Abstract
Reduced muscle mass and/or strength are risk factors for metabolic and musculoskeletal impairment. The present study evaluated anthropometric, metabolic, and musculoskeletal outcomes in females with and without sarcopenic-obesity parameters who underwent bariatric surgery during a 1-year follow-up. A prospective, single-center cohort study was conducted in females with obesity undergoing preoperative evaluation for surgery. In the preoperative period, females were allocated into obesity with sarcopenic-obesity parameters (SOP group, n = 15) and without sarcopenic-obesity parameters (obesity group, n = 21). Sarcopenic obesity parameters were defined as lower appendicular skeletal mass adjusted for weight (ASM/wt) and/or low handgrip strength (HGS). Anthropometric, metabolic, and musculoskeletal parameters were assessed before surgery and at 3 months, 6 months, and a 1-year after bariatric surgery. Weight loss was similar between groups (p > 0.05). Weight, body mass index, fat mass, body fat percentage, skeletal muscle mass, fat-free mass, fat-free mass index, HGS were reduced in both groups during the 1-year follow-up (p < 0.05). However, when muscle mass and strength were analyzed relative to body size, an improvement after bariatric surgery was found in both groups (p < 0.05). Total cholesterol, LDL-c, triglycerides, fasting glucose, glycated hemoglobin, insulin, and insulin resistance were reduced in both groups during the 1-year follow-up (p < 0.05). In addition, HDL-c serum concentration increased in females with and without sarcopenic-obesity parameters over the 1-year follow-up (p < 0.05). Both groups had decreased bone mineral density (BMD) at all sites (lumbar spine, femoral neck, and total femur) over the 1-year follow-up (p < 0.05). The highest quartile of ASM/wt was positively associated with BMD variables in a longitudinal analysis, suggesting that preserved ASM/wt in pre-surgery may be beneficial for BMD after 1 year of bariatric surgery. The results showed that bariatric surgery promotes similar musculoskeletal and metabolic changes in females with preserved muscle mass and strength or in females with sarcopenia-related parameters.
Similar content being viewed by others
Introduction
Surgical treatment for severe obesity has increased worldwide due to the increasing prevalence of obesity. Bariatric surgery is an effective treatment option for weight loss, improvement of comorbidities, and reduction of mortality1. Although several health benefits have been reported after bariatric surgery, many individuals do not experience the expected weight loss and improvement or remission of comorbidities, likely due to patients’ clinical conditions before surgery, such as age and comorbidities, and associated genetic factors2,3.
Classically, the preoperative evaluation for bariatric surgery is performed by an interprofessional team and includes the assessment of psychosocial factors, anthropometric and nutritional variables, complete screening for cardiovascular disease and obstructive sleep apnea, and a comprehensive metabolic panel4. However, it is important to emphasize that although obesity affects other systems, such as the musculoskeletal system, musculoskeletal evaluation is not a routinely recommended procedure in the workup for bariatric surgery.
Appendicular skeletal mass adjusted for weight (ASM/wt) in females with obesity was positively associated with handgrip strength (HGS) and bone mineral density (BMD)5. Bariatric surgery results in changes in body composition with loss of fat mass, skeletal muscle, and BMD6,7. A meta-analysis showed that individuals who underwent bariatric surgery lost 8 kg of lean body mass within 1 year of surgery8. Identifying patients at high risk of excessive muscle loss may help to develop strategies to limit muscle loss and complications after bariatric surgery9.
Sarcopenia-related parameters combined with high adiposity is a risk factor for several complications, including physical disability, falls, osteoporosis, fractures, cardiovascular and metabolic complications, and mortality risk10,11,12,13,14. Sarcopenia is strongly associated with advancing age, with 1 to 2% of skeletal muscle mass and 1.5 to 5% of muscle strength lost annually after age 50. However, regardless of age, low-grade chronic inflammation promoted by obesity is a risk factor for musculoskeletal disability and sarcopenia15.
Considering that the lack of studies investigating whether preoperative sarcopenic-obesity parameters disrupt the musculoskeletal and metabolic outcomes of bariatric surgery, this study evaluated anthropometric, metabolic, and musculoskeletal outcomes in females with low muscle mass and/or strength who underwent bariatric surgery for a 1-year follow-up. The hypothesis tested is that females with reduced muscle mass and/or strength before bariatric surgery have worse metabolic and musculoskeletal outcomes during a 1-year follow-up compared to females with only obesity alone.
Materials and methods
Ethical aspects
This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Research Ethics Committee of Lauro Wanderley University Hospital, Federal University of Paraiba (Reference number 2.548.555). All patients gave written informed consent. All procedures were conducted in agreement with the Resolution 466/2012 of the National Health Council and the International.
Design and subjects
Seventy-five participants were evaluated (convenience sample) before bariatric surgery; 44 participants underwent surgery. Male were excluded from the sample due to the small number of participants (n = 4). In addition, four females were lost to follow-up. Females with obesity, aged 18–60 years, with a body mass index ≥ 40 kg/m2 or ≥ 35 kg/m2 with comorbidities, previously referred to the bariatric surgery service of the Lauro Wanderley University Hospital were included in the study. This hospital is the only one in the State of Paraíba accredited by the public health system to perform bariatric surgery (sleeve gastrectomy or Roux-en-Y gastric bypass). Of these females, 9 underwent bariatric surgery using the sleeve gastrectomy and 27 underwent surgery using the Roux-in-Y gastric bypass surgery.
Participants were recruited and then divided into females with obesity and sarcopenic-obesity parameters (SOP group) and females with obesity (obesity group). Sarcopenic-obesity parameters were defined as low ASM/wt and/or HGS in the lowest quartile of the sample. All females had a high percentage of body fat. In addition, all participants had well-controlled comorbidities, were taking medications regularly, and had stable weight after dietary monitoring.
The occurrence of arrhythmias, cardiac transplantation, cardiac pacemakers, ischemic and non-ischemic cardiomyopathy, psychiatric disorders, and malignant neoplasms was used as exclusion criteria. Participants with neurological, osteoarticular, hepatic, pulmonary, and renal dysfunction were also excluded. The females underwent anthropometric, metabolic and body composition, and bone mass assessments before surgery and at 3, 6, and 12 months after bariatric surgery.
Clinical, anthropometric, and blood pressure measurements
Two questionnaires were administered to participants to collect information before and after bariatric surgery. The first questionnaire collected socio-demographic data, medical history (previous diseases, menopausal history, history of atraumatic bone fracture, duration of illness, and use of medications), and lifestyle (physical activity, dietary counseling, and smoking). The time of diagnosis of type 2 diabetes mellitus and hypertension was self-reported by the participants. The second questionnaire collected data on the type of surgery performed, surgical complications, and medications in use.
Participants were weighed in light clothing, barefoot, using a scale with an accuracy of 0.1 kg (Inbody 370). Height was measured to the nearest 0.5 cm using a stadiometer (Caumaq), and body mass index was calculated by dividing weight in kilograms by height in meters squared.
Calf and neck circumferences were measured using an inelastic tape. Calf circumference was measured in a sitting position, perpendicular to the long axis of the calf, by moving the tape up and down until the maximum circumference was found. Neck circumference was measured from the midpoint of the cervical spine to the anterior center of the neck. Weight loss was measured by subtracting the total weight measured at 3, 6, and 12 months from the baseline weight. Blood pressure was taken in the morning (8–11 am) in a quiet room, according to early studies16,17.
Body composition assessment
Bioelectrical impedance analysis (Inbody 370, Model JMW140, Chungcheongnam-do, KOREA), eight-point tactile electrodes, and multi-frequency (5 kHz, 50 kHz, 250 kHz) was used to assess body composition. It was recommended fast for 12 h, not to do strenuous physical exercises and not to be in the menstrual period. Fat mass (kg) and skeletal muscle mass (kg) of all body segments (arms, legs, and trunk), as well as fat-free mass and body fat percentage, were obtained from the manufacturer’s algorithm, using sex, age, weight, and height.
Appendicular skeletal mass (kg) was obtained by summing the skeletal muscle mass of both arms and legs. The following indices were calculated: muscle mass index (ASM/wt) and fat-free mass index (fat-free mass adjusted for height squared).
BMD, T-score, and Z-score at the lumbar spine (L1-L4), femoral neck, and total femur were assessed by dual-energy X-ray absorptiometry (DXA) using a properly calibrated densitometer (model Lunar 8743, Medical Systems Lunar, Madison, USA). DXA composition data were not used because the exam was not performed properly for whole body composition.
Physical function
HGS was measured in kilograms using a Jamar digital dynamometer (Sammons Preston Inc., IL, USA). Three measurements were taken in each hand, and the mean values of these three measurements in the dominant hand was used as the final value. The examination was performed with a 30-s rest between measurements18.
The six-minute walk (6MWT) test was used to measure physical performance. The space was demarcated every meters to facilitate the calculation of the distance covered. The gait speed was then calculated using the formula: speed (m/s) = distance covered in meters/360 seconds19. The test was performed on a flat surface in a closed, air-conditioned environment20.
Biochemical measurements
Blood samples were collected after a 12-h fast and without strenuous exercise for the previous 24 h. Fasting glucose, triglycerides, cholesterol, and high-density lipoprotein cholesterol (HDL-c) were measured using an automated enzymatic method (Autoanalyzer; Technicon, Tarrytown, NY, USA). Low-density lipoprotein cholesterol (LDL-c) was calculated using the Friedwald formula21. Insulin was determined by chemiluminescence immunoassay. Glycated hemoglobin (HbA1c) was determined by high-performance liquid chromatography, and high-sensitivity quantitative C-reactive protein (hs-CRP) was quantified by turbidimetry. Homeostasis model assessment insulin resistance (HOMA-IR) was used to measure insulin resistance and was calculated as fasting insulin (uU/L) x fasting glucose (mg/dL) divided by 22.5.
Statistical analysis
Baseline data and percent weight loss were analyzed by independent t test, Mann–Whitney, or chi-squared. Body composition, muscle function, biochemical variables, and bone mass were analyzed by mixed between-within-subjects ANOVA. Generalized estimating equations (GEE) models were used to prospectively examine the association between ASM/wt or HGS and BMD. The bone mineral density variables were used for the GEE models with normal distribution using the “Gaussian family” specification. Potential confounders included in the analysis were: age, body mass index, body fat, and HOMA-IR. Statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA), and differences between groups were considered statistically significant when p-value < 0.05. Missing values at three (n = 8 participants) and six (n = 7 participants) months were imputed using the mean of the variable.
Results
Baseline
Baseline characteristics regarding age, anthropometric measurements, blood pressure, and history of disease were similar between groups (Table 1). At baseline, females with SOP had reduced ASM/wt, total skeletal muscle mass, HGS, and HGS adjusted for body mass index, gait speed, L1-L4 BMD, femoral neck BMD, and HDL cholesterol, and higher HbA1c compared with females with obesity alone (Table 1). No differences were found in the rates of surgical Roux-in-Y gastric bypass and gastric sleeve surgery (p > 0.05, Table 1).
Follow-up after bariatric surgery
Weight loss, body composition, and muscle function after bariatric surgery
Both groups significantly decreased weight, body mass index, fat mass, body fat percentage, skeletal muscle mass, fat-free mass, fat-free mass index, gait speed, and HGS during the 1-year follow-up (p < 0.05, Table 2). The percentage of weight loss after 1 year of bariatric surgery was similar between groups (obesity: 24.3 ± 11.5 vs. sarcopenic-obesity parameters: 31.0 ± 9.9%, p = 0.09). Females in both groups showed increased ASM/wt, HGS adjusted for body mass index and gait speed during a 1-year follow-up (p < 0.05, Table 2). Although females with SOP had lower fat-free mass, lower skeletal muscle mass, lower ASM/wt, lower HGS, and lower HGS adjusted for body mass index in a cross-sectional comparison (p < 0.05, Table 2), no difference was found for the time x group interaction for these variables (I > 0.05, Table 2).
Both groups showed reduced BMD at all sites (lumbar spine, femoral neck, and total femur) during a 1-year follow-up period (p < 0.05, Table 3). Although females with SOP had lower BMD, Z-score, and T-score at the lumbar spine and femoral neck in a cross-sectional comparison (p < 0.05, Table 3), no difference was found for the time x group interaction for these variables (I > 0.05, Table 3).
Association between sarcopenia parameters and bone mineral density throughout follow-up
The highest quartile of ASM/wt was positively associated with L1-L4 BMD, femoral neck BMD, and femur BMD in a crude analysis and in models adjusted for age, body mass index, body fat percentage, and HOMA-IR (Table 4). On the other hand, L1-L4 BMD, femoral neck and total femur BMD were not associated with HGS over time (Table 4).
Metabolic and inflammatory profile after bariatric surgery
Total cholesterol, LDL-cholesterol, triglycerides, fasting glucose, HbA1c, insulin, and HOMA-IR were reduced in both groups over the 1-year follow-up (p < 0.05, Table 5). In addition, HDL-cholesterol increased in females with and without sarcopenic-obesity parameters over the 1-year follow-up (p < 0.05, Table 5). No difference was found for the time x group interaction for these variables, except for HDL cholesterol, where females with SOP had increased HDL cholesterol during the 1-year follow-up compared to females without sarcopenic obesity parameters (I = 0.017, Table 5).
Discussion
The results of this study showed that the percentage of weight loss, fat mass and body fat percentage, ASM/wt, gait speed, muscle mass and strength when properly analyzed divided by body size, and biochemical variables (glycemic, lipid, and inflammatory) were improved in females with and without sarcopenic-obesity parameters over a 1-year follow-up of bariatric surgery. In addition, the study demonstrated for the first time that females with sarcopenic-obesity parameters had lower BMD in L1-L4 and femoral neck in the preoperative period, both groups decreased BMD over time, and ASM/wt was positively associated with BMD over a 1-year follow-up of bariatric surgery.
Sarcopenia is a common disease in the elderly; however, young subjects with obesity may exhibit sarcopenia due to excessive weight gain, adipocyte hypertrophy, ectopic fat deposition in the muscle, inflammation, and insulin resistance22. In addition, a history of recent weight loss (including voluntary weight loss and a long-term restrictive diets), physical inactivity, and bariatric surgery may contribute to skeletal muscle mass loss22,23.
Low muscle mass has been reported in females with obesity and is associated with low HGS and BMD5. The present study evaluated parameters of sarcopenic obesity and not the diagnosis of sarcopenic obesity in middle-aged females who underwent bariatric surgery. There are several reasons for this: first, muscle mass and muscle function do not have the same clinical relevance during the aging process24; second, muscle strength and muscle mass are not congruent, i.e. muscle strength can decrease even if muscle mass is maintained or increased25; and lastly, there is no international consensus on a definition of sarcopenia26 and no clinical and research guidelines specific to Brazil. Therefore, it is reasonable to suggest that muscle mass and muscle strength need to be defined independently because they may have different clinical implications in middle-aged females.
It has been suggested that weight loss promoted by bariatric surgery results in changes in body composition with loss of fat mass, but also loss of skeletal muscle mass and bone mass6,7,27,28,29. The present study showed a significant decrease in weight, body mass index, total fat mass, body fat percentage, total skeletal muscle mass, fat-free mass, fat-free mass index and HGS in both groups over the 1-year follow-up period. However, when muscle mass and strength were analyzed relative to body size rather than in absolute terms, an improvement after bariatric surgery was found, suggesting that the assessment of absolute muscle mass and strength after surgery should be used with caution and that the adjusted assessment may be better applied.
Studies are needed to better understand the clinical implications of the loss of skeletal muscle mass that occurs after bariatric surgery. One of these gaps is the assessment of absolute skeletal muscle mass rather than relative skeletal muscle mass loss30,31. In the current study, there was a loss of total skeletal muscle mass during the follow-up, but when considering the ASM/wt, there was an increase in relative muscle mass. These data are consistent with a previous study that reported an improvement in the proportion of fat mass to muscle mass in the group that lost more than 50% of excess weight, despite a decrease in absolute muscle mass32.
In the present study, although there was a decrease in absolute HGS, an increase in HGS adjusted for body mass index was observed in both groups during a 1-year follow-up period. Similarly, a prospective cohort showed a 9% decrease in absolute muscle strength and a 32% increase in HGS adjusted for body mass index in the 12 months after Roux-in-Y gastric bypass surgery33. Here, we have demonstrated that females with SOP had lower HGS and HGS adjusted for body mass index than females without SOP because this is part of the criteria for group definition. However, both groups showed a decrease in HGS and an increase in HGS adjusted for body mass index during 1 year of follow-up. Whether strength training before and after bariatric surgery can have beneficial effects on HGS in SOP patients remains to be determined.
Worldwide guidelines for bariatric surgery have recommended that the cardiovascular risk profile of patients with obesity must be assessed prior to surgery. However, there is no formal recommendation for bone and muscle assessment before a bariatric surgery procedure34,35. It is already documented that bariatric surgery, sarcopenia, and obesity increase the risk of bone compromise and bone fracture10,28,29,36. Although the negative repercussions of sarcopenic obesity on bone are already recognized, to our knowledge, no studies have assessed the association between sarcopenic obesity parameters and bariatric surgery. In the current study, BMD was reduced at all sites (L1-L4, femoral neck, and total femur), as well as Z-score and T-score in females with and without sarcopenic-obesity parameters.
Comparing the two groups during a 1-year follow-up, females with SOP had lower BMD, Z-score, and T-score in the L1-L4 and femoral neck than the obesity group. This data is important considering that bariatric surgery increases the risk of bone fracture during follow-up due to nutritional factors (low calcium intake and vitamin D deficiency), hormonal factors (decreased estrogen, leptin, insulin, amylin, and increased parathyroid hormone), and bone architecture changes37. Fracture risk appears to be higher after two to five years of bariatric surgery and after Roux-in-Y gastric bypass than sleeve gastrectomy28,29. Furthermore, it is important to emphasize that not only does the presence of metabolic factors increase mortality, but osteoporosis and fractures are also risk factors for higher mortality38,39. In fact, whether sarcopenic obesity or sarcopenic-obesity parameters before bariatric surgery increases bone fracture and higher mortality remains to be elucidated.
In the present study, females with obesity from both groups displayed a decrease in total cholesterol, LDL-c, triglycerides, fasting glucose, HbA1c, insulin, HOMA-IR, and an increase in HDL-cholesterol over the 1-year follow-up. This finding corroborates with early studies reporting metabolic improvement after bariatric surgery40,41. However, there were no differences between the two groups regarding metabolic and inflammatory profiles.
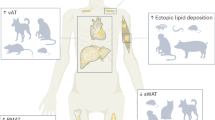
Given that muscle mass or strength may affect BMD, a prospective analysis of the association was performed to answer this gap. The findings showed that females with the highest quartile of ASM/wt had a positive association with BMD. Muscle mass and muscle strength should both be assessed in pre-surgery in middle-aged females, since the proper diagnosis of sarcopenic obesity requires appropriate follow-up by a multidisciplinary health care team. The results of this study are summarized in Fig. 1.
Effects of preoperative sarcopenia-related parameters on the musculoskeletal and metabolic outcomes after bariatric surgery. Females with preserved muscle mass and strength or females with sarcopenia-related parameters had reduced metabolic disorders and decreased bone mineral density during a 1-year follow-up after bariatric surgery. In addition, the findings showed that although muscle mass and strength have decreased over a 1-year follow-up, there was an improvement in the muscle mass and strength when analyzed relative to body size. Lastly, the findings showed the highest quartile of ASM/wt was positively associated with BMD variables in a longitudinal analysis. ASM/weight appendicular skeletal mass adjusted weight, HGS handgrip strength, SMM skeletal muscle mass, BMD bone mineral density, BMI body mass index.
The number of participants is a limitation of the study. The COVID-19 pandemic stopped bariatric surgery in several hospital and clinics. Our sample consisted of females, and extrapolating these results to males would not be appropriate. We evaluated parameters of sarcopenic obesity and not the diagnosis of sarcopenic obesity, which could interfere with the results. Unfortunately, we have a lot of heterogeneity in determining cutoff points for low ASM/wt and HGS. We do not have a formal recommendation for low ASM/wt and HGS in middle-aged individuals. However, our study brings the relevance of the association of ASM/wt and HGS variables with BMD outcomes. Studies that include not only bone mass but also bone quality and metabolism would be needed.
Despite the limitations, to our knowledge, this was the first study to evaluate individuals with parameters related to sarcopenic obesity and their clinical responses during follow-up. Furthermore, this current study suggests that a better musculoskeletal stratification should be performed before bariatric surgery to identify individuals with a greater propensity to lose bone mass during the follow-up of this surgery, thus promoting a better clinical management of these cases.
Conclusion
Bariatric surgery promoted weight loss, improved body fat percentage, and improved glucose, lipid, and inflammatory marker in females with and without sarcopenic-obesity parameters. Although skeletal muscle mass and HGS decreased throughout the follow-up, there was an improvement in the muscle mass and strength when analyzed relative to body size. The highest quartile of ASM/wt was positively associated with BMD variables in a longitudinal analysis, suggesting that preserved ASM/wt in pre-surgery may be beneficial for BMD after 1 year of bariatric surgery.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ASM:
-
Appendicular skeletal mass
- ASM/wt:
-
Appendicular skeletal mass adjusted for weight
- BMD:
-
Bone mineral density
- hs-CRP:
-
High sensitivity C-reactive protein
- HGS:
-
Handgrip strength
- HbA1c:
-
Glycated hemoglobin
- HDL-cholesterol:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model assessment-insulin resistance
- LDL-cholesterol:
-
Low-density lipoprotein cholesterol
References
Sjostrom, L. Bariatric surgery and reduction in morbidity and mortality: Experiences from the SOS study. Int. J. Obes. (Lond) 32(Suppl 7), S93-97. https://doi.org/10.1038/ijo.2008.244 (2008).
Molero, J. et al. Prevalence of low skeletal muscle mass following bariatric surgery. Clin. Nutr. ESPEN 49, 436–441. https://doi.org/10.1016/j.clnesp.2022.03.009 (2022).
Gupta, S. R., Zhou, Y., Wadden, T. A., Berkowitz, R. I. & Chao, A. M. A systematic review of genetic correlates of weight loss after bariatric surgery. Obes. Surg. 31, 4612–4623. https://doi.org/10.1007/s11695-021-05585-6 (2021).
Benalcazar, D. A. & Cascella, M. Obesity Surgery Preoperative Assessment and Preparation (StatPearls Publishing, 2023).
Crispim Carvalho, N. N. et al. Relationship between skeletal muscle mass indexes and muscular function, metabolic profile and bone mineral density in women with recommendation for bariatric surgery. Diabetes Metab. Syndr. Obes. 12, 2645–2654. https://doi.org/10.2147/DMSO.S213643 (2019).
Maimoun, L. et al. Body composition changes in the first month after sleeve gastrectomy based on gender and anatomic site. Surg. Obes. Relat. Dis. 13, 780–787. https://doi.org/10.1016/j.soard.2017.01.017 (2017).
Gomez-Ambrosi, J. et al. Dissociation of body mass index, excess weight loss and body fat percentage trajectories after 3 years of gastric bypass: Relationship with metabolic outcomes. Int. J. Obes. (Lond) 41, 1379–1387. https://doi.org/10.1038/ijo.2017.134 (2017).
Nuijten, M. A. H. et al. The magnitude and progress of lean body mass, fat-free mass, and skeletal muscle mass loss following bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 23, e13370. https://doi.org/10.1111/obr.13370 (2022).
Mastino, D. et al. Bariatric surgery outcomes in sarcopenic obesity. Obes. Surg. 26, 2355–2362. https://doi.org/10.1007/s11695-016-2102-7 (2016).
Scott, D. et al. Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5–10 years in community-dwelling older adults. Calcif. Tissue Int. 99, 30–42. https://doi.org/10.1007/s00223-016-0123-9 (2016).
Batsis, J. A., Mackenzie, T. A., Barre, L. K., Lopez-Jimenez, F. & Bartels, S. J. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 68, 1001–1007. https://doi.org/10.1038/ejcn.2014.117 (2014).
Park, S. H. et al. Sarcopenic obesity as an independent risk factor of hypertension. J. Am. Soc. Hypertens. 7, 420–425. https://doi.org/10.1016/j.jash.2013.06.002 (2013).
Baumgartner, R. N. et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 12, 1995–2004. https://doi.org/10.1038/oby.2004.250 (2004).
Kim, T. N. et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: The Korean sarcopenic obesity study. Clin. Endocrinol. (Oxf) 78, 525–532. https://doi.org/10.1111/j.1365-2265.2012.04433.x (2013).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31. https://doi.org/10.1093/ageing/afy169 (2019).
Crispim Carvalho, N. N. et al. Effects of preoperative sarcopenia-related parameters on cardiac autonomic function in women with obesity following bariatric surgery: A one-year prospective study. Nutrients https://doi.org/10.3390/nu15122656 (2023).
Carvalho, N. N. C. et al. Impact of arterial hypertension and type 2 diabetes on cardiac autonomic modulation in obese individuals with recommendation for bariatric surgery. Diabetes Metab. Syndr. Obes. 12, 1503–1511. https://doi.org/10.2147/DMSO.S204414 (2019).
Otto, M. et al. Handgrip strength as a predictor for post bariatric body composition. Obes. Surg. 24, 2082–2088. https://doi.org/10.1007/s11695-014-1299-6 (2014).
DePew, Z. S., Karpman, C., Novotny, P. J. & Benzo, R. P. Correlations between gait speed, 6-minute walk distance, physical activity, and self-efficacy in patients with severe chronic lung disease. Respir. Care 58, 2113–2119. https://doi.org/10.4187/respcare.02471 (2013).
Agarwala, P. & Salzman, S. H. Six-minute walk test: Clinical role, technique, coding, and reimbursement. Chest 157, 603–611. https://doi.org/10.1016/j.chest.2019.10.014 (2020).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972).
Donini, L. M. et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes. Facts 15, 321–335. https://doi.org/10.1159/000521241 (2022).
Lynch, D. H. et al. Multimodal diagnostic approaches to advance precision medicine in sarcopenia and frailty. Nutrients https://doi.org/10.3390/nu14071384 (2022).
Isoyama, N. et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 9, 1720–1728. https://doi.org/10.2215/CJN.10261013 (2014).
Goodpaster, B. H. et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 61, 1059–1064. https://doi.org/10.1093/gerona/61.10.1059 (2006).
Zanker, J. et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J. Cachexia Sarcopenia Muscle 14, 142–156. https://doi.org/10.1002/jcsm.13115 (2023).
Gagnon, C. & Schafer, A. L. Bone health after bariatric surgery. JBMR Plus 2, 121–133. https://doi.org/10.1002/jbm4.10048 (2018).
Pereira, C. et al. Fracture risk after bariatric surgery: A systematic literature review and meta-analysis. Endocr. Pract. 28, 58–69. https://doi.org/10.1016/j.eprac.2021.09.007 (2022).
de Holanda, N. C. P. et al. Secondary hyperparathyroidism, bone density, and bone turnover after bariatric surgery: Differences between roux-en-Y gastric bypass and sleeve gastrectomy. Obes. Surg. 31, 5367–5375. https://doi.org/10.1007/s11695-021-05739-6 (2021).
Matos, O. et al. Changes in bone mass and body composition after bariatric surgery. Gynecol. Endocrinol. 36, 578–581. https://doi.org/10.1080/09513590.2020.1762558 (2020).
Ruthes, E. M. P. et al. Lean mass and strength profile of women submitted to bariatric surgery: Comparison of the EWGSOP2 and FNIH classification for sarcopenia - ASBS program phase II. Gynecol. Endocrinol. 38, 868–873. https://doi.org/10.1080/09513590.2022.2119956 (2022).
Sivakumar, J. et al. Body composition differences between excess weight loss >/= 50% and < 50% at 12 months following bariatric surgery. Obes. Surg. 32, 2556–2566. https://doi.org/10.1007/s11695-022-06128-3 (2022).
Alba, D. L. et al. Changes in lean mass, absolute and relative muscle strength, and physical performance after gastric bypass surgery. J. Clin. Endocrinol. Metab. 104, 711–720. https://doi.org/10.1210/jc.2018-00952 (2019).
Mechanick, J. I. et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists - Executive Summary. Endocr. Pract. 25, 1346–1359. https://doi.org/10.4158/GL-2019-0406 (2019).
Busetto, L. et al. Practical recommendations of the obesity management task force of the european association for the study of obesity for the post-bariatric surgery medical management. Obes. Facts 10, 597–632. https://doi.org/10.1159/000481825 (2017).
Scott, D. et al. Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: The concord health and ageing in men project. J. Bone Miner. Res. 32, 575–583. https://doi.org/10.1002/jbmr.3016 (2017).
Krez, A. N. & Stein, E. M. The skeletal consequences of bariatric surgery. Curr. Osteoporos. Rep. 18, 262–272. https://doi.org/10.1007/s11914-020-00579-2 (2020).
Mattisson, L., Bojan, A. & Enocson, A. Epidemiology, treatment and mortality of trochanteric and subtrochanteric hip fractures: Data from the Swedish fracture register. BMC Musculoskelet. Disord. 19, 369. https://doi.org/10.1186/s12891-018-2276-3 (2018).
Management of Osteoporosis in Postmenopausal Women: The Position Statement of The North American Menopause Society. Management of osteoporosis in postmenopausal women: The 2021 position statement of the North American Menopause Society. Menopause 28, 973–997. https://doi.org/10.1097/GME.0000000000001831 (2021).
Brethauer, S. A. et al. Bariatric surgery improves the metabolic profile of morbidly obese patients with type 1 diabetes. Diabetes Care 37, e51-52. https://doi.org/10.2337/dc13-1736 (2014).
Piche, M. E., Tardif, I., Auclair, A. & Poirier, P. Effects of bariatric surgery on lipid-lipoprotein profile. Metabolism 115, 154441. https://doi.org/10.1016/j.metabol.2020.154441 (2021).
Acknowledgements
de Brito Alves, JL thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their research productivity fellowship.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: N.N.C.C. and J.L.B.A. Formal analysis and investigation: N.N.C.C., V.J.B.M. and A.C.P.A.N. Data acquisition: N.N.C.C.; Writing—original draft preparation: N.N.C.C. and J.L.B.A. Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crispim Carvalho, N.N., Martins, V.J.B., Filho, J.M. et al. Effects of preoperative sarcopenia-related parameters on the musculoskeletal and metabolic outcomes after bariatric surgery: a one-year longitudinal study in females. Sci Rep 13, 13373 (2023). https://doi.org/10.1038/s41598-023-40681-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40681-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.