Abstract
By affecting core neurobiological systems early in development, early life adversities (ELAs) might confer latent vulnerability to future psychopathologies. This coordinate-based meta-analysis aims to identify significant convergent alterations in functional connectivity of the amygdala related to ELAs across resting-state and task-based fMRI-studies. Five electronic databases were systematically searched until 22 October 2020, retrieving 49 eligible studies (n = 3162 participants). Convergent alterations in functional connectivity related to ELAs between the amygdala and the anterior cingulate cortex (ACC) and left hippocampus were found. Sub-analyses based on hemisphere and direction showed that connectivity seeded in the right amygdala was affected and, moreover, revealed that connectivity with ACC was decreased. Analyses based on paradigm and age showed that amygdala-ACC coupling was altered during resting state and that amygdala–left hippocampus connectivity was mostly affected during task-based paradigms and in adult participants. While both regions showed altered connectivity during emotion processing and following adverse social postnatal experiences such as maltreatment, amygdala-ACC coupling was mainly affected when ELAs were retrospectively assessed through self-report. We show that ELAs are associated with altered functional connectivity of the amygdala with the ACC and hippocampus. As such, ELAs may embed latent vulnerability to future psychopathologies by systematically affecting important neurocognitive systems.
Similar content being viewed by others
Introduction
The potential impact of unfavourable environmental conditions during childhood is well established across literature. By affecting core neural networks for threat, stress and autobiographical memory processing early in development, exposure to such early life adversities (ELAs) may shape one’s vulnerability to future psychopathologies1,2,3,4,5. After all, these neural recalibrations might serve to cope with the negative environments during childhood, but may become maladaptive under certain conditions later in life2,6. Both human and animal research into the neurobiological correlates of ELAs has highlighted the amygdala as key convergence site3,4,7,8. In general, amygdala activity supports emotion processing and salience detection, particularly stimuli that are associated with danger9,10. As these processes critically rely on continuous interactions between the amygdala and other sensory and regulatory brain regions, ELAs may impact these dynamic networks as well. Indeed, extensive evidence has linked alterations in amygdala-prefrontal circuits, playing a central role in integrating information and regulating emotional responses11,12,13,14, to ELAs3,4,5,15,16,17,18,19,20—ranging from prenatal stress exposure to childhood maltreatment. Similarly, altered connections between the amygdala and the hippocampus, synergistically involved in stress responsiveness and emotional memory consolidation21,22,23,24,25, have also been reported in relation to ELAs3,26,27,28,29,30. As changes in these important circuits have been observed across stress-related psychopathologies (e.g. Refs.22,31,32,33), this may suggest a mediating role of these circuits in conferring risk for pluripotent transdiagnostic trajectories in relation to ELAs.
Alterations in amygdala connectivity in participants with ELAs have been highly heterogeneous. This pertains not only to the direction of amygdala coupling3 but also to the target regions20. Likewise, original studies often prioritized investigating the effects on amygdala coupling to specific regions of interest and thereby potentially overlook broader effects on a whole-brain level (e.g. Ref.3). In this study, we therefore conducted a quantitative summary of individual findings on a whole-brain level to capture these heterogeneous findings. By pooling data from multiple studies and analysing coordinates of altered connectivity, we aimed to comprehensively evaluate the variability and convergence of findings across different regions to identify consistent patterns of affected amygdala connectivity in relation to ELAs. Following current recommendations for coordinate-based meta-analyses (CBMAs)34,35,36, this study is the first to decipher the overall effect of ELAs on amygdala network connectivity. In this context, we consider ELAs more broadly as developmental risk factors acting early in life and therefore included both prenatal exposures (e.g. substance exposure) and postnatal experiences (e.g. childhood maltreatment or poverty). In line with our previous meta-analyses on neural alterations related to ELAs4,5, we performed a coordinate-based meta-analysis using activation likelihood estimation (ALE; for an explanation, see Ref.37)36,38,39 to consolidate this yet inconclusive literature and assess robust effects of altered amygdala connectivity across samples and analytic approaches. Based on the seed-based amygdala connectivity literature (for a recent review, see Ref.3), we hypothesize that particularly the amygdala-PFC circuit and amygdala-hippocampus circuit are affected in relation to ELAs. As such, these functional connectivity phenotypes might serve as stratification markers for early detection and treatment of the potentially long-lasting effects of ELAs throughout life.
Results
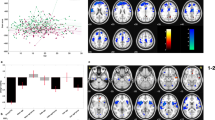
The global ALE analysis on the overall effect of ELAs was based on 45 experiments (3162 participants) and revealed convergence within the anterior cingulate cortex (ACC; L ACC: ALE-value: 0.0180, Z-score: 3.99; R ACC: ALE-value: 0.0167, Z-score: 3.78) (Fig. 1A, Table 1) and the left hippocampus (ALE-value: 0.0236, Z-score: 4.76) (Fig. 1B, Table 1).
ALE analyses for functional alteration in connectivity with the amygdala as seed-region revealed two significant clusters of convergence, with the first within the (A) ACC (BA24), with peaks at [− 6, 36, 8], ALE = 0.0180 and at [2, 38, 10], ALE = 0.0167, and the second cluster within the (B) left hippocampus ([− 30, − 26, − 10], ALE = 0.0236). The ALE map was computed in MNI152 and thresholded (pcluster-level < 0.05, puncorrected < 0.001). L = left, R = right. ALE analyses were conducted using GingerALE 3.0.2 (https://www.brainmap.org/ale/) and results were visualized using Mango 4.1 (https://mangoviewer.com/).
Additional sub-analyses were performed if a sufficient number of experiments (n ≥ 17) was available35. Separating the analyses by paradigm (resting-state or task-based) indicated convergent altered coupling of the amygdala with the ACC during resting-state (25 experiments, n = 1725; ALE-value: 0.0163, Z-score: 4.16) and with the left hippocampus during task-based paradigms (22 experiments; n = 1601; ALE-value: 0.0204; Z-score: 4.92) in relation to ELAs. Both regions showed altered connectivity in the context of emotion processing as well (21 experiments, n = 1569; L hippocampus: ALE-value: 0.0204, Z-score: 4.97; L ACC: ALE-value: 0.0125, Z-score: 3.69). Analyses based on direction of altered connectivity (increase or decrease) revealed significant convergence within the ACC (30 experiments, n = 2175; L ACC: ALE-value: 0.0146, Z-score: 3.87; R ACC: ALE-value: 0.0138, Z-score: 3.76) reflecting a consistent decrease in connectivity in relation to ELAs, while no cluster for increase was found (32 experiments, n = 2065). Separating by hemisphere revealed convergence within the left hippocampus related with ELAs (ALE-value: 0.0214, Z-score: 4.93) and within the ACC (L ACC: ALE-value: 0.0174, Z-score: 4.34; R ACC: ALE-value: 0.0140, Z-score: 3.74) for connectivity analyses seeded in the right amygdala (31 experiments, n = 2175), but no cluster appeared for the left amygdala (35 experiments, n = 2592). Subdividing the experiments by age (children or adults), revealed convergence within the left hippocampus for adults (21 experiments, n = 1683; ALE-value: 0.023, Z-score: 5.11) and within the left ACC for children (24 experiments, n = 1479; ALE-value: 0.0155; Z-score: 4.18). Only including experiments with healthy participants (29 experiments, n = 2207), to ensure no confounding effects of psychopathology, corroborated two clusters within the left ACC (ALE-value: 0.0174 and 0.0153, Z-score: 4.12 and 3.76) and one within the left hippocampus (ALE-value: 0.0209, Z-score: 4.65).
Separate analyses per ELA-subtype revealed convergence in the left hippocampus for postnatal adverse social experiences such as maltreatment and trauma in adults (20 experiments, n = 1642; ALE-value: 0.0231, Z-score: 5.09), which also resulted in convergently decreased connectivity with the ACC in relation to ELAs (21 experiments, n = 1669; ALE-value: 0.0142, Z-score: 3.90). No additional convergence was revealed for further sub-specifications of these ELAs. Lastly, differentiating based on the type of assessment indicated that amygdala-ACC connectivity was altered in relation to ELAs in studies relying on subjective self-reports (26 experiments, n = 1861; ACE-value: 0.0146, Z-score: 3.66) and in participants that were assessed retrospectively (31 experiments, n = 2061; ACE-value: 0.0146, Z-score: 3.62).
Functional decoding confirmed the involvement of these clusters during positive and negative emotion processing, as well as higher cognitive functions (see supplement and Fig. S1).
Discussion
Our meta-analysis provides a comprehensive overview of neuroimaging studies investigating the association of ELAs with brain connectivity using the amygdala as seed-region. We have demonstrated robust evidence for decreased amygdala-ACC and altered amygdala-hippocampus connectivity in connection with ELAs, that support a certain level of equifinality of ELAs in these neural adaptations40. As such, this study complements previous meta-analyses reporting neural alterations associated with ELAs4,5,41 and offers more insight into the mechanisms of how ELAs might become embedded into the human brain.
The overall analysis revealed significant alterations in functional connectivity related to ELAs between the amygdala and the ACC, as well as between the amygdala and the left hippocampus—which is in line with previous studies3,4,5,18,42,43,44. Subsequent sub-analyses were applied to further specify these results by restricting them based on paradigm, direction, hemisphere, age, disease status, ELA-subtype or -assessment.
For the ACC, these sub-analyses revealed a predominant decrease in amygdala-ACC connectivity mainly arising from the right amygdala during resting-state related to postnatal, social ELAs in healthy participants—especially when these ELAs were retrospectively assessed through self-report. This has several implications. First, as the ACC can be recruited to inhibit negative emotional processing in the amygdala14, decreased connectivity between the amygdala and ACC might indicate that the effectivity of this emotional stress resolution is affected in relation to ELAs—which may result in altered stress reactivity22,45,46,47 and memory extinction48. Second, as the right amygdala is mainly responsible for global, dynamic detection of (negative) emotional stimuli due to a faster habituation rate49,50,51,52, this further suggests that ELAs are related to altered automatic, emotional stress responses, which may lead to a more sustained emotional responding49,53. This aspect is corroborated by the fact that altered amygdala-ACC connectivity associated with ELAs primarily arises during the resting-state paradigm, a measure of intrinsic brain connectivity29. Overall, the altered resting-state connectivity pattern seems to be consistent across developmental stages, which is in line with previous studies23. Moreover, alterations in functional connectivity between the amygdala and ACC, as well as the hippocampus for that matter, are particularly associated with postnatal social ELAs, such as maltreatment. This intuitively makes sense: severe and prolonged trauma experiences can have severe (neural) consequences, and are correlated in space and time—a line of reasoning that is supported by literature3,54. It must however be taken into account that most of the studies investigated postnatal social ELAs and that the planned analysis for either socio-economic ELAs or prenatal exposures could not be performed due to an insufficient number of experiments. Lastly, the observation of altered amygdala-ACC connectivity primarily in individuals who self-report ELAs, might reinforce the hypothesis that such alterations contribute to enhanced cognitive biases that intensify the subjective evaluation of ELAs. This complements recent findings on different risk pathways for prospective and retrospective assessments55, and a superiority of the impact of subjectively experienced ELA-burden on the development of psychopathology56.
For the hippocampus, the sub-analyses further specified the results to an altered connectivity between right amygdala–left hippocampus. This alteration was predominantly observed in task-based experiments involving healthy adults who experienced postnatal, social ELAs. Interestingly, contributors to the task-based effect mainly employed an emotion processing paradigm, which further implicates this link between amygdala and hippocampus in emotional memory processing21. It also raises the assumption that ELAs might foster vulnerability to future psychopathologies via increased emotional memory consolidation21,22. The observation that this altered connectivity in relation to ELAs mainly arises in adult participants parallels previous literature showing hippocampal alterations in adults, but not in children57. Given the positive correlation between ELAs and future stress throughout life58, it might be that this cumulative effect of stress only manifests itself in adulthood—probably mediated by chronic stress-induced hippocampal glucocorticoid exposure33,47,59,60,61.
Multiple theoretical frameworks have tried to capture the range of relationships between ELAs and neural adaptations. These include the latent vulnerability framework2, implicating that changes in neurocognitive systems in relation to ELAs, reflecting an adaptation to these negative early environments, alter one’s vulnerability to future mental health problems. In later life, exposure to stress or challenge might unveil these vulnerabilities, thereby manifesting as clinical symptoms. Furthermore, the allostatic load model56 suggests that intense and enduring exposure to adversities can disrupt the body's ability to maintain homeostasis, resulting in a dysregulation of the stress response56. In addition, the cumulative stress model62 emphasizes the accumulation of stressors over time, implying that the combined effect of multiple stressors can have a significant impact on health outcomes. Overall, these models are not mutually exclusive in conceptualizing the complex and currently incompletely understood nature and consequences of ELAs, and instead might influence and complement each other. For example, chronic stress exposure during childhood can alter the allostatic load of physiological systems, such as amygdala-hippocampal coupling. These changes may act as latent vulnerabilities, thereby modifying responses to future stressors2 and in turn impacting the allostatic load even more. Another conceptualization is the dimensional model of adversity, implicating that different stressors, such as threat- and deprivation-related ELAs, might act on qualitatively different mechanisms to increase the risk for specific psychopathologies20,63,64,65. While intriguing, this framework remains to be elucidated by future meta-analyses, given that most of the included studies encompass multi-faceted adversities with only few studies making use of this suggested dimensional approach63. Importantly, in order to establish a clear, coherent and consistent model to conceptualize the neural adaptations in relation to ELAs in its entirety, the understanding of the exact mechanisms by which ELAs impact the brain should be advanced in future neurodevelopmental studies.
It is important to highlight the heterogeneous nature of ELAs. It comprises many exposures that are often interlinked with each other and share commonalties3,66. As such, the ultimate effect of ELAs reflects an intricate interplay between the exposure and one´s characteristics, such as genetic make-up and personality factors, and its socioenvironmental embedding, e.g. social support67. So while we aimed to stratify the results on amygdala connectivity as much as possible, we must acknowledge that—by following stringent guideline criteria for inclusion34,35,68—the number of available experiments was insufficient for further identification of potential moderators, such as sex, other subtypes including psychopathology, timing of ELAs, prenatal ELAs or any specification of direction within the sensitivity analyses. Concerning prenatal ELAs, while their exclusion yielded no significant result in the pooled analysis, further direction-, task-, and sample-specific sub-analyses do reveal the same convergence clusters in the ACC and the hippocampus (Table 1), supporting the robustness of the presented results. We also acknowledge that the effects—including the potential teratogenic effects69—of prenatal substance exposure, such as marijuana or cocaine, on the developing brain may differ from those of other ELAs, like maltreatment or poverty. However, as both types of ELAs are linked to changes in similar brain areas4,70,71, as well as to altered amygdala connectivity72, this seems to suggests some level of equifinality. Future studies should further look into this. Of note, a potential limitation might be that no correction for multiple testing across all sensitivity analyses was performed, as it was considered as too conservative for this purpose.
Furthermore, due to the heterogeneity and interrelatedness of the different types of ELAs included in this meta-analysis, our findings may rather point to the amygdala as being a nosologically unspecific network hub targeted by many kinds of adversities with effects being present independent of specific samples. Thus, its affected connections to the hippocampus and ACC reflect alterations suggestive of transdiagnostic phenotypes that may imply a latent vulnerability signature, which unfolds during system-challenging stressful situations2. This understanding is well in line with therapeutic studies that show neurotrophic changes in the amygdala following electroconvulsive therapy73,74 across (patient) samples or other methodological considerations74. In light of this, we speculate that these recalibrations of amygdala connectivity as reported here might represent shared mechanisms of ELAs, that may be considered as a transdiagnostic risk correlate.
Given the recent discussion on different risk pathways dependent on ELA assessment55,75, the number of studies permitted a separate analysis on subjective and retrospectively assessed ELAs. However, the specification of neural embedding of ELAs that were either objectively or prospectively assessed was not possible and should therefore be focused in future meta-analyses. As the convergent effects reported in our work were mainly driven by findings from healthy participants and are thus not confounded by psychopathology, they should be assumed to reflect latent vulnerability signatures as similar alterations have been reported in clinical populations76. However, we cannot rule out that these altered neural phenotypes in relation to ELAs might be unrelated to psychological functioning altogether nor that they might reflect compensatory mechanisms supporting adaptive functioning later in life. After all, such recalibrated responses may be either adaptive or maladaptive based on environmental conditions6.
Previous studies indicate that amygdala-prefrontal connectivity develops with age, with the occurrence of a valence shift in task-related amygdala-prefrontal connectivity around the age of 10 years77. We therefore ensured that our result of decreased amygdala-ACC coupling still holds when participants under 10 years of age are excluded (see Table S5)—even though it must be noted that the literature is not consistent in reporting such age-related alterations of functional connectivity78,79,80. To further investigate the normative developmental pattern of this circuit, as well as to clearly delineate whether the neural recalibration associated with ELAs—mostly seen in children and adolescents—presents a delay of maturation or an acceleration, longitudinal studies are warranted5.
Taken together, our current meta-analysis provides robust evidence for decreased amygdala-ACC and altered amygdala-hippocampus connectivity in relation to ELAs. These results are in line with previous research (for a recent review, see Ref.3) and fits well within the theoretical framework of latent vulnerability2. This inherent neural plasticity to environmental exposures also holds a promise, as it might potentially enable normative recalibration and thereby promote resilience67,81,82,83. While initial evidence does exist in relation to the reversibility of the structural and functional alterations associated with ELAs4,67,84, future (longitudinal) studies should examine this neural malleability in light of potential therapeutic interventions.
Methods and materials
This meta-analysis was preregistered with PROSPERO (CRD42018107773) and integrates all neuroimaging studies on the relation between ELAs and task-specific and resting-state brain connectivity using the amygdala as seed region. The study was conducted according to the PRISMA guidelines and current consensus guidelines for CMBAs34,35,36.
All screening, evaluation and data extraction procedures were performed by two independent authors (EK, NH) to reduce the chances of selection bias, and disagreements were resolved by consensus. The review protocol and data can be accessed upon request.
Search strategy and study selection
Relevant articles published until October 2020 were identified through a comprehensive literature search using five databases (EMBASE, MEDLINE, PsychINFO/PsychARTICLES, Scopus and Web of Science). Search strategies were composed of the search terms ‘neuroimaging’ or ‘MRI’ AND ‘preterm birth’, ‘prenatal exposure’ or ‘adverse childhood experience’ with associated synonyms, using the keywords appropriate to each individual database (for full search terms, see Supplementary Table 1). In line with the definition of ELAs as deviations from the expected environment that require adaptation85 and that brain development does not start at birth but at conception86, we conceptualized ELA in this manuscript as developmental risk factors acting early in life and therefore included both pre- and postnatal exposures and specified the analyses in further steps (see below). Additional articles were identified by reference tracking of all included studies and consultation of relevant review articles17,19,71,87,88.
Studies were selected if (1) peer-reviewed, original articles were published in English language; (2) human brain connectivity was measured using the amygdala as seed-region; (3) prenatal exposures and/or postnatal experiences were assessed; and (4) whole-brain results with stereotactic coordinates were reported or if not, provided by the authors. As such, from the 7195 unique publications that were initially identified, 119 publications were included in the qualitative synthesis (Fig. 2; a detailed overview of the included and excluded studies is in Table 2 and Supplementary Table 2, respectively).
Data extraction and quality assessment
The following variables were extracted: bibliographic information, sample characteristics (e.g. sample size, age and sex) and methodological specifics regarding ELA assessment and fMRI procedures (e.g. acquisition, paradigm and analyses) (see Table 2). Criteria for quality assessment were based on best-practice guidelines21,22. Studies passed the stringent quality assessment if (1) sample characteristics were properly described; (2) ELA subtype and assessment was reported; (3) details about fMRI paradigm (resting-state or task-based) and acquisition (scanner, settings and seed-region) were provided; and (4) details about fMRI processing (motion correction), analyses (whole-brain and where applicable, contrast(s) of interest) and results (coordinates of peak foci of activation in MNI or Talairach space) were provided. When details were not explicitly mentioned in the article itself, corresponding authors were contacted.
After quality assessment, 49 unique publications were included in this meta-analysis, which comprised 3162 participants (weighted mean = 19.93 years), with 6 studies (12%) reporting exposure to prenatal adversities and 43 studies (88%) reporting exposure to postnatal adversities (Fig. 2; a detailed overview of the included and excluded studies is in Table 2 and Supplementary Table 2, respectively). Participants’ age for prenatal studies (n = 335) ranged from 30.1 days to 16.6 years (weighted mean = 3.12 years), with 47% identified as male and 100% reported as healthy. Participants’ age for postnatal studies (n = 2946) ranged from 77.8 days to 40.6 years (weighted mean = 21.8 years), with 47% identified as male and 58% reported as healthy. Of these postnatal studies, 44% reported on functional connectivity of children (n = 1479, weighted mean = 11.9 years), and 56% of that of adults (n = 1683, weighted mean = 29.02 years). Twenty-six (53%) studies reported on a task-based paradigm, with 96% using emotion recognition and processing tasks.
Analytical procedures
Coordinate-based ALE analyses89 were conducted using GingerALE 3.0.2 based on CBMA consensus guidelines34,35,36. Activation likelihood estimation is amongst the most common algorithm for CMBAs and determines the convergence of reported coordinates across different experiments. This analysis considers activation foci not as single point but rather as centers of spatial probability distributions. An activation likelihood map is created based on the voxel-by-voxel union of these distributions, tested for statistical significance against randomly generated sets of foci36,89. ALE is a reliable way of combining results from multiple studies38 and was used successfully in previous CMBAs on the neurological consequences of ELAs (e.g. Refs.4,5).
First, Talairach coordinates were converted to MNI coordinates using the Lancaster transform (icbm2tal) function in GingerALE. For each analysis, coordinates from separate contrasts were grouped into one experiment to limit within-group effects39. As the samples of several studies (partially) overlapped, coordinates were organized by subjects to limit within-subject effects as well38. Full-width at half maximum (FWHM) was subject-based89 and the modeled activation maps were computed using as more conservative mask size. Cluster-level interference thresholding was used based on uncorrected p-values (p < 0.001), with a cluster-level family wise error corrected threshold of p < 0.05 and 1000 permutations. Results were visualized using Mango 4.1.
A global ALE analysis was performed across all experiments (n = 49 studies) to assess the relation between ELAs and functional brain connectivity irrespective of direction, hemisphere or paradigm. Regarding the latter, the joint analysis across heterogeneous designs, i.e. task-dependent and task-independent, reflects alterations of network connectivity across several mental states that are internally and externally determined. In a further step, we therefore specified the analyses into task-based (n = 22 studies) and resting-state (n = 27 studies) to delineate whether reported changes reflect common disturbances in neural mechanisms or paradigm-specific effects90. Additional sub-analyses based on direction (decrease: n = 31 studies, increase: n = 34 studies), hemisphere (left amygdala seed: n = 37 studies, right amygdala seed: n = 33 studies), age (adults: n = 23 studies, children: n = 26 studies), and disease status (healthy: n = 31 studies) were performed to further specify the effect (see Supplementary Table 4 for included studies per analysis). Moreover, separate sub-analyses on social ELAs (n = 35 studies) only, such as maltreatment, trauma, violence exposure as well as negative parenting, and on all postnatal ELAs excluding prenatal adversities were carried out (for a complete overview, see Supplementary Tables 3 and 4). Also, given recent evidence of a stronger link of subjective when compared to objective reports75 and that prospectively and retrospectively assessed populations may follow different risk pathways55, such sub-analyses were added as well (subjective self-report: n = 29 studies and retrospective report: n = 34 studies).
Subsequently, the resulting ALE clusters were functionally decoded using all eligible BrainMap experiments (n = 19,044) on healthy subjects coded in terms of all behavioral domains (cognition, action, perception, emotion, and interoception) and paradigm classes to avoid preselection bias91,92,93,94. Of note, this database reflects activity and not connectivity, and emotion regulation is not included. For functional characterization, we considered forward inference using a binomial test (significant at p < 0.05) that determines in which domains and classes the probability of finding activation in the respective cluster [P (Activation|Task)] was significantly higher than the overall chance, i.e., across the entire database [P(Activation)].
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Kessler, R. et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 197(5), 378–385 (2010).
McCrory, E. & Viding, E. The theory of latent vulnerability: Reconceptualizing the link between childhood maltreatment and psychiatric disorder. Dev. Psychopathol. 27(2), 493–505 (2015).
Holz, N. E. et al. Early social adversity, altered brain functional connectivity, and mental health. Biol. Psychiatry. 93(5), 430–441 (2023).
Kraaijenvanger, E. et al. Impact of early life adversities on human brain functioning: A coordinate-based meta-analysis. Neurosci. Biobehav. Rev. 113, 62–76 (2020).
Pollok, T. M. et al. Neurostructural traces of early life adversities: A meta-analysis exploring age- and adversity-specific effects. Neurosci. Biobehav. Rev. 135, 104589 (2022).
Fenneman, J., Frankenhuis, W. E. & Todd, P. M. In which environments is impulsive behavior adaptive? A cross-discipline review and integration of formal models. Psychol. Bull. 148(7–8), 555–587 (2022).
Zhang, W., Zhang, J., Holmes, A. & Pan, B. Amygdala circuit substrates for stress adaptation and adversity. Biol. Psychiatry. 89(9), 847–856 (2021).
Bilek, E. et al. Deficient amygdala habituation to threatening stimuli in borderline personality disorder relates to adverse childhood experiences. Biol. Psychiatry. 86(12), 930–938 (2019).
Phelps, E. & LeDoux, J. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48(2), 175–187 (2005).
LeDoux, J. E. Brain mechanisms of emotion and emotional learning. Curr. Opin. Neurobiol. 2(2), 191–197 (1992).
Etkin, A., Egner, T. & Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15(2), 85–93 (2012).
Cieslik, E., Mueller, V., Eickhoff, C., Langner, R. & Eickhoff, S. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci. Biobehav. Rev. 48, 22–34 (2015).
Gasquoine, P. Localization of function in anterior cingulate cortex: From psychosurgery to functional neuroimaging. Neurosci. Biobehav. Rev. 37, 340–348 (2013).
Shackman, A. et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 12, 154–167 (2011).
Tost, H., Champagne, F. A. & Meyer-Lindenberg, A. Environmental influence in the brain, human welfare and mental health. Nat. Neurosci. 18(10), 1421–1431 (2015).
McLaughlin, K. A., Weissman, D. & Bitrán, D. Childhood adversity and neural development: A systematic review. Annu. Rev. Dev. Psychol. 1(1), 277–312 (2019).
Herzberg, M. & Gunnar, M. Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. Neuroimage 209, 116493 (2020).
VanTieghem, M. & Tottenham, N. Neurobiological programming of early life stress: Functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr. Top. Behav. Neurosci. 38, 117–136 (2018).
Teicher, M., Samson, J., Anderson, C. & Ohashi, K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 17(10), 652 (2016).
Colich, N., Rosen, M., Williams, E. & McLaughlin, K. Biological aging in childhood and adolescence following experiences of threat and deprivation. Psychol. Bull. 146(9), 721–764 (2020).
Phelps, E. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 14(2), 198–202 (2004).
De Quervain, D., Schwabe, L. & Roozendaal, B. Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nat. Rev. Neurosci. 18, 7–19 (2017).
Rozendaal, B., McEwen, B. & Chattarji, A. Stress, memory and the amygdala. Nat. Rev. Neurosci. 10, 423–433 (2009).
Yang, Y. & Wang, J. Z. From structure to behavior in basolateral amygdala-hippocampus circuits. Front. Neural Circuits. 31, 11 (2017).
Pitkänen, A., Pikkarainen, M., Nurminen, N. & Ylinen, A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: A review. Ann. N. Y. Acad. Sci. 911(1), 369–391 (2006).
Vaisvaser, S. et al. Neural traces of stress: Cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front. Hum. Neurosci. 7, 313 (2013).
Ghosh, S., Laxmi, R. & Chatterji, S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J. Neurosci. 33(17), 7234–7244 (2013).
van der Werff, S. J. A. et al. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol. Med. 43(9), 1825–1836 (2013).
Brady, R. G. et al. The effects of prenatal exposure to neighborhood crime on neonatal functional connectivity. Biol. Psychiatry. 92(2), 139–148 (2022).
DeCross, S. N., Sambrook, K. A., Sheridan, M. A., Tottenham, N. & McLaughlin, K. A. Dynamic alterations in neural networks supporting aversive learning in children exposed to trauma: Neural mechanisms underlying psychopathology. Biol. Psychiatry. 91(7), 667–675 (2022).
Shin, L. & Liberzon, I. The neurocircuitry of fear, stress and anxiety disorders. Neuropsychopharmacology 35, 169–191 (2009).
Satterthwaite, T. et al. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivity. Mol. Psychiatry. 21(7), 894–902 (2016).
Lupien, S., McEwen, B., Gunnar, M. & Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009).
Müller, V. et al. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161 (2018).
Eickhoff, S. et al. Behavior, sensititivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 15(137), 70–85 (2016).
Eickhoff, S., Bzdok, D., Laird, A., Kurth, F. & Fox, P. Activation likelihood estimation meta-analysis revisited. Neuroimage 59(3), 2349–2361 (2012).
Cortese, S., Castellanos, F. & Eickhoff, S. What are neuroimaging meta-analytic procedures?. Epidemiol. Psychiatr. Sci. 22(2), 121–123 (2013).
Turkeltaub, P. et al. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 33(1), 1–13 (2012).
Turkeltaub, P., Eden, G., Jones, K. & Zeffiro, T. Meta-analysis of functional neuroanatomy of single-word reading: Method and validation. Neuroimage 16(3), 765–780 (2002).
Cicchetti, D. & Rogosch, F. Equifinality and multifinality in developmental psychopathology. Dev. Psychopathol. 8, 597–600 (1996).
Doretto, V. & Scivoletto, S. Effects of early neglect experience on recognition and processing of facial expressions: A systematic review. Brain Sci. 6(1), e10 (2018).
Rinne-Albers, M. et al. Anterior cingulate cortex grey matter volume abnormalities in adolescents with PTSD after childhood sexual abuse. Eur. Neuropsychopharmacol. 27(11), 1163–1171 (2017).
Calem, M., Bromis, K., McGuire, P., Morgan, C. & Kempton, M. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage 14, 471–479 (2017).
Zhai, Z. et al. Childhood trauma moderates inhibitory control and anterior cingulate cortex activation during stress. Neuroimage 185, 111–118 (2019).
Liberzon, I. & Abelson, J. Context processing and the neurobiology of post-traumatic stress disorder. Neuron 92(1), 14–30 (2016).
Logue, M. et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatry. 83, 244–253 (2018).
Frodl, T. & O’Keane, V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function an hippocampal structure in humans. Neurobiol. Dis. 52, 24–37 (2013).
Phelps, E., Delgado, M., Nearing, K. & LeDoux, J. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43(6), 897–905 (2004).
Wright, C. et al. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. NeuroReport 12, 379–383 (2001).
Glascher, J. & Adolphs, R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J. Neurosci. 23, 10274–11082 (2003).
Markowitsch, H. Differential contribution of right and left amygdala to affective information processing. Behav. Neurol. 11, 233–244 (1998).
Wager, T., Luan Phan, K., Liberzon, I. & Taylor, S. Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. Neuroimage 19, 513–531 (2003).
Sergerie, K., Chochol, C. & Armony, J. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 32(4), 811–830 (2008).
Herzog, J. & Schmahl, C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front. Psychiatry. 9, 420 (2018).
Baldwin, J., Reuben, A., Newbury, J. & Danese, A. Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiat. 76(6), 584–593 (2019).
Danese, A. & McEwen, B. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 106(1), 29–39 (2012).
Tottenham, N. & Sheridan, M. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front. Hum. Neurosci. 3, 68 (2010).
Monninger, M. et al. The long-term impact of early life stress on orbitofrontal cortical thickness. Cereb. Cortex 30(3), 1307–1317 (2020).
Sapolsky, R., Uno, H., Rebert, C. & Finch, C. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 10, 2897–2902 (1990).
Seckl, J., Dickson, K., Yates, C. & Fink, G. Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Res. 561, 332–337 (1991).
Gilbertson, M. et al. Smaller hippocampal volume predicts pathological vulnerability to psychological trauma. Nat. Neurosci. 5, 1242–1247 (2002).
McLaughlin, K. A., Sheridan, M. A., Humphreys, K. L., Belsky, J. & Ellis, B. J. The Value of dimensional models of early experience: Thinking clearly about concepts and categories. Perspect. Psychol. Sci. 16(6), 1463–1472 (2021).
Sheridan, M., Shi, F., Miller, A., Salhi, C. & McLaughlin, K. Network structure reveals clusters of associations between childhood adversities and development outcomes. Dev. Sci. 23(5), e12934 (2020).
LeMoult, J. et al. Meta-analysis: Exposure to early life stress and risk for depression in childhood and adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 59(7), 842–855 (2019).
Miller, A. et al. Dimensions of deprivation and threat, psychopathology, and potential mediators: A multi-year longitudinal analysis. J. Abnorm. Psychol. 127(2), 160–170 (2018).
Smith, K. & Pollak, S. Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspect. Psychol. Sci. 16(1), 67–93 (2020).
Holz, N., Tost, H. & Meyer-Lindenberg, A. Resilience and the brain: A key role for regulatory circuits linked to social stress and support. Mol. Psychiatry. 25, 1–18 (2020).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. & Groiup, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6(7), e1000097 (2009).
Little, B., Sud, N., Nobile, Z. & Bhattacharya, D. Teratogenic effects of maternal drug abuse on developing brain and underlying neurotransmitter mechanisms. Neurotoxicology 86, 172–179 (2021).
Salzwedel, A., Chen, G., Chen, Y., Grewen, K. & Gao, W. Functional dissection of prenatal drug effects on baby brain and behavioral development. Hum. Brain Mapp. 41(17), 4789–4803 (2020).
Pulli, E. et al. Prenatal exposures and infant brain: Review of magnetic resonance imaging studies and a population description analysis. Hum. Brain Mapp. 40(6), 1987–2000 (2019).
Grewen, K., Salzwedel, A. P. & Gao, W. Functional connectivity disruption in neonates with prenatal marijuana exposure. Front. Hum. Neurosci. 9, 601 (2015).
Camilleri, J. et al. Electroconvulsive therapy modulates grey matter increase in a hub of an affect processing network. Neuroimage Clin. 25, 102114 (2020).
Janouschek, H. et al. Meta-analytic evidence for volume increases in the medial temporal lobe after electroconvulsive therapy. Biol. Psychiatry. 90(4), e11–e17 (2021).
Danese, S. & Widom, C. Objective and subjective experiences of child maltreatment and their relationships with psychopathology. Nat. Hum. Behav. 4(8), 811–818 (2020).
Kaul, D., Schwab, S., Mechawar, N. & Matosin, N. How stress physically re-shapes the brain: Impact on brain cell shapes, numbers and connections in psychiatric disorders. Neurosci. Biobehav. Rev. 124, 193–215 (2021).
Gee, D. G. et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 110(39), 15638–15643 (2013).
Zhang, Y., Padmanabhan, A., Gross, J. & Menon, V. Development of human emotion circuits investigated using a big-data analytic approach: Stability, reliability, and robustness. J. Neurosci. 39(36), 7155–7172 (2019).
Gabard-Durnam, L. et al. The development of human amydala functional connectivity at rest from 4 to 23 years: A cross-sectional study. Neuroimage 15(95), 193–207 (2014).
Jalbrzikowski, M. et al. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: Associations with anxiety and depression. Biol. Psychiatry. 82(7), 511–521 (2017).
Cowan, C., Callaghan, B., Kan, J. & Richardson, R. The lasting impact of early-life adversity on individuals and their descendants: Potential mechanisms and hope for intervention. Genes Brain Behav. 15(1), 155–168 (2016).
Harrison, E. & Baune, B. Modulation of early stress-induced neurobiological changes: A review of behavioural and pharmacological interventions in animal models. Transl. Psychiatry. 4, e390 (2014).
Leal, A. & Silvers, J. Neurobiological markers of resilience to early-life adversity during adolescence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 6(2), 238–247 (2021).
van Wingen, G. et al. Persistent and reversible consequences of combat stress on the mesofrontal circuit and cognition. PNAS 109(38), 15508–15513 (2012).
Sun, Y., Fang, J., Wan, Y., Su, P. & Tao, F. Association of early-life adversity with measures of accelerated biological aging among children in China. JAMA Netw. Open. 3(9), e2013588 (2020).
Nelson, C. The implications of early adversity even before birth. JAMA Netw. Open. 3(1), e1920030 (2020).
Morie, K., Crowley, M., Mayes, L. & Potenza, M. Prenatal drug exposure from infancy through emerging adulthood: Results from neuroimaging. Drug Alcohol Depend. 198, 39–53 (2019).
Rotem-Kohavi, N., Williams, L. & Oberlander, T. Advanced neuroimaging: A window into the neural correlates of fetal programming related to prenatal exposure to maternal depressoin and SSRIs. Semin. Perinatol. 44(3), 151223 (2020).
Eickhoff, S. et al. Coordinate-based ALE meta-analysis of neuroimging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30(9), 2907–2926 (2009).
Nickl-Jockschat, T. et al. Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Struct. Funct. 220(4), 2355–2371 (2015).
Laird, A. et al. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 29(46), 14496–14505 (2009).
Wensing, T. et al. Neural correlates of formal thought disorder: An activation likelihood estimation meta-analysis. Hum. Brain Mapp. 38, 4946–4965 (2017).
Cortese, S. et al. Functional decoding and meta-analytic connectivity modeling in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 80(2), 896–904 (2016).
Fox, P., Lancaster, J., Laird, A. & Eickhoff, S. Meta-analysis in human neuroimaging: Computational modeling of large-scale databases. Annu. Rev. Neurosci. 37, 409–434 (2014).
Acknowledgements
This work was gratefully supported by the German Research Foundation (Grant number DFG HO 5674/2-1, GRK2350/1; N.E.H. and T.B.) and the Ministry of Science, Research and the Arts of the State of Baden-Württemberg, Germany (Special support program SARS CoV-2 pandemic) as well as the Radboud Excellence Fellowship to N.E.H. Further, T.B. gratefully acknowledges grant support by the German Federal Ministry of Education and Research (01EE1408E ESCAlife; FKZ 01GL1741[X] ADOPT; 01EE1406C Verbund AERIAL; 01EE1409C Verbund ASD-Net; 01GL1747C STAR; 01GL1745B IMAC-Mind), by the German Research Foundation (TRR 265/1), by the Innovative Medicines Initiative Joint Undertaking (IMI JU FP7 115300 EU-AIMS; grant 777394 EU-AIMS-2-TRIALS) and the European Union—H2020 (Eat2beNICE, Grant 728018; PRIME, Grant 847879). SBE acknowledges funding by the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” and the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 945539 (HBP SGA3). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
N.E.H. was responsible for study concept and design. E.J.K. and N.E.H. conducted the literature search. E.J.K., N.E.H. and S.B.E. analysed the data and all authors drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
EJK, NEH and SBE report no financial relationships with commercial interests. TB served in an advisory or consultancy role for Actelion, Hexal Pharma, Lilly, Lundbeck, Medice, Novartis and Shire. He received conference support or speaker’s fees from Lilly, Medice, Novartis and Shire. He has been involved in clinical trials conducted by Shire and Viforpharma. He received royalties from Hogrefe, Kohlhammer, CIP Medien and Oxford University Press. All other authors do not have any competing interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kraaijenvanger, E.J., Banaschewski, T., Eickhoff, S.B. et al. A coordinate-based meta-analysis of human amygdala connectivity alterations related to early life adversities. Sci Rep 13, 16541 (2023). https://doi.org/10.1038/s41598-023-43057-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43057-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.