Abstract
The increasing demand for natural products and biotechnological activities from bees facilitate their widespread use in food preservation and beneficial effects on humans. This study aimed to prepare and characterize the nano-capsules of Qaluiobia (PQG) governorates propolis extracted with water, ethanol and supercritical fluid-carbon dioxide at 50 °C with co-solvent. Propolis bioavailability was analyzed and introduced to prepare crackers to extend their shelf life. Nano-encapsulation was examined using transmission electron microscopy (TEM), differential scanning calorimetry (DSC) and antioxidant activity. Ethanol and supercritical fluid-carbon dioxide (SCF-CO2) at 50 °C with ethanol as co-solvent recorded higher yield, antioxidant activities, total phenolics and total flavonoids. SCF-CO2 extracts had a higher flavonoid concentration. It was revealed that propolis nano-capsules had high-temperature stability and cytotoxic effects against the three tested human cancer cell lines (i.e. PC3, MCF7 and HePG2). The higher overall acceptability of crackers fortified with PQG was achieved with SCF-CO2 at 50 °C and ethanol extract nano-capsules, i.e. 86.57% and 86.29% respectively. The higher ability to retain antioxidant activity reduces the increase of peroxide value (PV), preventing rancidity and increasing the shelf life of crackers during the storage period. Practical application: This study can provide a suitable method for extracting bioactive compounds from propolis, and improve the biological properties and activities by nano-encapsulation, also reveals the extent of its use as a natural antioxidant and anticancer and its application in bakery products as a functional food.
Similar content being viewed by others
Introduction
Propolis is a complex resinous produced by honey bees and has highly different physical characteristics depending mainly on the environmental conditions1. It is well known that the biochemical compounds in propolis contain phenolic compounds, vitamins, carbohydrates, hydrocarbons and carboxylic acids, mainly responsible for therapeutic effects and high antioxidant activity2,3.
Raw propolis is not soluble in water and is extracted by solvents. Propolis is purified to preserve its polyphenolic fraction. These polyphenolic compounds are essential for healing effects4. The ingredients extracted from propolis exhibit many biological activities and health benefits and can be used as functional ingredients in food and medicine4.
Propolis is complex and contains 30% wax, 50% resin (mainly flavonoids and phenolic acid derivatives), 10% aromatic oils, 5% other organic residues and 5% pollen5. The chemical composition of propolis, especially related to its components and their polarity, makes it challenging to use in a free or non-encapsulated form in various applications. The extraction yield of bioactive components depends on the extraction methods and organic solvents (i.e. methanol, chloroform, ethanol and ethyl-ether). Many researchers have studied propolis processing using conventional methods6,7. However, despite its use, it has some shortcomings, mainly due to the quality of processing resulting in the presence of solvent residues in the final product.
Supercritical fluid (SCF) extraction is an innovative technology that has gained widespread interest due to increasingly restrictive environmental regulations (i.e. the use of low temperatures, reduced solvent use and energy consumption)8,9,10. This technology has spread in multiple industrial areas due to its unique advantages and SCF characteristics11,12. EPE has the potential to be used as a natural additive with antimicrobial and antioxidant characteristics in toast bread13. Still, SCF is a promising alternative technique in the fine chemistry, foodstuff fields and pharmaceuticals14.
Nanotechnology is an innovative technique for various applications in nano-food and nano-medicine. Nano-particles with encapsulation produce small size, large surface area and protection, with high bioavailability compared to their bulk counterparts15.
Nano-capsulation can be obtained using emulsification, high-pressure homogenization, evaporation, spray drying, ultrasonication, high-speed stirring, micro-emulsion and ball milling16.
This work evaluated Qaluibia (PQG) propolis extraction produced methods using extraction methods (i.e. water, ethanol 95% and one green technology SCF-CO2 at 50 °C) as the extractants were used. The physicochemical properties, nano-capsulation, cytotoxic activity of propolis and their application as functional bakery products were also studied.
Materials and methods
Materials and chemicals
Egyptian raw honey bee’s propolis was collected from Delta region Qaluiobia Governorate (PQG) and kept in dark sterile glass containers at room temperature until further use. All chemicals and reagents i.e. Folin–Ciocalteu reagent, 2,2-diphenyl-1-picryl hydrazyl radical (DPPH), Tween 20 (T20), Ethanol, Aluminum chloride, Potassium acetate, linoleic acid, Gallic acid, Sodium alginate, Butylated hydroxyltoluene (BHT), Sodium Thiocyanate, Ascorbic acid, Quercetin, Methanol, Phosphate buffer, Ammonium thiocyanate, Ferrous chloride, Potassium ferricyanide, Trichloroacetic acid, Ferric chloride, Sodium carbonate, Aluminum chloride, Formic acid, Acetonitrile, Phosphotungestic acid, Chloroform, Glacial acetic acid, Sodium thiosulphate were of analytical grade and purchased from Sigma Aldrich Chemical (Merck KGaA, Darmstadt, Germany).
The work does not involve humans or conduct experiments on live animals.
Methods
Extraction methods
Propolis water extracts (PWE)
Honey bees propolis powders (10 g) were suspended in 100 ml distilled water and shaken for 24 h at 24 °C. The suspension was then centrifuged at 3000 rpm for 20 min at 4 °C, and the supernatants were collected after several extractions (3 times) under the same conditions, dialyzed against distilled water, and lyophilized (LABCONCO, Freeze dryer, Console, 12 L −50 °C, 240 V, Catalog No. 7754030, USA)17. The yield of water extract was defined as the mass of water extract of propolis obtained by dry mass of raw propolis used in percentage.
Propolis ethanolic extracts (PEE)
Ethanolic extracts of propolis (PEE) were obtained using the method of Yong and Masaharu18 with some modification where, (10 g) propolis powder was mixed with 250 methanol (95%) at room temperature in a dark place for 24 h. The mixture was centrifuged (3000 rpm, for 10 min) to obtain the supernatant. The propolis yield was calculated based on the initial amount of dry propolis in percentage.
Propolis supercritical fluid carbon dioxide extracts (PSCF-CO2)
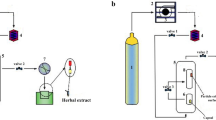
The extracts of propolis were carried out at temperatures of 50 °C and 250 bar pressure using a laboratory-scale unit in National Research Centre (Speed TM SFE-2/4, Applied separations, Built in conjunction with the USDA1- USA). Ten grams of propolis powder were mixed with ethanol 15% (w/w) as co-solvent. The CO2 was pumped into the reactor, which was supported by two: 300 vessels and kept for 30 min to allow complete contact and guarantee that the operational conditions of temperature and pressure were stabilized. The CO2 mass flow rate was 1.0 g/min. The samples were collected, and the process line was washed with the used ethanol to recover the extract deposited. The global extract yield Xo (%) (extraction + cleaning process) to the initial mass of raw material (drybases)19.
At the end of the extraction methods, the yield of propolis was collected and calculated as follows:
where: W 1 is the weight of propolis extracts (g) and W2 is the weight of dried raw propolis powder (g).
Preparation of nano-capsules
Ethanol and SCF-CO2 Propolis extracts were encapsulated into nano-forms using ultrasound and high-speed homogeniser (PRO, USA) methods, according to Vasiliki and Constantina20. Nano-emulsion was prepared by adding one gram propolis extract to 10 ml deionized water (water (1:1) with addition of 0.1% T20. The water was gradually added with stirring using a magnetic stirrer (2000 rpm at room temperature) to avoid the formulation of bubbles during mixing till complete dissolving, then homogenized using high-speed homogenizer (Model: 400ELPC, PRO Scientific Inc., 01-02411ELPC HOMOGENIZER, USA) at 18,000 rpm for 30 min in the presence of an ice water bath to reduce the temperature of the mixture. The sample was stored at 4 °C for 24 h before encapsulation. For encapsulation, 50 ml sodium alginate solution (prepared by adding 3 g sodium alginate to 100 ml deionized water and mixed by magnetic stirrer at 2000 rpm for 60 min and left in a refrigerator at 4 °C to form a gel) were gradually added to 10 ml nano extract emulsion (5:1) using homogenizer (2000 rpm, 10 min) and treated by ultrasound (Sonics & Materials Inc., Newton, Connecticut, USA) for 30 min at 30 °C then the encapsulated emulsion was packed in brown packages at 4 °C till use.
Chemical properties of propolis extracts and their nano-capsules
Total phenolic content (TPC)
The total phenolic content (TPC) of three extracts and their nano-capsules were determined spectrophotometrically using Folin-Ciocalteu reagent according to the method described by Ebrahimzadeh et al.21 with some modification. The extracted samples and their nano-capsules (0.5 ml) were mixed separately with Folin-Ciocalteu reagent (5 ml with distilled water at a rate of 1:10) for 3 min then; 3 ml of 2% sodium carbonate (1 M) was added. The mixture was left for 15 min, and the polyphenols were determined by an automated UV–VIS spectrophotometer at 765 nm and the results were calculated using a Gallic acid calibration curve (0–100 mg/l). The blank was prepared using the same procedure with 0.5 ml of pure water in place of the extract. The results are expressed as equivalents to Gallic acid (mg GAE/g dry extract).
Total flavonoid content (TFC)
The total flavonoid content (TFC) of three extracts and their nano-capsules were determined according to the method described by Huang et al.22, and Ebrahimzadeh et al.21, Nabavi et al.23. The propolis extracts and their nano-capsules (0.5 ml) were mixed separately with 1.5 ml methanol, 0.1 ml of 10% aluminium chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. They then left at room temperature for 10 min. The absorbance of the mixture was measured at 415 nm on a UV/visible spectrophotometer. The quercetin (µg/g) was used as a standard for the calibration curve.
Determination of radical-scavenging activity by (DPPH)
Radical scavenging activity of tested extracts ability was assayed using the method of Hatano et al.24. Different concentrations of three extracts (i.e.10, 20, 30, 40, 50 and 60 µg/ml) were added to reaction solution DPPH (1 ml) (0.2 mM). The mixture was shaken forcibly and left at room temperature for 30 min, and then the absorbance of the solution was measured spectrophotometrically at 517 nm.
As is the absorbance of the sample; Ac is the absorbance of control in the absence of the sample.
Nano-capsules of ethanol and SCF-CO2 with (60 µg/ml) were determined as above.
Reducing power (RP)
The reducing power (RP) of three extracts were determined following the method of Oyaizu25. Briefly, different concentrations of extracts (10, 20, 30, 40, 50 and 60 µg/ml) were mixed with 5 ml phosphate buffer (0.2 M, pH 6.6) and 2.5 ml potassium ferricyanide (1%) and compared with the same concentration of BHT and ascorbic acid in ethanol (95%) with 2.5 ml of sodium phosphate buffer (200 mM, pH 6.6). The reaction mixture was incubated at 50 °C for 20 min. Aliquots of 1% trichloroacetic acid (5 ml) were added to the mixtures, then centrifuged at 2000 rpm for 10 min, and the absorbance of the pink color mixture was recorded spectrophotometrically at 700 nm.
Nano-capsules of ethanol and SCF-CO2 with (60 µg /ml) were determined as above.
Total antioxidant activity (TAA)
Water, Ethanol and CO2 extracts total antioxidant activity (TAA) were determined using a linoleic acid system26. The reaction mixture at different extract concentrations (i.e.10, 20, 30, 40, 50 and 60 µg/ml) were separately treated with linoleic acid (0.13 ml), phosphate buffer 0.2 M (pH 7.0, 10 ml) and ethanol (99.8%). The mixture was adjusted to 25 ml by distilled water. Then incubated at 40 °C for 10 min and the oxidation rate was measured using thiocyanate method27. The obtained solutions were added to a mixture of 10 ml ethanol (75%) with 0.2 ml ammonium thiocyanate (30%), 0.2 ml of sample solution and 0.2 ml of ferrous chloride solution (20 mM in 3.5% HCl), stirring for 3 min and the peroxide value was measured using a spectrophotometer (JASCO, Corporation Model V-730, S.N. A112961798, Tokyo, Japan) at 500 nm. The percent inhibition of linoleic peroxidation is calculated as 100 – (Abs increase of sample/Abs increase of control X100). The commercial antioxidants butylated hydroxytoluene (BHT) and ascorbic acid were used.
Nano-capsules of ethanol and SCF-CO2 with (60 µg/ml) were determined as above.
Physical properties of nano-capsules
The nano-capsules formed were tested by their efficiency, yield, TEM, DSC effect as follows:
Encapsulation efficiency (EE)
Encapsulation efficiency (% encapsulation) was calculated following Busch et al.28 based on the total amount of phenolic in encapsulated propolis per total amount of phenolic in propolis extract. Encapsulation efficiency can be calculated by the following formula:
where x is the total amount of phenol in encapsulated propolis (%); Y is the total amount of phenol in propolis extract (%).
Encapsulation yield (EY)
The encapsulation yield (EY) is another factor to be considered during an encapsulation process. The effectiveness of encapsulation yield (EY) was measured using Paviani et al.19 method and calculated as follows:
Transmission electron microscopy (TEM)
Propolis extracts and their nano-capsules' morphological characteristics were tested by TEM (1400, JEOL, Japan) using Saloka et al.29 method. The nano-particles suspensions were dripped onto a 400-mesh copper grid coated with Forvar and stained by 2% phosphotungstic acid30. The samples were air-dried at room temperature for more than 2 h before analyzing on the TEM.
Thermal stability (DSC)
Thermal stability of propolis extract and their nano- capsules were measured using a differential scanning calorimeter (DSC) (Mettler Toledo, SWITZERLAND) according to Hazra et al.31 as follows. Ten milligrams of both samples were placed in aluminium crucibles under a flow of nitrogen gas (40 ml/min). A dynamic scan was performed at a heating rate of 10 °C/min over a temperature range of −150 to 300 °C. Evaporation enthalpies were calculated by peak area integration of DSC profiles, and the results were compared with the estimated vaporization enthalpy of major components.
LC–ESI–MS/MS analysis of propolis forms
The analysis of crude propolis, nano-capsules propolis extracts by slovent and SCF-CO2 at 50 °C was performed using liquid chromatography–electro-spray ionization–tandem mass spectrometry (LC–ESI–MS/MS) with an Exion LC/AC system for separation and SCIEX Triple Quad 5500+ MS/MS system equipped with an electro-spray ionization (ESI) for detection. The instrument data were collected and processed using the SCIEX OS 1.6.10.40973 software.
The separation of the targeted analyses was performed with a Discovery® BIO Wide Pore C18-5 Column (4.6 × 250 mm, 5 µm). The mobile phases were consisted of two eluents A: 0.1% formic acid in water; B: 0.1% formic acid in acetonitrile (LC grade). The mobile phase gradient was programmed as follows: 5% B at 0 min, 5–25% B from 0.0 to 60.0 min, 25–5% B from 60 to 65 min, 5% from 65 to 70. The flow rate was 1.0 ml/min and the injection volume was 5 µl. For MS/MS analysis, negative ionization mode was applied with a scan (EMS-IDA-EPI) from 150 to 800 Da with the following parameters: curtain gas: 25 psi; ion spray voltage: −4500; source temperature: 400 °C; ion source gas 1 & 2 were 55 psi.
Cytotoxic effect on human cell lines
Cell viability was assessed by the mitochondrial-dependent reduction of yellow MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] to purple formazan32.
Cells from (National Cancer Institute, Egypt) were suspended in DMEM-F12 medium (for HePG2, MCF7, and PC3) beside one normal cell line (BJ1), 1% antibiotic–antimycotic mixture (10,000 U/ml Potassium Penicillin, 10,000 µg/ml Streptomycin Sulfate and 25 µg/ml Amphotericin B) and 1% l-glutamine at 37 °C under 5% CO2.
Cells were batch cultured for 10 days, then seeded at concentration of 10 × 103 cells/well in fresh complete growth medium in 96-well microtiter plastic plates at 37 °C for 24 h under 5% CO2 using a water-jacketed CO2dioxide incubator (Sheldon, TC2323, Cornelius, OR, USA). Media was aspirated, fresh medium (without serum) was added, and cells were incubated either alone (negative control) or with different concentrations of sample to give a final concentration of (100–50–25–12.5–6.25–3.125–0.78 and 1.56 µg/mL). After 48 h of incubation, the medium was aspirated, 40 µL MTT salt (2.5 µg/mL) were added to each well and incubated for a further 4 h at 37 °C under 5% CO2. To stop the reaction and dissolve the formed crystals, 200 µL of 10% Sodium dodecyl sulfate (SDS) in deionized water were added to each well and incubated overnight at 37 °C.
A positive control which composed of 100 µg/ml was used as a known cytotoxic natural agent that gives 100% lethality under the same conditions33. The absorbance was then measured using a microplate multi-well reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA) at 595 nm and a reference wavelength of 620 nm. The percentage of change in viability was calculated according to the formula:
Using the SPSS 11 program, the IC50 was determined using probit analysis. The degree of selectivity of the synthetic compounds was expressed as SI = IC50 of the pure compound in a normal cell line/IC50 of the same pure compound in a cancer cell line, where IC50 is the concentration required to kill 50 percent of a cell population.
Application
Crackers preparation
With slight modification, five types of crackers were prepared according to the method of Benjakula and Karnjanapratum34. The control crackers were prepared using 62.5% wheat flour (72%), 1.16% salt, 0.23% sugar, 0.37% baking powder, 15% sunflower oil, 0.187% paprika and 20.25% water to produce the dough. The ingredients were mixed at a low speed for 3 min. The resulting cracker dough was sheeted to a thickness of a 0.4 mm and cut into a rectangle (2.4 × 7.3 cm2). The shaped cracker dough was baked in an electric oven at 120 °C for 30 min (SL-Shel-LAB-1370FX). In the same way, the remaining cracker samples were prepared with the same previous ingredients to produce 1.8 g of four types of crackers containing 0.6 g of nano-capsules of PEE (Propolis ethanolic extract), PSCF-E (Propolis Supercritical Extract at 50 °C), PNC-EE (Propolis Nano-Capsule Ethanolic Extract) and PNC-SCFE (Propolis Nano-capsule Supercritical Extract at 50 °C) (1.8 g capsule contain 0.6 g propolis extract of their nano-forms).
Sensory evaluation of crackers
All samples of the crackers were sensory assessed by ten-member panels for appearance, color, thickness, texture, shrinkage, taste and odor using the method described by Smith35.
Storage stability of crackers
The stability of the stored cracker samples (at 25 °C for 90 days) was determined every month compared to the control sample using the DPPH and PV previous described methods.
Determination of radical-scavenging activity using (DPPH)
The oils from each cracker sample were extracted by n-hexane (25 ml) that was evaporated after keeping it in a refrigerator for 24 h using rotary evaporator36. The antioxidant activity of the crackers’ residue was determined as described previously.
Determination of peroxide value (PV)
Peroxide value of all cracker samples fortified with propolis extracts and their nano-forms were determined by extracting the oil from samples with n-hexane, and then PV was performed according to AOAC36. Two mg of oil were added and mixed in a solution containing chloroform-glacial acetic acid (30 ml, 3:2 v/v) and sodium thiosulphate (1 ml, 0.1 M) until the disappearance of yellow color. PV (meq/kg) was calculated as follows:
where; C is sodium thiosulphate concentration (mol/l), V and VO volumes of sodium thiosulphate blank respectively (ml), and m is the mass of cracker sample extracts (mg).
Statistical analysis
Results were expressed as means ± standard deviation (n = 3) and ANOVA variance analysis with average comparison Duncan’s37 Multiple Range set to ˂0.05. All the statistical processes were conducted by the Statistical Package for Social Science (SPSS, V21.0) for Windows (SPSS, Inc., Chicago, IL, USA).
Results and discussion
Physical and chemical properties of propolis extracts
Efficiency of extraction yield methods
The yield of the different extraction methods was determined as tabulated in Table 1.
In the above Table, the propolis extraction yield differences were shown as affected by the three extraction methods. Significant differences among values (p ˂ 0.05) were found in the extracted yields of the brown propolis (PQG). Water extraction had a lower yield, due to the high polarity of water resulting from polarization of its molecule what makes this solvent suitable for extraction of lyphylic organic compounds present in propolis. Although the polarity of water was high, the yield was low because it was not the only factor affecting the extraction efficiency38. The yield of water extraction of PQG was 14.63 g/100 g dw and agreed with that obtained by Biscaia and Ferreira39, at 14.3%.
The highest yields obtained with SCF-CO2 with co-sovent at 50 °C and 250 bar for PQG was 24.26%. These high pressures increase the density and polarity of carbon dioxide which increase its capacity to extract more polar components19.
TPC and TFC of propolis extracts
The optimum phenolic content varied according to the plant types and active compounds40 in which Phenolic compounds can be classified into polar and weak-polar41. The most crucial feature of propolis extracts was polyphenols in their structure, which is an indication for high biological activity. The total contents of polyphenols and flavonoids in extracts of the propolis obtained using the three extraction methods are shown in Table 2.
Results showed that the highest TPC value for CO2 extract at 50 °C was 395.22 ± 4.1, followed by ethanolic sample 318.36 ± 3.8, while the water samples had the lowest activity.
When the total flavanoids amount (TFC) was examined, it was found that samples extracted with CO2 extractor at 50 °C had the highest flavonoid value being 745.42 ± 8. All supercritical extracts presented higher flavonoids concentration to the initial value found in three extracted method,). Flavonoid level in ethanol extract is lower than that extracted by CO2 as ethanol belongs to the group of less polar solvent. While, water extract showed lower value for TFC being 366.51 ± 3.9.
The total concentration of polyphenols or flavonoids was not the only factor responsible for antioxidant properties but also, the chemical nature of polyphenols and the presence of other compounds contributed to the overall antioxidant capacity of the extracts42.
Antioxidant activity of propolis extracts
DPPH, reducing power (RP), and total antioxidant activity (TAA)
The antioxidant properties determined using DPPH, reducing power methods and TAA of propolis extracts at different extracting methods in Figs. 1, 2 and 3 have been thoroughly investigated and compared. Propolis extracts were characterized by the high or similar values of scavenging activity, RP, and TAA when compared to the synthetic BHT and ascorbic acid in vitro studies. Propolis extracts DPPH scavenging activity ranged from about 51.16% to 96.54% using different extraction methods. PQG antioxidant activities in CO2 extract at 50 °C were higher than other extracts in Fig. 1.
Reducing power was related to antioxidant activity because antioxidants can give off their electrons to reduce reactive radicals in Fig. 2. Thus, the reducing power could indicate the antioxidative potential of prospective antioxidants. The increase in the absorbance values for the method of determining reducing power was due to the phytochemical components present.
At the same time, RP had a slight increase from 0.624 to 0.692 at ethanolic extract and from 0.472 to 0.484 (Abs. at 700 nm) for SCF-CO2.
SCF-CO2 extraction showed the highest value (92.17%) followed by ethanol being 85.09%. while; sample extracted by water had lower TAA content. These results were in agreement with those reported by Paviani et al.19. Generally, water extract showed lower values for TPC, TFC and TAA than the others extracted methods; therefore it was excluded from the nano procedure.
Antioxidant activity of Propolis nano-capsule bioactive compounds
Propolis nano-capsules had significant differences among values in Table 3 (p ˂ 0.05) and had the highest TPC and TFC values for CO2 extract at 50 °C equal to 381.86 GAE/g and736.92 mg PE/g, respectively. This was followed by the ethanolic sample (286.12 GAE/g and 561.58 mg PE/g, respectively.
The propolis extract obtained using ethanolic and supercritical fluid CO2 methods differ significantly between each other in terms of antioxidant activity (Table 3). Sodium alginate-propolis ethanolic extract nano-capsules significantly decreased by nearly 10%, 14%, 10, 15% and 10% for TPC, TFC, RP, DPPH and TAA, respectively than ethanolic free extracts. While, the percentage decrease between the SCF-CO2 extract and its nano-capsules was lower (3.5, 1, 2.5, 4 and 3%), respectively. The nano-capsules of SCF-CO2 extract maintained the stability of the bioactive compound despite the ethanolic extract in all antioxidants parameter.
Falcão et al.43 proved that European propolis samples (Russia and Italy) had similar polyphenol components and antioxidant activity of Brazilian propolis, which contained fewer polyphenols and fewer antioxidant properties.
In addition, SCF-CO2 presents good yields and nano-particles preserves the physico-chemical characteristics of the components to be extracted14, and improve the bioavailability of the polyphenolic compound44.
Physical properties of nano-capsules
Nano-capsules formed were tested by their efficiency, yield, TEM, DSC, LC–ESI–MS/MS and cytotoxic effect as follows:
Encapsulation efficiency (%)
The encapsulation efficiency of Propolis ethanol and SCF-CO2 at 50 °C extracts was 84.56% and 95.89%, respectively. This was in agreement with Chen et al.45, who reported an adequate encapsulation efficiency of at least 80%.
Encapsulation yield (%)
The encapsulation yield of Propolis SCF-CO2 extract at 50 °C was significantly higher than that of the ethanolic extract. The nano-capsules of PQG-SCF-CO2 extract at 50 °C were 95.89% more effective than ethanol extract (84.56%) based on the basic structure of total flavonoid (736.92). The nano-capsules were also more hydrophobic than the total phenols (381.86). The possibility that sodium ALg bound the hydroxyl groups found in flavonoids with hydrogen bonds46 was supported by these data.
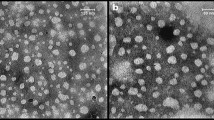
Transmission electron microscopy (TEM)
The morphology and particle size of the propolis nano ethanolic and SCF-CO2 at 50 °C extracts were measured using TEM. These results are shown in Figs. 4A, B. Propolis extracts had a large particle size in the micrometre range, with an average particle size between 0.03 and 0.09 μm for the ethanol extract and 0.02 to 0.07 μm for the SCF-CO2 extract at 50 °C. The propolis ethanol extract in sodium-ALg nano-particles was homogenous, had no aggregation, had a round shape and smooth surface and were discrete, with an average ranging from 18.87 to 39.12 nm, as shown using a TEM image in Fig. 4C. The SCF-CO2 extract at 50 °C had spherical shape, had no aggregation and homogenous, with an average ranging from 1.49 to 36.14 nm in Fig. 4D, compared to the encapsulated ethanol extract47. This result may be due to the anionic carboxylic groups of sodium-ALg, which caused strong electrostatic repulsion between the particles48.
Differential scanning calorimetry (DSC)
Three steep sorbents at temperatures representing melting points of 90.27, 128.64 and 134.11 °C are shown in the thermal schematic diagram DSC of Propolis in Fig. 5A. The extracted compounds represented a considerable area starting at 67.10 °C and ending at 123.62 °C. This sharp heat absorption peak starts from the melting point of the weak melt transition at 90.27 °C. Therefore, the extract in its free form (un-capsulated) could not be used in food applications as an additive because could not torelate high temperature49.
While the DSC thermogram of ethanol extract nano-capsules had four melting points in Fig. 5b (i.e. 107.24, 128.91, 178.05 and 184.82 °C), the Propolis tolerance ranged from 110.04 to 163.84 °C. It was more resistant to exposure to high temperatures than the un-capsulated Propolis sample, in which a crystalline state was not formed. This proved the success of the packaging process and the thermal stability against temperature47.
On the other hand, the SCF-CO2 nano-capsules had higher thermal stability than the other two samples. Three peaks with thermal exposure from 149.90 to 201.58 °C are shown in Fig. 5C, with a melting point of 167.67 °C. The ability of SCF-CO2 nano-capsules was proven by these results to be used in food applications, especially in bakery products.
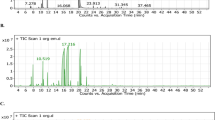
LC–ESI–MS/MS analysis of propolis nano-capsules
The polyphenolic profile of different extracts could be determined using LC–ESI–MS/MS50. The LC–ESI–MS/MS analysis of Propolis nano-capsules of the ethanol and SCF-CO2 extracts at 50 °C are shown in Fig. 6A–C and Table 4. Heneicosapentaenoic acid, ketotricla-bendazole and heneicosapentaenoic acid were detected in all three propolis forms.
The highest concentration of flavonoids and phenolics occurred in crude propolis i.e. glycetein, quercetin, kaemferol, ethyl p-coumarate, and 3,4-dimethoxycinnamic acid were observed in both crude propolis and nano-capsules SCF-CO2 extracts at 50 °C. The absence of most compounds was indicated in the ethanol extract. The presence of 30, 49, 50, 52, 56 and 66 derivatives in all propolis samples in Table 4 with a similar retention time (RT) was evidenced.
The chromatograms (A, B and C) contain the peaks corresponding with the generally expected phenolic compounds were found in nano-capsules from SCF-CO2 at 50 °C extracts in Fig. 6. The highest component was kaempferol in the crude propolis at an RT of 56 min, and the lowest component was in the ethanol extract of PQG (285.1507 and 215.3376 m/z), respectively in Fig. 6.
These compounds were observed in several types of propolis sourced from different parts of the world51.
The biological properties of propolis were confirmed due to its high phenolic and flavonoid content, making it a food ingredient with high antioxidant activity. These data indicate that propolis may play a critical role in health beneficial effects.
Cytotoxic effect of human cell lines
Egyptian propolis extracts were examined against the human tumour prostate cell line (PC3), human hepatocellular carcinoma cell line (HePG2) and human Caucasian breast adenocarcinoma (MCF7). The antitumoural effects of propolis on the three cells are documented in Fig. 7A, B.
(A,B) Anticancer effect of propolis extracts and their nano-capsules. IC50 lethal concentration of the sample that causes 50% death of PC3: prostate cell line; MCF7 human Caucasian breast adenocarcinoma, HePG2 human hepatocellular carcinoma cell line, positive control Adriamycin (doxorubicin) after 48 h.
Results in Fig. 7A showed that SCF-CO2 propolis extract nano-capsule increase in the inhibitory activity for the three cancer cells was 98.3, 72.3 and 55.4% for PC3, MCF7 and HePG2 respectively compared to ethanol extract and their nano-capsules being (25.3, 23.5 and 22.3%, respectively). These results indicated that there is a direct correlation between TPC concentration and cancer cell inhibition due to the presence of highest antioxidant compounds (most notably TPC and TFC)52.
On the other hand, IC50 described the lethal concentration of the sample which causes 50% death of cells in 48 h compared to the positive control (i.e. Doxorubicin, an anticancer drug for hematologic and solid tumors, was used as the positive control).
Results in Fig. 7B showed that the lower values belonged to SCF-CO2 propolis nano-capsule in the case of HePG2 (90.8 µg/mL), PC3 (99.2 µg/mL), and MCF7 (124.6 µg/mL) followed by SCF-CO2 extract for HePG2 cells (63.8 µg/mL), MCF7 cells (78.4 µg/mL) and PC3 cells (116.6 mg/mL).
While IC50 for ethanol extract was (44.6 µg/mL) for PC3 cells followed by (47 g/mL) and (75.9 µg/mL) for MCF7cells and PC3 respectively .The lower was for ethanol nano-capsule extract in all cells.
The recommendation of NCI stated that IC50 of crude extract, if incubated from 48 to 72 h, has cytotoxic activity in vitro less than 20 and 4 µg/ml pure compounds53.
Different types of honey and propolis extracts have been indicated in many reports to significantly inhibit cell growth and reduce the differentiation or proliferation of cells from various tumour cell lines54. Cancer cell inhibition should be evaluated to develop new anticancer drugs. Based on these criteria, nano-capsule CO2 propolis extracts were assessed for their inhibition as candidate anticancer cancer drugs for human hepatocellular, prostate and human Caucasian breast adenocarcinoma.
Application
Sensory evaluation of crackers fortified with propolis extracts
The sensory scores of crackers fortified with propolis extracts and their nano-capsules are shown in Table 5 and Fig. 8.
The control sample was characterized by the highest (p ˂ 0.05) total overall acceptability (86.84%) among the cracker samples tested (Table 5). At the same time, the lower scores of crackers fortified with Propolis ethanol extract than the two nano-capsules sample may be due to the gum taste of the propolis extract. The overall acceptability of crackers fortified with Propolis SCF-CO2 at 50 °C and ethanol extracts nano-capsules was 86.57 and 86.29, respectively.
These crackers (FE-EEN and FP-SCFN) obtained the highest score, probably because they acquired the fragility and porosity properties of the sodium alginate capsules and their ability to bind water and reduce its content55. For a long time, propolis bioactive ingredients have been used in many applications. Many new functional food products have appeared in the markets in recent years to respond to health problems faced by consumers.
Storage stability of fortified cracker samples
Antioxidant activity of stored crackers
The DPPH scavenging activity of the stored cracker samples fortified with propolis extracts and their nano-capsules were measured compared with the control samples during the storage period of 90 days, as shown in Table 6.
A clear and noticeable significant decrease in DPPH (p ˂ 0.05) between the fortified crackers with ethanol and SCF-CO2 at 50 °C extracts with increasing storage time are shown in Table 6. From zero to 90 days of storage, the decrease was between 54.58 and 36.18% for the ethanol extract and 76.84% and 48.33% for the SCF-CO2 extract. This was due to the high sensitivity of the un-capsulated extract to storage period. The cracker samples fortified with nano-capsules of ethanol and SCF-CO2 at 50 °C extracts had slight decreases, from 62.43 to 55.07% and from 90.72 to 84.79% after 90 days storage respectively, due to the ability of encapsulation to protect the extract from exposure to high temperatures.
These results were indications that crackers fortified with nano-capsulate propolis SCF-CO2 extract at 50 °C had a higher ability to retain antioxidant activity during the storage period, followed by the crackers fortified with propolis nano-capsulate ethanol extract. The encapsulation process was able to store the propolis extract for nine months in solid-state form, as mentioned by Ticiano et al.56.
Peroxide value (PV) of the crackers
The changes in PV of the five stored cracker samples that occurred during the storage period are shown in Table 7. The increase of peroxide values was relatively low in the cracker fortified with PQG ethanol and SCF-CO2 at 50 °C extracts nano-capsules, being 0.11 and 0.08, respectively. This result occurred because of the ability of capsules to prevent the oxidation of fat and rancidity, increasing the shelf life of crackers.
Conclusions and prospects
Currently, there is a significant interest directed towards the bioactive compounds of plants to build up a robust immune system. This future vision led researchers to discover a novel technique for extracting these bioactive compounds facilitate and increase the yield, improving the process without degradation. The functional food market has expanded over the years due to the demand of consumers for healthy food products, reducing the use of synthetic preservatives and antioxidants and improving the quality of products57.
Nano-capsulation technologies in the food industry, mainly nano-structured food ingredients, improve solubility, sensory properties and stability during storage58,59. Controlling food safety and risk assessments of novel materials added to food encourage many countries to establish powerful platforms60. However, the toxicological repercussions of these new materials and ethical issues are still limited and remain to be tested.
We choose nano-capsules of ethanolic and SCF-CO2 extracts at 50 °C in this study. These nanocapsules were characterized by the high extraction yields and supercritical extracts, which provided higher flavonoid concentrations than EEP. Such a result indicates essential compounds in the fractionation of propolis compounds, as made by several authors before being commercialised. Propolis nano-capsules (SCF-CO2 at 50 °C extracts) had robust inhibitory effects on human tumour growth, potentially preventing oxidative damage and inducing apoptosis and immune stimulation. The crackers fortified with these two propolis extract nano-forms proved their overall acceptability, improved their stability and extended their shelf life. This product could be considered a new functional food that the food industry could process to improve human health. Finally, numerous futures expand for nano-capsulation in the food industry will enhance the standard of living of people.
Data availability
Tip 1: In general, different methods were used to extract the bioactive ingredients in propolis, and the best extraction method was to use supercritical fluid carbon dioxide as shown in Table 1. The data issued in Table 2 also shows the content of phenols and total flavonoids for those extracts, and Figs. 1 and 2 show the antioxidant activity of those extracts through reducing power and DPPH, and Fig. 3 confirms the total antioxidant activity of the extracts. And the comparison between them (free and encapsulated extracts in nano-capsules). Table 3 shows the antioxidant activity of the extracts before and after nano-encapsulation, which showed significant differences for the encapsulated extracts higher than the un-encapsulated ones. The physical and chemical properties of those free and encapsulated extracts were studied, and the encapsulation efficiency was studied. Figure 4 shows the morphological composition of the particles before and after nano-encapsulation. Figure 5 shows the thermal stability of the extracts before and after nano-encapsulation to determine the extent to which they can be applied in the field of foods that are exposed to high temperatures. The toxicity of these extracts on cancer cells of the liver and prostate was also studied, and their effects on cell vitality and inhibition of cancer cells were studied. Finally, the applied study was done by adding it to crackers (bakery products) by comparing the alcoholic extract and the extract with CO2 before and after nano-encapsulation, and studying the extent of storage stability for those products that contain natural antioxidants, which were favored by consumers. Tip 2: Data is "on demand" and all data in a study is available to scholars in the same field. Tip 3: All data we create is disclosed. "All the data mentioned in this paper are available and help in understanding the importance of the study."
References
Bankova, V. S., De-Castro, S. L. & Marcucci, M. C. Recent advances in chemistry and plant origin. Apidologie 2000(31), 3–15. https://doi.org/10.1051/apido:2000102 (2000).
Kolayli, S. & Keskin, M. Natural bee products and their apitherapeutic applications. Stud. Nat. Prod. Chem. 66, 175–196. https://doi.org/10.1016/B978-0-12-817907-9.00007-6 (2020).
El Adaouia, T. R., Djebli, N., Chenini, H., Sahin, H. & Kolayli, S. In vivo and in vitro anti-diabetic activity of ethanolic propolis extract. J. Food Biochem. https://doi.org/10.1111/jfbc.13267 (2020).
Huang, S., Zhang, C. P., Wang, K., Li, G. Q. & Hu, F. L. Recent advances in the chemical composition of propolis. Molecules 19, 19610–19632. https://doi.org/10.3390/molecules191219610 (2014).
Heleno, S. A., Martins, A., Queiroz, M. J. R. P. & Ferreira, I. C. F. R. Bioactivity of phenolic acids: Metabolites versus parent compounds, a review. Food Chem. 173, 501–513. https://doi.org/10.1016/j.foodchem.2014.10.057 (2015).
Devequi-Nunes, D. et al. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE 13, e0207676. https://doi.org/10.1371/journal.pone.0207676 (2018).
Sforcin, J. M. Biological properties and therapeutic applications of propolis. Phyther. Res. 30, 894–905. https://doi.org/10.1002/ptr.5605 (2016).
Trusheva, B., Trunkova, D. & Bankova, V. Dierent extraction methods of biologically active components from propolis; a preliminary study. Chem. Cent. J. https://doi.org/10.1186/1752-153x-1-13 (2007).
Machado, B. A. S. et al. Determination of parameters for the supercritical extraction of antioxidant compounds from green propolis using carbon dioxide and ethanol as co-solvent. PLoS ONE 10, e0134489. https://doi.org/10.1371/journal.pone.0134489 (2015).
Manjare, S. D. & Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2, 463–484. https://doi.org/10.1016/j.mset.2019.04.005 (2019).
Walczak-skierska, J. The influence of plant material enzymatic hydrolysis and extraction conditions on the polyphenolic profiles and antioxidant activity of extracts: A green and efficient approach. Molecules 25, 2074. https://doi.org/10.3390/molecules25092074 (2020).
Machado, B. A. S. et al. Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS ONE 11, e0145954. https://doi.org/10.1371/journal.pone.0145954 (2016).
Khabbazi, S. H., Mansouripour, S. & Saremnezhad, S. The effect of propolis extract as a valuable natural additive on the quality characteristics of toast bread. Food Sci. Nutr. https://doi.org/10.1002/fsn3.3500 (2023).
Essien, S. O., Young, B. & Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 97, 156–169. https://doi.org/10.1016/j.tifs.2020.01.014 (2020).
Vaidyanathan, R., Kalishwaralal, K., Gopalram, S. & Gurunathan, S. Nano silver: The burgeoning therapeutic molecule and its green synthesis. Biotechnol. Adv. 27, 924–937. https://doi.org/10.1016/j.biotechadv.2009.08.001 (2009).
Chowdhury, S. M. et al. Cell specific cytotoxicity and uptake of grahene nanoribbons. Biomaterials 34, 282–293. https://doi.org/10.1016/j.biomaterials.2012.09.057 (2013).
Nagai, T., Inoue, R., Inoue, H. & Suzuki, N. Preparation and antioxidant properties of water extract of propolis. Food Chem. 80(1), 29–33. https://doi.org/10.1016/S0308-8146(02)00231-5 (2003).
Yong, K. P. & Masaharu, I. Preparation of water and ethanolic extracts of propolis and evaluation of the preparations. Biosci. Biotechnol. Biochem. 62, 2230–2232. https://doi.org/10.1271/bbb.62.2230 (1998).
Paviani, L. C. et al. Supercritical CO2 extraction of raw propolis and its dry ethanolic extract. Braz. J. Chem. Eng. 29(02), 243–251. https://doi.org/10.1590/S0104-66322012000200005 (2012).
Vasiliki, P. & Constantina, T. Study of formulation and stability of co-surfactant free water-in-olive oil nano- and submicron emulsions with food grade non-ionic surfactants. J. Am. Oil Chem. Soc. 91, 79–88. https://doi.org/10.1007/s11746-013-2356-3 (2014).
Ebrahimzadeh, M.A., Pourmorad, F. & Hafezi, S. Antioxidant activities of Iranian corn silk. Turk. J. Biol. 32, 43–49. https://dergipark.org.tr/tr/download/article-file/121359 (2008).
Huang, T., Gao, P. & Hageman, M. J. Rapid screening of antioxidants in pharmaceutical formulation development using cyclic voltammetry—Potential and limitations. Curr. Drug Disc. Tech. 1, 173–179. https://doi.org/10.2174/1570163043335117 (2004).
Nabavi, S.M., Ebrahimzadeh, M.A., Nabavi, S.F., Hamidinia, A. & Bekhradnia, A.R. Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. Pharmacol. Online 2, 560–567. https://pharmacologyonline.silae.it/files/archives/2008/vol2/53_Ebrahimzadeh.pdf (2008).
Hatano, T., Kagawa, H., Yasuhara, T. & Okuda, T. Two new flavonoids and other constituents in Licorice root: Their relative astringency and radical scavenging effects. Chem. Phar. Bull. 36, 2090–2097. https://doi.org/10.1248/cpb.36.2090 (1988).
Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. J. Nutr. 44, 307–315. https://doi.org/10.5264/eiyogakuzashi.44.307 (1986).
Osawa, T., Namiki, M., A novel type of antioxidant isolated from leaf wax of eucalyptus leaves. Agric. Biol. Chem. 45, 735–739. https://www.jstage.jst.go.jp/article/bbb1961/45/3/45_3_735/_pdf (1981).
Mitsuda, H., Yasumoto, K. & Twaki, K. Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyo Shokuryo 19, 210–216. https://doi.org/10.4327/jsnfs1949.19.210 (1966).
Busch, V. M. et al. Propolis encapsulation by spray drying: Characterization and stability. LWT 75, 227–235. https://doi.org/10.1016/j.lwt.2016.08.055 (2017).
Saloka, S., Darmadji, P., Setiaji, B., Pranoto, Y. & Anal, A.K. Encapsulation of coconut shell liquid smoke in chitosan-maltodextrin based nanoparticles. Int. Food Res. J. 20, 1269–1276. http://www.ifrj.upm.edu.my/20%20(03)%202013/35%20IFRJ%2020%20(03)%202013%20Satrijo%20(299).pdf (2013).
Grenha, A., Seijo, B., Serra, C. & Remuñán-López, C. Chitosan nanoparticle loaded mannitol microspheres: Structure and surface characterization. Biomacromol 8(7), 2072–2079. https://doi.org/10.1021/bm061131g (2007).
Hazra, A., Alexander, K., Dollimore, D. & Riga, A. Characterization of some essential oils and their key components: Thermoanalytical techniques. J. Therm. Anal. Calorim. 75, 317–330. https://doi.org/10.1023/B:JTAN.0000017352.86803.6d (2004).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65(1–2), 55–63. https://doi.org/10.1016/0022-1759(83)90303-4 (1983).
El-Menshawi, B.S., Fayad, W., Mahmoud, K., El-Hallouty, S.M., El-Manawaty, M., Hägg, M.O. & Linder, S. Screening of natural products for therapeutic activity against solid tumors. Indian J. Exp. Biol. 48, 258–264. http://nopr.niscair.res.in/bitstream/123456789/7402/1/IJEB%2048%283%29%20258-264.pdf (2010).
Benjakula, S. & Karnjanapratum, S. Characteristics and nutritional value of whole wheat cracker fortified with tuna bone bio-calcium powder. Food Chem. 259, 181–187. https://doi.org/10.1016/j.foodchem.2018.03.124 (2018).
Smith W.H. Biscuits, Crackers and Cookies, eBook: Document: English. Vol. 1–2. 1095. https://www.worldcat.org/title/biscuits-crackers-and-cookies/oclc/690414551 (Applied Science Publishers, 1972).
AOAC International, Latimer, G.W. (Eds.) Official Methods of Analysis. 20th edn. https://www.yumpu.com/en/document/read/65659513/free-download-pdf-official-methods-of-analysis-of-aoac-international-20th-aoac-2016-gigapaper (AOAC International, 2016).
Duncan, D. B. Multiple range and multiple F tests. Biometrics 11(1), 1–42. https://doi.org/10.2307/3001478 (1955).
Almeida, P. P., Mezzomo, N. & Ferreira, S. R. S. Extraction of Mentha spicata L. volatile compounds: Evaluation of process parameters and extract composition. Food Bioprocess. Tech. 5(2), 548–559. https://doi.org/10.1007/s11947-010-0356-y (2012).
Biscaia, D. & Ferreira, S. R. S. Propolis extracts obtained by low pressure methods and supercritical fluid extraction. J. Supercrit. Fluids 51, 17–23. https://doi.org/10.1016/j.supflu.2009.07.011 (2009).
Margeretha, I., Suniarti, D.F., Herda, E. & Mas’ud, Z.A. Optimization and comparative study of different extraction methods of biologically active components of Indonesian propolis Trigona spp. J. Nat. Prod. 5, 233–242. https://repository.ipb.ac.id/bitstream/handle/123456789/76473/Optimization%20and%20comparative%20study%20of%20different%20ext.pdf?sequence=1&isAllowed=y (2012).
Malekhosseini, P. et al. Development of casein-based nanoencapsulation systems for delivery of epigallocatechin gallate and folic acid. Food Sci. Nutr. 7, 519–527. https://doi.org/10.1002/fsn3.827 (2019).
Sun, C., Wu, Z., Wang, Z. & Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid. Based Complement. Altern. Med. 9, 595–393. https://doi.org/10.1155/2015/595393 (2015).
Falcão, S., Vale, N., Gomes, P., Domingues, M. R. M. & Freire, C. Phenolic profiling of Portuguese propolis by liquid chromatography coupled with mass spectrometry: Uncommon propolis rich with flavonoid glycosides. Phytochem. Anal. 24, 309–318. https://doi.org/10.1002/pca.2412 (2013).
Mojtaba, Y., Mahdi, S., Sara, S., Nasim, K. & Amir, M. M. Encapsulation Systems for delivery of flavonoids: A review biointerface. Res. Appl. Chem. 11(6), 13934–13951. https://doi.org/10.33263/BRIAC116.1393413951 (2021).
Chen, C. R. et al. Precipitation of sub-micron particles of 3,5-diprenyl-4-hydroxynnamic acid in Brazilian propolis from supercritical carbon dioxide anti-solvent solutions. J. Supercrit. Fluids 50, 176–182. https://doi.org/10.1016/j.supflu.2009.06.001 (2009).
Wu, P. et al. Spray drying of Rhdomyrtus tomentosa (Ait) Hassk. flavonoids extract: optimization and physiochemical, morphological, and antioxidant properties. Int. J. Food Sci. https://doi.org/10.1155/2014/420908 (2014).
Farag, T. K., El-Houssiny, A. S., Abdel-Rahman, E. H. & Hegazi, A. G. A. New approach to the treatment of lumpy skin disease infection in cattle by using propolis. Encapsul. ALG NPS. 8(12), 1346. https://doi.org/10.17582/journal.aavs/2020/8.12.1346.1355 (2020).
Do-Nascimento, T. G. et al. Modified release microcapsules loaded with red Propolis extract obtained by spray-dryer technique. J. Therm. Anal. Calorim. 138(5), 3559–3569. https://doi.org/10.1007/s10973-019-08287-5 (2019).
Bankova, V., Bertelli, D., Borba, R., Conti, B. J. & Barbosa da-Silva, C. I. Standard methods for Apis mellifera propolis research. J. Apic. Res. 58, 1–49. https://doi.org/10.1080/00218839.2016.1222661 (2019).
Soraia, I. F. et al. Phenolic profiling of Portuguese propolis by LC–MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytochem. Anal. 24(4), 203–2018. https://doi.org/10.1002/pca.2412 (2012).
Fabris, S. et al. Antioxidant properties and chemical composition relationship of European and Brazilian propolis. Pharmacol. Pharm. 4(1), 46–51. https://doi.org/10.4236/pp.2013.41006 (2013).
Owen, R. W. et al. Theantioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 36(10), 1235–1247 (2000).
Boik, J. Natural Compounds in Cancer Therapy: Promising Non Toxic Antitumor Agents From Plants and Other Natural Sources. https://doi.org/10.1093/ajcn/75.5.955a (Oregon Medical Press, 2001).
Vatansever, H. S., Sorkun, K. & Ismet, S. D. G. Propolis from Turkey induces apoptosis through activating caspases in human breast carcinoma cell lines. Acta Histochem. 112(6), 546–556. https://doi.org/10.1016/j.acthis.2009.06.001 (2010).
Purnomo, H.A., Subaryono, B.S.B.U. & Paul, N. Institutional arrangement for quality improvement of the Indonesian Gracilaria seaweed. AACL Bioflux 13, 2798–2806. http://www.bioflux.com.ro/aacl (2020).
Ticiano, G., do-Nascimento, P.F., da-Silva, L.F.A., Ouisianny, G., da-Rocha, I.C.C., de-Moraes, P., Túlio, F.A.L.M., Irinaldo, D.B., Luciano A.M.G., Camila, B.D., Eduardo, J.F., Eduardode-Jesus, O., Alex, T.Z. & David, G.W. Polymeric nanoparticles of Brazilian red propolis extract: Preparation, characterization, antioxidant leishmanicidal. Activ. Nanoscale Res. Lett. 11, 301. https://doi.org/10.1186/s11671-016-1517-3 (2016).
Quilaqueo, M. et al. Inclusion of piperine in β-cyclodextrin complexes improves their bioaccessibility and in vitro antioxidant capacity. Food Hydrocoll. 91, 143–152. https://doi.org/10.1016/j.foodhyd.2019.01.011 (2019).
Ponce, A.G., Ayala-Zavala, J.F., Marcovich, N.E., Vazquez, F.J. & Ansorena, M.R. The Netherlands: Nanotechnology Trends in the Food Industry: Recent Developments, Risks, and Regulation. 113–141. https://doi.org/10.1016/B978-0-12-811441-4.00005-4 (2018).
Pisoschi, A. M. et al. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity—A critical view. Eur. J. Med. Chem. 157, 1326–1345. https://doi.org/10.1016/j.ejmech.2018.08.076 (2018).
Nile, S. H. et al. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano-Micro Lett. 12, 1–34. https://doi.org/10.1007/s40820-020-0383-9 (2020).
Acknowledgements
The authors wish to thank the Academy of Scientific Research and Technology (ASRT) in Egypt and the National Research Centre (NRC) Institute of Biology and Agricultural Biotechnology (IBBA) in Italy. These institutions supported and funded this research throughout the bilateral project between Egypt and Italy in the project “Preparation and Utilization of Nano-Propolis Active Compounds, their Biological Function and Application”.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.A.A.: visualization,writing and reviewing, project administration. K.F.M.: methodology, formal analysis, design. M.F.S.: investigation, writing and reviewing. V.L.: supervision and final approval. L.P.: methodology, and design. E.I.S.: formal analysis and supervision. M.A.I.: methodology, formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amin, A.A., Mahmoud, K.F., Salama, M.F. et al. Characterization and stability evaluation of Egyptian propolis extract nano-capsules and their application. Sci Rep 13, 16065 (2023). https://doi.org/10.1038/s41598-023-42025-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42025-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.