Abstract
This study aimed to investigate the relationship between venous blood parameters and respiratory functions in patients with amyotrophic lateral sclerosis (ALS) and develop a model to predict respiratory impairment for individual patients with ALS. A total of 416 ALS patients were included in the study, and various hematologic and biochemical laboratory parameters as well as demographic and clinical factors were collected and compared. A multivariable logistic regression model was constructed to assess the association between FVC and venous blood biomarkers and clinical factors. The results showed that along with onset age, bulbar-onset, disease duration, BMI, eosinophil count (EO#), basophil count (BASO#), creatinine (CREA), uric acid (URCI) and low-density lipoprotein cholesterol/high-density lipoprotein cholesterol (LDL/HDL) ratio were associated with reduced FVC. The area under the ROC curve is 0.735 for the test set and 0.721 for the validation set. The study also developed a relatively acceptable model for predicting respiratory impairment in ALS patients. These findings suggest that EO#, BASO#, CREA, URIC and LDL/HDL ratio can be useful in assessing FVC in ALS and can be easily accessible, accurate, and low-cost parameters.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder with a variable natural history, which affects 0.6–3.8 per 100,000 person/year worldwide1. The initial clinical presentation and evolution of ALS is heterogeneous2. First symptoms usually occur in the fifth or sixth decade of life1. Most patients die within 3–5 years after onset, generally due to respiratory failure3. Thus, monitoring respiratory function is of significant importance for ALS patients4, 5.
Recently, several biochemical parameters have been demonstrated to be related to respiratory impairment6,7,8,9,10. EMPOWER study founded a weak longitudinal correlation between vital capacity and plasma creatinine6. Adriano Chiò et. al. suggested that lower albumin and creatinine (CREA), levels were strongly related to forced vital capacity (FVC)7. And cross-sectional study from Japan suggested that FVC was associated with serum levels of total cholesterol, low-density lipoprotein cholesterol (LDL), CREA, and urate8. Study from USA and Poland also showed that plasma CREA correlated with FVC9, 10. These studies suggested that there were some correlations between hematologic and biochemical laboratory parameters and respiratory function. However, the latest study did not confirm a correlation between plasma CREA and FVC11. The contradictory results suggested that further studies of blood biomarkers and FVC are needed.
The aim of the present study was to analyze a potential utility of readily available, relatively inexpensive, and rapid to determine laboratory parameters in the assessment of respiratory impairment in patients with ALS; and to develop and validate a model of hematologic and biochemical parameters for predicting respiratory impairment for patients with ALS.
Methods
Ethics statement
This study protocol was reviewed and approved by Ethics Committee of West China Hospital, Sichuan University, approval number 2020-842 and 2021-799, and all the participants signed written informed consent. All protocols and procedures of our research were conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations.
Patients
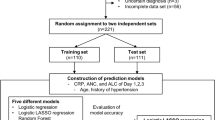
Patients from two cohorts were included in our study. In the retrospective cohort, 319 patients with ALS were enrolled from 1 January 2015 to 30 December 2020. And in the prospective cohort, 97 participants from 1 March 2017 to 30 October 2021 were enrolled. Our medical center was a large, regional referral ALS clinic located in Southwest China. The diagnosis of ALS was made according to the revised-El Escorial criteria for probable or definite ALS12, 13. Patients with other medical or neurological diseases and patients with missing baseline hematologic and biochemical values were excluded. Patients with manifestations of neoplastic disorders or patients who could not perform respiratory function were also excluded.
Clinical variables
The clinical analysis included age, sex, age at ALS onset, disease duration, onset body region (limb/bulbar), diagnostic level (probable/definite), blood pressure and comorbidities. We extracted data on comorbidities present at diagnosis as listed in medical records. Charlson comorbidity index (CCI) was used to evaluate comorbid conditions14. Weight and height were measured following international guidelines, and body mass index (BMI) was calculated as weight (kg)/height (m)2 at the time of pulmonary function tests. High blood pressure was defined as systolic blood pressure (SBP) above 140 mmHg and/or diastolic blood pressure (DBP) above 90 mmHg in this study.
Venous blood parameters were obtained using an automated hematology analyzer (KX-21 N, Sysmex America, Lincolnshire, IL, USA). Hematologic data on platelet count (PLT), white blood count (WBC), neutrophil count (NEUT#), lymphocyte count (LYMPH#), monocyte count (MONO#), eosinophil count (EO#), and basophil count (BASO#) as well as and biochemical parameters on albumin (ALB), glucose (GLU), urea (UREA), creatinine (CREA), uric acid (URIC), triglyceride (TG), cholesterol (CHOL), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and creatine kinase (CK) were obtained. PLR was calculated as the ratio of PLT to LYMPH# and NLR as the ratio of NEUT# to LYMPH#. LDL/HDL was measured as the ratio of LDL to HDL. FVC was measured using a standard volumetric spirometer, with the patient in a standing position. Spirometer was quality controlled using 2005 criteria15. The spirometry methods produce comparable measurements of forced vital capacity (FVC)16. At least three acceptable manoeuvres were performed. Obstructive physiology was defined as the presence of airflow limitation with FEV1/FVC < 0.7017. For participants without airflow limitation (FEV1/FVC ≥ 0.7), restrictive physiology was operationalized as FVC < 80% predicted17.
Statistical analysis
(1) Correlation analysis: Continuous variables with normal distribution were reported as mean ± standard deviation; non-normal variables were presented as median (interquartile range [IQR]). The linear correlations of these variables and the FVC were calculated by Spearman. For categorical variables, the correlations were performed by the nonparametric test (Wilcoxon rank sum test). For normal distribution data, differences were performed by Independent Samples T-Test. For non-normal distribution, differences were assessed for significance using the Mann–Whitney test or chi-squared tests. (2) The training set and the test set: The retrospective cohort was divided into two independent sets by random sampling: a training set and a test set. The training set included 70% (220/319) of the retrospective cohort. Four participants with missing data were deleted. The test set included 30% (95/319) of the retrospective cohort. Models were developed using the characteristics in the training set. To verify the model, the test set was used to validate the models for internal validation, and the prospective cohort for external validation, respectively. In order to improve the robustness of the model, fivefold cross-validation was used. And down sampling method was used to deal with the class imbalance problem. (3) Logistic regression: Logistic regression was used to construct a model to predict FVC. For factors among each other (NEUT# vs. WBC, onset.age vs. age, LDL vs. CHOL) with rho > 0.75 in correlation analysis, only one factor was screened out in the next stage of the analysis. For groups of factors (weigh, height and BMI; NEUT#, LYMPH#, and PLR; PLT, LYMPH#, and NLR; LDL, HDL and LDL/HDL; SP, DP and hypertension) with strong clinical correlation, only BMI, PLR, NLR, LDL/DHL and hypertension were screened out for the next stage of analysis. Correlation analysis between each variable and FVC was carried out to determine factor for logistic regression. Then, a total of 23 variables including gender, disease duration, onset age, site of onset, diagnostic level, CCI, blood pressure category (hypertension or not), BMI, WBC, NLR, PLR, MONO#, EO#, BASO#, ALB, GLU, UREA, CREA, URIC, TG, CHOL, LDL/HDL, and CK were included to construct the model. Based on the results of backward stepwise regression method combined with Akaike information criterion (AIC), the equation of FVC for predicting respiratory impairment in ALS patients were established. Heatmap of FVC was depicted. Receiver operating characteristic (ROC) curves was used to assess the prediction accuracy of the model. All statistical analyses were performed using SPSS 24.0 (IBM, Chicago, IL, USA) and R 4.1.0 (www.rproject.org).
Results
There were 92 male patients (69.70%) with FVC < 3.062 and 40 female patients (30.30%) with FVC < 2.266 in the retrospective cohort (Table 1). In the prospective cohort, there were a similar percentage of male (66.67%) and female (33.33%) ALS individuals with abnormal FVC (Table 1). There were significant differences in age, onset age, weight,
BMI, PLR, TG and HDL when stratified by FVC either in the retrospective cohort or in the prospective cohort (all P < 0.05, Table 1). Statistically significant correlations were observed between older age, later onset age, lower weight, lower BMI, lower levels of PLR and TG, and higher levels of HDL with reduced FVC.
To further analyze the correlation between these factors and FVC, we developed a multivariable logistic regression model for the association between the hematologic, biochemical laboratory parameters, clinical factors and FVC in ALS patients in the test set. The regression equation was created from the Estimate values obtained by z-value and was presented in Table 2. Moreover, we constructed a heatmap to show the relation between these variables in the equation and FVC (Fig. 1).
Heatmap of FVC in this study. The grey histogram represents values of FVC. Below the histogram are factors in the logistic regression model equation. The brightness of the color varied upon the value for continuous variables. FVC forced vital capacity, BMI body mass index, BASO# basophil count, EO# eosinophil count, CREA creatinine, URIC uric acid, LDL low-density lipoprotein cholesterol, HDL high-density lipoprotein cholesterol, LDL/HDL the ratio of LDL to HDL.
Model FVC = 0.022 + 0.199 BMI + 1.029 EO# + 16.960 BASO# + 0.043 CREA level + 0.004 URIC level + 0.260 LDL/HDL + 0.021 disease duration + 0.101 Onset.age + 0.839 Site.of.onset 2 (bulbar onset) (Table 2).
Receiver operating characteristic (ROC) curve with 9 predictive variables revealed that the area under the curve was 73.5% in the test set (Table 3, Fig. 2). And the ROC curve has standard error of 0.013 with 95% confidence interval as 0.710–0.760 in the test set. To validate the model, we tried to predict the data from the prospective cohort and calculated the ROC curve, which yielding a concordance statistic of 0.721 (95% CI 0.616–0.825) (Table 3, Fig. 3).
Discussion
Respiratory failure is the main cause of death in ALS patients3. It is of significance to find some easily accessible, accurate, and low-cost parameters to assess the respiratory function. We created and validated a multivariable logistic regression model for the association between FVC and the venous blood biomarkers and clinical factors in ALS patients. Our single-site study found that FVC related to hematologic and biochemical laboratory parameters. EO#, BASO#, CREA, LDL /HDL and URIC are easily accessible, accurate, and low-cost parameters useful in assessment of the FVC in ALS.
To the best of our knowledge, there are few studies about models predicting respiratory function in ALS patients11, 18. In our model, onset age, site of onset, disease duration, BMI, CREA, LDL /HDL and URIC were factors previously reported to predict respiratory impairment6,7,8,9, 18. In addition, EO# and BASO# were two new features predicting to FVC in ALS. This prediction model could be useful in clinical settings in which the respiratory function is not available.
Older age and bulbar onset are consistently reported to have poorer outcomes19. BMI, onset age and disease duration were factors correlated with ALS prognostication either in retrospective or in prospective studies20,21,22,23. Recently, a large multinational study with participants aged 40 years and over founded that low BMI was one of the most influential risk factors for chronic airflow obstruction24, which implicated a potential reason for low BMI associated with impaired FVC in this study.
Lower level of CREA was related to impaired FVC in ALS in this study, which was consistent with previous studies7,8,9. In these studies, Ken Ikeda et.al found that the annual decline of FVC ≥ 30% was significantly linked to baseline serum levels of CREA8. Serum CREA is a product of nonenzymatic catabolism of creatine phosphate in muscles, and is transported from muscle through the circulation to the kidneys25.
A decreased level of plasma CREA in ALS patients is expected because of the variation in muscle mass observed in these patients. Moreover, CREA levels are correlated with lean body mass in healthy individuals26 and with BMI in ALS individuals7.
The mean TG level was significantly lower among patients with a lower FVC but not statistical significance at multivariable analysis. We confirmed that decreased LDL/HDL ratio was correlated with decreased FVC, as reported by previous studies8, 18. Whereas in a Dutch study, authors found that mean CHOL and LDL levels were lower in patients with FVC < 70%. Higher serum LDL/HDL ratio was correlated with increased survival27. Study from French reported that serum levels of CHOL and LDL were significantly increased in ALS patients, and the elevation of LDL /HDL ratio was associated with prolonged survival28. Ethical and environmental backgrounds may lead to different lipid levels in patients with ALS. Multicenter, prospective study are needed to elucidate the relationship between lipid and respiratory function.
Study from Japan showed that the rapid worsening of annual FVC was associated with serum levels of URIC8. Interestingly, the level of URIC was not statistically significant between FVC normal group and FVC lower group but independently related to decreased FVC at multivariable analysis in our study. Study from USA found strong hazard ratios relating plasma CREA and ALSFRS-R when using trajectories of all three measures of plasma CREA, plasm URIC, and ALSFRS-R to predict time to death. These studies suggest complex biochemical interactions exist in ALS patients. More studies are warranted to better understand the metabolic mechanisms of disease progression in ALS.
Peripheral EO# is an important clinical biomarker in the management of asthma and chronic obstructive pulmonary disease (COPD)29, 30. For persistent childhood asthma patients, normal lung function and serum EO# at baseline are clinical prognostic indicators of remission by adulthood31. For COPD patients, counts of 4% or greater or 300 cells per μL or more might identify a deleterious effect of inhaled corticosteroids withdrawal32. Furthermore, retrospective cohort study also shown that ALS patients concomitant with COPD relates to poor outcome23. One possibility of EO# predicting respiratory in this study is that some ALS patients coexisting with COPD, the other possibility is that ALS patient concomitant with some indirect factors which correlates with EO# and FVC. Further studies are warranted to understand the mechanism underpinning the association of EO# and FVC in ALS.
In respiratory disease, BASO# plays a distinct role in the pathogenesis of allergic and nonallergic33. Recently, authors found that the number of circulating BASO# was significantly elevated in patients with aspirin-exacerbated respiratory disease34. Except for respiratory disease, Katie Lunnon et al. have found increased numbers of BASO# in people with MCI and AD, Yet the basophil counts are within the normal acceptable range35. More studies are needed to clarify the mechanisms of BASO# and respiratory impairment in ALS and other neurodegenerative disorders.
Our results should be interpreted with caution and a number of limitations should be borne in mind. Firstly, the prediction model derived from the retrospective cohort could not analyze data potentially important to risk of FVC. For example, eosinophils and uric acid values measured at admission may be affected by the other conditions coexisted with ALS, which we did not analyze. Secondly, possible ALS patients were excluded in this study, thus the prediction model is only suitable for probable and definite ALS patients. Thirdly, the model should be tested on multicenter, prospective cohorts to study the validity and predictability.
In conclusion, our study indicates that EO#, BASO#, CREA, LDL/HDL and URIC might be important ALS biomarkers and may be used to assess FVC in ALS patients when lung function was not available. This finding needs to be validated in prospective, multi-site studies.
Data availability
All data generated or analysed during this study are included in this published article. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Longinetti, E. & Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 32(5), 771–776 (2019).
Eisen, A. et al. Cortical influences drive amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 88(11), 917–924 (2017).
Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers. 3, 17071 (2017).
Soriani, M. & Desnuelle, C. Care management in amyotrophic lateral sclerosis. Revue neurologique 173(5), 288–299 (2017).
Andersen, P. et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)-revised report of an EFNS task force. Eur. J. Neurol. 19(3), 360–375 (2012).
Küffner, R. et al. Crowdsourced analysis of clinical trial data to predict amyotrophic lateral sclerosis progression. Nat. Biotechnol. 33(1), 51–57 (2015).
Chiò, A. et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: A population-based study. JAMA Neurol. 71(9), 1134–1142 (2014).
Ikeda, K., Hirayama, T., Takazawa, T., Kawabe, K. & Iwasaki, Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: A cross-sectional study. Intern. Med. (Tokyo, Japan) 51(12), 1501–1508 (2012).
Mitsumoto, H. et al. Plasma creatinine and oxidative stress biomarkers in amyotrophic lateral sclerosis. Amyotroph. Later. Scler. Frontotemporal Degener. 21, 263–272 (2020).
Chełstowska, B. & Kuźma-Kozakiewicz, M. Biochemical parameters in determination of nutritional status in amyotrophic lateral sclerosis. Neurol. Sci. 41(5), 1115–1124 (2020).
Morgadinho, J. et al. Plasma creatinine level does not predict respiratory function in amyotrophic lateral sclerosis. J. Neuromuscul. Dis. 8, 795–799 (2021).
Brooks, B. R., Miller, R. G., Swash, M. & Munsat, T. L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Later. Scler. Other Mot. Neuron Disord. 1(5), 293–299 (2000).
Agosta, F. et al. The El Escorial criteria: Strengths and weaknesses. Amyotroph. Later. Scler. Frontotemporal Degener. 16, 1–7 (2015).
Charlson, M., Pompei, P., Ales, K. & MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40(5), 373–383 (1987).
Miller, M. et al. Standardisation of spirometry. Eur. Respir. J. 26(2), 319–338 (2005).
Wever, A., Britton, M., Hughes, D., Van der Plas, K. & Wever-Hess, J. Clinical evaluation of five spirometers. Monaghan M403, Pneumoscreen, Spirotron, Vicatest and Vitalograph. Eur. J. Respir. Dis. 62(2), 127–137 (1981).
Bhatt, S. et al. Discriminative accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA 321(24), 2438–2447 (2019).
Chiò, A. et al. Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology 73(20), 1681–1685 (2009).
Couratier, P. et al. Epidemiology of amyotrophic lateral sclerosis: A review of literature. Revue neurologique 172(1), 37–45 (2016).
Moglia, C. et al. Early weight loss in amyotrophic lateral sclerosis: Outcome relevance and clinical correlates in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 90(6), 666–673 (2019).
Yu, C. et al. The clinical assessment of amyotrophic lateral sclerosis patients’ prognosis by ZNF512B gene, neck flexor muscle power score and body mass index (BMI). BMC Neurol. 18(1), 211 (2018).
Ngo, S., Steyn, F. & McCombe, P. Body mass index and dietary intervention: Implications for prognosis of amyotrophic lateral sclerosis. J. Neurol. Sci. 340, 5–12 (2014).
Requardt, M., Görlich, D., Grehl, T. & Boentert, M. Clinical determinants of disease progression in amyotrophic lateral sclerosis-A retrospective cohort study. J. Clin. Med. 10(8), 1623 (2021).
Burney, P. et al. Prevalence and population attributable risk for chronic airflow obstruction in a large multinational study. Am. J. Respir. Crit. Care Med. 203, 1353–1365 (2020).
Wyss, M. & Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 80(3), 1107–1213 (2000).
Swaminathan, R., Ho, C., Chu, L. & Donnan, S. Relation between plasma creatinine and body size. Clin. Chem. 32(2), 371–373 (1986).
Sutedja, N. et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 82(6), 638–642 (2011).
Dupuis, L. et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70(13), 1004–1009 (2008).
Korevaar, D. et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: A systematic review and meta-analysis. Lancet Respir. Med. 3(4), 290–300 (2015).
Roche, N. et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment data from the FLAME trial. Am. J. Respir. Crit. Care Med. 195(9), 1189–1197 (2017).
Wang, A., Datta, S., Weiss, S. & Tantisira, K. Remission of persistent childhood asthma: Early predictors of adult outcomes. J. Allergy Clin. Immunol. 143(5), 1752-1759.e1756 (2019).
Watz, H. et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: A post-hoc analysis of the WISDOM trial. Lancet Respir. Med. 4(5), 390–398 (2016).
Salter, B. et al. Expression of activation markers in circulating basophils and the relationship to allergen-induced bronchoconstriction in subjects with mild allergic asthma. J. Allergy Clin. Immunol. 137(3), 936-938.e937 (2016).
Stevens, W. et al. Studies of the role of basophils in aspirin-exacerbated respiratory disease pathogenesis. J. Allergy Clin. Immunol. 148(2), 439-449.e435 (2021).
Lunnon, K. et al. Mitochondrial dysfunction and immune activation are detectable in early Alzheimer’s disease blood. J. Alzheimer’s Dis. JAD 30(3), 685–710 (2012).
Acknowledgements
We thank the Amyotrophic lateral sclerosis patients for their participation in our study.
Funding
This work was supported by the Basic Conditions Platform Construction Project of Sichuan Science and Technology Department (2019JDPT0015), the 1.3.5 Project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18003), the National Key Research and Development Program of China (2018YFC2000400), the National Natural Science Foundation of China (91849118, 31760299, and 82260289) and the Miaozi Project in Science and Technology Innovation Program of Sichuan Province (2020JDRC0057).
Author information
Authors and Affiliations
Contributions
X.H.H. and J.Y. conceptualization, data curation, formal analysis, methodology, software, validation, visualization, writing-original draft, and writing-review and editing. H.Y.H. data curation, formal analysis, methodology and funding acquisition. J.M.F., X.L.D., Q.Z.Z. and Q.Y.S. data curation, formal analysis and methodology. Y.M.X. and C.Y.H. conceptualization, funding acquisition, project administration, supervision and writing-review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, X., Yang, J., Feng, J. et al. Venous blood parameters in determination of respiratory impairment in amyotrophic lateral sclerosis. Sci Rep 13, 15695 (2023). https://doi.org/10.1038/s41598-023-42075-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42075-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.