogenetic variation in thermal sensitivity shapes insect ecological responses to climate change. Curr. Opin. Insect Sci. 41, 17–24 (2020).
Moyano, M. et al. Linking individual physiological indicators to the productivity of fish populations: A case study of Atlantic herring. Ecol. Indicat. 113, 106146 (2020).
Pörtner, H. O. & Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 (2007).
Rubalcaba, J. G., Verberk, W. C. E. P., Hendriks, J. A., Saris, B. & Woods, H. A. Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proc. Natl. Acad. Sci. 170, 31963–31968 (2020).
Casselman, M. T., Anttila, K. & Farrell, A. P. Using maximum heart rate as a rapid screening tool to determine optimum temperature for aerobic scope in Pacific salmon Oncorhynchus spp. J. Fish Biol. 80, 358–377 (2012).
Chen, N. et al. Different transcriptomic responses to thermal stress in heat-tolerant and heat-sensitive pacific abalones indicated by cardiac performance. Front. Physiol. 9, 1 (2019).
Eliason, E. J. & Anttila, K. Temperature and the Cardiovascular System. In Fish Physiology (eds. Gamperl, A. K., Gillis, T. E., Farrell, A. P. & Brauner, C. J.) vol. 36 235–297 (Academic Press, 2017).
Hofmann, G. E. & Todgham, A. E. Living in the now: Physiological mechanisms to tolerate a rapidly changing environment. Ann. Rev. Physiol. 72, 127–145 (2010).
Somero, G. N. The physiology of global change: Linking patterns to mechanisms. Ann. Rev. Mar. Sci. 4, 39–61 (2012).
Stillman, J. & Somero, G. Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): Correlation of physiology, biochemistry and morphology with vertical distribution. J. Experim. Biol. 199, 1845–1855 (1996).
Farrell, A. P. From Hagfish to Tuna: A perspective on cardiac function in fish. Physiol. Zool. 64, 1137–1164 (1991).
Farrell, A. P., Eliason, E. J., Sandblom, E. & Clark, T. D. Fish cardiorespiratory physiology in an era of climate change. Can. J. Zool. 87, 835–851 (2009).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Hatton, I. A., Dobson, A. P., Storch, D., Galbraith, E. D. & Loreau, M. Linking scaling laws across eukaryotes. Proc. Natl. Acad. Sci. 116, 21616–21622 (2019).
Killen, S. S. et al. Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am. Nat. 187, 592–606 (2016).
Killen, S. S., Atkinson, D. & Glazier, D. S. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 13, 184–193 (2010).
Jerde, C. L. et al. Strong evidence for an intraspecific metabolic scaling coefficient near 0.89 in fish. Front. Physiol. 10, 1 (2019).
Glazier, D. S. Variable metabolic scaling breaks the law: from ‘Newtonian’ to ‘Darwinian’ approaches. Proc. R. Soc. B: Biol. Sci. 289, 20221605 (2022).
White, C. R., Phillips, N. F. & Seymour, R. S. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2, 125–127 (2006).
Lindmark, M., Ohlberger, J. & Gårdmark, A. Optimum growth temperature declines with body size within fish species. Glob. Change Biol. 28, 2259–2271 (2022).
Harrison, J. F. et al. White paper: An integrated perspective on the causes of hypometric metabolic scaling in animals. Integrat. Comp. Biol. https://doi.org/10.1093/icb/icac136 (2022).
Norin, T. & Gamperl, A. K. Metabolic scaling of individuals vs. populations: Evidence for variation in scaling exponents at different hierarchical levels. Funct. Ecol. 32, 379–388 (2018).
Norin, T. Growth and mortality as causes of variation in metabolic scaling among taxa and taxonomic levels. Integrat. Comp. Biol. 63, 1448–1459 (2022).
White, C. R., Alton, L. A., Bywater, C. L., Lombardi, E. J. & Marshall, D. J. Metabolic scaling is the product of life-history optimization. Science 377, 834–839 (2022).
Glazier, D. S. Activity alters how temperature influences intraspecific metabolic scaling: Testing the metabolic-level boundaries hypothesis. J. Comp. Physiol. B 190, 445–454 (2020).
Ohlberger, J., Mehner, T., Staaks, G. & Hölker, F. Intraspecific temperature dependence of the scaling of metabolic rate with body mass in fishes and its ecological implications. Oikos 121, 245–251 (2012).
Lillywhite, H. B., Zippel, K. C. & Farrell, A. P. Resting and maximal heart rates in ectothermic vertebrates. Comp. Biochem. Physiol. Part A: Mol. Integrat. Physiol. 124, 369–382 (1999).
Streicher, J., Cox, C. & Birchard, G. Non-linear scaling of oxygen consumption and heart rate in a very large cockroach species (Gromphadorhina portentosa): Correlated changes with body size and temperature. J. Experim. Biol. 215, 1137–1143 (2012).
Blawas, A. M., Nowacek, D. P., Rocho-Levine, J., Robeck, T. R. & Fahlman, A. Scaling of heart rate with breathing frequency and body mass in cetaceans. Philos. Trans. R. Soc. B: Biol. Sci. 376, 20200223 (2021).
Seymour, R. S. Scaling of cardiovascular physiology in snakes. Am. Zool. 27, 97–109 (1987).
Dawson, T. H. Allometric relations and scaling laws for the cardiovascular system of mammals. Systems 2, 168–185 (2014).
Seymour, R. S. & Blaylock, A. J. The principle of laplace and scaling of ventricular wall stress and blood pressure in mammals and birds. Physiol. Biochem. Zool. 73, 389–405 (2000).
Stahl, W. R. Scaling of respiratory variables in mammals. J. Appl. Physiol. 22, 453–460 (1967).
Clark, T. D. & Farrell, A. P. Effects of body mass on physiological and anatomical parameters of mature salmon: Evidence against a universal heart rate scaling exponent. J. Experim. Biol. 214, 887–893 (2011).
Schulte, P. M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Experim. Biol. 218, 1856–1866 (2015).
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M. & Charnov, E. L. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001).
Schulte, P. M., Healy, T. M. & Fangue, N. A. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702 (2011).
Hardison, E. A., Kraskura, K., Van Wert, J., Nguyen, T. & Eliason, E. J. Diet mediates thermal performance traits: Implications for marine ectotherms. J. Experim. Biol. 224, jeb242846 (2021).
Kellermann, V. et al. Comparing thermal performance curves across traits: how consistent are they?. J. Experim. Biol. 222, jeb193433 (2019).
Ørsted, M., Jørgensen, L. B. & Overgaard, J. Finding the right thermal limit: A framework to reconcile ecological, physiological and methodological aspects of CTmax in ectotherms. J. Experim. Biol. 225, jeb244514 (2022).
Eliason, E. J., Schwieterman, G. D. & Van Wert, J. Chapter 4: Applied Aspects of the Cardiorespiratory System. In Conservation Physiology for the Anthropocene—A Systems Approach Part A vol. 39A (Academic Press, 2022).
Anttila, K., Casselman, M. T., Schulte, P. M. & Farrell, A. P. Optimum temperature in Juvenile Salmonids: Connecting subcellular indicators to tissue function and whole-organism thermal optimum. Physiol. Biochem. Zool. 86, 245–256 (2013).
Eliason, E. J., Clark, T. D., Hinch, S. G. & Farrell, A. P. Cardiorespiratory collapse at high temperature in swimming adult sockeye salmon. Conserv. Physiol. 1, cot008–cot008 (2013).
Sinclair, B. J. et al. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures?. Ecol. Lett. 19, 1372–1385 (2016).
Twiname, S. et al. Mismatch of thermal optima between performance measures, life stages and species of spiny lobster. Sci. Rep. 10, 21235 (2020).
Lefevre, S., Wang, T. & McKenzie, D. J. The role of mechanistic physiology in investigating impacts of global warming on fishes. J. Experim. Biol. 224, 238840 (2021).
Gilbert, M. J. H., Adams, O. A. & Farrell, A. P. A sudden change of heart: Warm acclimation can produce a rapid adjustment of maximum heart rate and cardiac thermal sensitivity in rainbow trout. Curr. Res. Physiol. 5, 179–183 (2022).
Gilbert, M. J. H. et al. Rapid cardiac thermal acclimation in wild anadromous Arctic char (Salvelinus alpinus). J. Experim. Biol. 225, jeb244055 (2022).
Ekström, A. et al. Cardiac oxygen limitation during an acute thermal challenge in the European perch: Effects of chronic environmental warming and experimental hyperoxia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 311, R440–R449 (2016).
Eschmeyer, W. N. & Herald, E. S. A Field Guide to Pacific Coast Fishes: North America. (Houghton Mifflin Harcourt, 1999).
Fossen, E. I. F., Pélabon, C. & Einum, S. Genetic and environmental effects on the scaling of metabolic rate with body size. J. Experim. Biol. 222, jeb193243 (2019).
Glazier, D. S. Activity affects intraspecific body-size scaling of metabolic rate in ectothermic animals. J. Comp. Physiol. B 179, 821–828 (2009).
Glazier, D. S. A unifying explanation for diverse metabolic scaling in animals and plants. Biol. Rev. 85, 111–138 (2010).
Glazier, D. S. Scaling of metabolic scaling within physical limits. Systems 2, 425–450 (2014).
Clark, T. D., Sandblom, E., Cox, G. K., Hinch, S. G. & Farrell, A. P. Circulatory limits to oxygen supply during an acute temperature increase in the Chinook salmon (Oncorhynchus tshawytscha). Am. J. Physiol.-Regul. Integr. Comp. Physiol. 295, 1631–1639 (2008).
Clark, T. D. et al. Physiological benefits of being small in a changing world: Responses of Coho Salmon (Oncorhynchus kisutch) to an acute thermal challenge and a simulated capture event. PLoS ONE 7, e39079 (2012).
Wootton, H. F., Morrongiello, J. R., Schmitt, T. & Audzijonyte, A. Smaller adult fish size in warmer water is not explained by elevated metabolism. Ecol. Lett. 24, 1177–1188 (2022).
Verberk, W. C. E. P. et al. Shrinking body sizes in response to warming: Explanations for the temperature–size rule with special emphasis on the role of oxygen. Biol. Rev. 96, 247–268 (2021).
Gardner, J. L., Peters, A., Kearney, M. R., Joseph, L. & Heinsohn, R. Declining body size: A third universal response to warming?. Trends Ecol. Evol. 26, 285–291 (2011).
Silva-Garay, L. & Lowe, C. G. Effects of temperature and body-mass on the standard metabolic rates of the round stingray, Urobatis halleri (Cooper, 1863). J. Experim. Mar. Biol. Ecol. 540, 151564 (2021).
Li, Q. et al. Effects of temperature on metabolic scaling in black carp. PeerJ 8, e9242 (2020).
Xiong, W. et al. Effects of temperature on metabolic scaling in silver carp. J. Experim. Zool. Part A: Ecol. Integrat. Physiol. 337, 141–149 (2022).
Tirsgaard, B., Behrens, J. W. & Steffensen, J. F. The effect of temperature and body size on metabolic scope of activity in juvenile Atlantic cod Gadus morhua L. Comp. Biochem. Physiol. Part A: Mol. Integrat. Physiol. 179, 89–94 (2015).
Christensen, E. A. F., Svendsen, M. B. S. & Steffensen, J. F. The combined effect of body size and temperature on oxygen consumption rates and the size-dependency of preferred temperature in European perch Perca fluviatilis. J. Fish Biol. 97, 794–803 (2020).
Enders, E. C., Boisclair, D., Boily, P. & Magnan, P. Effect of body mass and water temperature on the standard metabolic rate of juvenile yellow perch, Perca flavescens (Mitchill). Environ. Biol. Fish. 76, 399–407 (2006).
Little, A. G. et al. Maxed out: Optimizing accuracy, precision, and power for field measures of maximum metabolic rate in fishes. Physiol. Biochem. Zool. 93, 243–254 (2020).
Norin, T. & Clark, T. D. Measurement and relevance of maximum metabolic rate in fishes: Maximum metabolic rate in fishes. J. Fish Biol. 88, 122–151 (2016).
Rummer, J. L., Binning, S. A., Roche, D. G. & Johansen, J. L. Methods matter: considering locomotory mode and respirometry technique when estimating metabolic rates of fishes. Conserv Physiol 4, cow008 (2016).
Steell, S. C., Van Leeuwen, T. E., Brownscombe, J. W., Cooke, S. J. & Eliason, E. J. An appetite for invasion: digestive physiology, thermal performance and food intake in lionfish (Pterois spp.). J. Experim. Biol. 222, jeb209437 (2019).
Carlisle, J. G., Schott, J. W. & Abramson, N. J. Fish Bulletin No. 109. The Barred Surfperch (Amphistichus argenteus Agassiz) in Southern California. (1960).
Adams, O. A. et al. An unusually high upper thermal acclimation potential for rainbow trout. Conserv. Physiol. 10, coab101 (2022).
Messmer, V. et al. Global warming may disproportionately affect larger adults in a predatory coral reef fish. Glob. Change Biol. 23, 2230–2240 (2017).
Steinhausen, M. F., Sandblom, E., Eliason, E. J., Verhille, C. & Farrell, A. P. The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). J. Experim. Biol. 211, 3915–3926 (2008).
Bishop, C. M. Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Philos. Trans. R Soc. Lond. B Biol. Sci. 352, 447–456 (1997).
Bartholomew, G. A. & Tucker, V. A. Size, body temperature, thermal conductance, oxygen consumption, and heart rate in australian varanid lizards. Physiol. Zool. 37, 341–354 (1964).
Chen, Z., Farrell, A. P., Matala, A. & Narum, S. R. Mechanisms of thermal adaptation and evolutionary potential of conspecific populations to changing environments. Mol. Ecol. 27, 659–674 (2018).
Mottola, G., Kristensen, T. & Anttila, K. Compromised thermal tolerance of cardiovascular capacity in upstream migrating Arctic char and brown trout—are hot summers threatening migrating salmonids?. Conserv. Physiol. 8, coaa101 (2020).
Twardek, W. M. et al. Field assessments of heart rate dynamics during spawning migration of wild and hatchery-reared Chinook salmon. Phil. Trans. R. Soc. B 376, 20200214 (2021).
Anttila, K. et al. Association between swimming performance, cardiorespiratory morphometry, and thermal tolerance in Atlantic salmon (Salmo salar L.). Front. Mar. Sci. 1, 1 (2014).
Schwieterman, G. D., Hardison, E. A. & Eliason, E. J. Effect of thermal variation on the cardiac thermal limits of a eurythermal marine teleost (Girella nigricans). Curr. Res. Physiol. 5, 109–117 (2022).
Anttila, K. et al. Atlantic salmon show capability for cardiac acclimation to warm temperatures. Nat. Commun. 5, 1–7 (2014).
Sandblom, E. et al. Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat. Commun. 7, 11447 (2016).
White, C. R. & Kearney, M. R. Metabolic scaling in animals: Methods, empirical results, and theoretical explanations. Compreh. Physiol. 4, 231–256 (2014).
Franklin, C. E. & Davie, P. S. Sexual maturity can double heart mass and cardiac power output in male rainbow trout. J. Experim. Biol. 1, 139–148 (1992).
Darveau, C.-A., Suarez, R. K., Andrews, R. D. & Hochachka, P. W. Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 417, 166–170 (2002).
Hochachka, P. W., Darveau, C.-A., Andrews, R. D. & Suarez, R. K. Allometric cascade: A model for resolving body mass effects on metabolism. Comp. Biochem. Physiol. Part A: Mol. Integrat. Physiol. 134, 675–691 (2003).
Edmunds, N. B., McCann, K. S. & Laberge, F. Relative heart size and fish foraging ecology in a lake food web. Can. J. Fish. Aquat. Sci. 75, 1477–1484 (2018).
Farrell, A. P. et al. Effects of exercise training and coronary ablation on swimming performance, heart size, and cardiac enzymes in Rainbow Trout, Oncorhynchus mykiss. Can. J. Zool. 68, 1174–1179 (1990).
Gillooly, J. F., Gomez, J. P. & Mavrodiev, E. V. A broad-scale comparison of aerobic activity levels in vertebrates: Endotherms versus ectotherms. Proc. R. Soc. B. 284, 20162328 (2017).
Thorarensen, H., Gallaugher, P. & Farrell, A. P. Cardiac Output in Swimming Rainbow Trout, Oncorhynchus mykiss, Acclimated to Seawater. Physiol. Zool. 69, 139–153 (1996).
Audzijonyte, A. et al. Is oxygen limitation in warming waters a valid mechanism to explain decreased body sizes in aquatic ectotherms?. Glob. Ecol. Biogeogr. 28, 64–77 (2019).
Pauly, D. The gill-oxygen limitation theory (GOLT) and its critics. Sci. Adv. 7, eabc6050 (2021).
Luo, Y. et al. Ventilation frequency reveals the roles of exchange surface areas in metabolic scaling. Physiol. Biochem. Zool. 93, 13–22 (2020).
Horrell, H. D. et al. Relationship between capillaries, mitochondria and maximum power of the heart: A meta-study from shrew to elephant. Proc. R. Soc. B: Biol. Sci. 289, 20212461 (2022).
Cannas, M., Schaefer, J., Domenici, P. & Steffensen, J. F. Gait transition and oxygen consumption in swimming striped surfperch Embiotoca lateralis Agassiz. J. Fish Biol. 69, 1612–1625 (2006).
Ferreira, E. O., Anttila, K. & Farrell, A. P. Thermal optima and tolerance in the Eurythermic Goldfish (Carassius auratus): Relationships between whole-animal aerobic capacity and maximum heart rate. Physiol. Biochem. Zool. 87, 599–611 (2014).
Fernández-Aldecoa, R., Ladah, L., Morgan, S., Morgan, S. & Filonov, A. Delivery of zooplankton to the surf zone during strong internal tidal forcing and onshore winds in Baja California. Mar. Ecol. Prog. Ser. 625, 15–26 (2019).
Sunday, J. M. et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. PNAS 111, 5610–5615 (2014).
CA Marine Species Portal, Section 3.1.2.1.8. Marine Protected Areas. Table 3–1. Marine Protected Areas listed north to south containing Amphistichinae. https://marinespecies.wildlife.ca.gov/barred-surfperch-and-redtail-surfperch/management/.
Washburn, L., Gotschalk, C. & Salazar, D. SBC LTER: Ocean: Currents and Biogeochemistry: Moored CTD and ADCP data from Naples Reef Mooring (NAP), ongoing since 2001 (2022). 10.6073/PASTA/11CE26BB3AB7AFA41A2F4BFB7836CBCA.
Hoshijima, U. & Hofmann, G. E. Variability of seawater chemistry in a kelp forest environment is linked to in situ transgenerational effects in the purple sea urchin, Strongylocentrotus purpuratus. Front. Mar. Sci. 6, 1 (2019).
Hanson, R. C. & Stanley, J. G. The effects of hypophysectomy and temperature acclimation upon the metabolism of the central mudminnow, Umbra limi (Kirtland). Comp. Biochem. Physiol. 33, 871–879 (1970).
Klicka, J. Temperature acclimation in goldfish: Lack of evidence for hormonal involvement. Physiol. Zool. 38, 177–189 (1965).
Killen, S. S. et al. Guidelines for reporting methods to estimate metabolic rates by aquatic intermittent-flow respirometry. J. Experim. Biol. 224, jeb242522 (2021).
Svendsen, M. B. S., Bushnell, P. G. & Steffensen, J. F. Design and setup of intermittent-flow respirometry system for aquatic organisms: How to set up an aquatic respirometry system. J. Fish Biol. 88, 26–50 (2016).
Chabot, D., Steffensen, J. F. & Farrell, A. P. The determination of standard metabolic rate in fishes: Measuring SMR in fishes. J. Fish Biol. 88, 81–121 (2016).
Christensen, E. A. F., Stieglitz, J. D., Grosell, M. & Steffensen, J. F. Intra-specific difference in the effect of salinity on physiological performance in European Perch (Perca fluviatilis) and its ecological importance for fish in estuaries. Biology 8, 1–17 (2019).
Muggeo, V. M. R. Estimating regression models with unknown break-points. Stat. Med. 22, 3055–3071 (2003).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Fox, J. & Weisberg, S. An R: Companion to Applied Regression. (Sage, 2019).
Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. (2019).
Acknowledgements
We thank Eliason Lab members, Gretchen Hofmann, and Christopher Jerde for conceptual and statistical advice. We thank, Jessica Madden, Kyle Emery, Jennifer Dugan and her lab, Claire Anderson, Lucy Johnson, Yvette Gaytan, Terra Dressler, Gail Schwieterman, Bashir Ali, Dana Cook, Jason Johns, and local fishers for help fishing. We thank Lucy Johnson, Yvette Gaytan, Gail Schwieterman, Jacey Van Wert, Jessica Madden, Terra Dressler, Andrea Chandler, Cameron Blair, Bella Giglio for help with experiments and animal care. We also thank David Davis at the University of California, Santa Barbara for technical facility assistance. This work was supported by Santa Barbara Coastal Long Term Ecological Research project under National Science Foundation Cooperative Agreement # OCE-1831937, the Schmidt Foundation Mentorship Award, UCSB, Worster Family Award, UCSB, Faculty Research Grant, UCSB, and National Science Foundation Graduate Research Fellowship Program to E.A.H. Any opinions, findings or recommendations expressed in this article are those of the author(s) and do not necessarily reflect the view of the National Science Foundation.
Author information
Authors and Affiliations
Contributions
All authors conceptualized and designed this study, contributed to data collection, and validated the data. K.K. performed the formal analysis, curated data, and produced figures. Resource acquisition and formal administration of the project was led by E.J.E. The manuscript was first drafted by K.K and all authors edited, reviewed, and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kraskura, K., Hardison, E.A. & Eliason, E.J. Body size and temperature affect metabolic and cardiac thermal tolerance in fish. Sci Rep 13, 17900 (2023). https://doi.org/10.1038/s41598-023-44574-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44574-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.