Abstract
Management of malignant hemispheric stroke (MHS) after cardiothoracic surgery (CTS) remains difficult as decision-making needs to consider severe cardiovascular comorbidities and complex coagulation management. The results of previous randomized controlled trials on decompressive surgery for MHS cannot be generally translated to this patient population and the expected outcome might be substantially worse. Here, we analyzed mortality and functional outcome in patients undergoing decompressive hemicraniectomy (DC) for MHS following CTS and assessed the impact of perioperative coagulation management on postoperative hemorrhagic and cardiovascular complications. All patients that underwent DC for MHS resulting as a complication of CTS between June 2012 and November 2021 were included in this observational cohort study. Outcome was determined according to the modified Rankin Scale (mRS) score at 1 and 3–6 months. Clinical and demographic data, anticoagulation management and postoperative hemorrhagic and thromboembolic complications were assessed. In order to evaluate a predictive association between clinical and radiological parameters and the outcome, we used a multivariate logistic regression analysis. Twenty-nine patients undergoing DC for MHS after CTS with a female-to-male ratio of 1:1.9 and a median age of 60 (IQR 49–64) years were identified out of 123 patients undergoing DC for MHS. Twenty-four patients (83%) received pre- or intraoperative substitution. At 30 days, the in-hospital mortality rate and neurological outcome corresponded to 31% and a median mRS of 5 (5–6), which remained stable at 3–6 months [Mortality: 42%, median mRS: 5 (4–6)]. Postoperatively, 15/29 patients (52%) experienced new hemorrhagic lesions and Bayesian logistic regression predicting mortality (mRS = 6) after imputing missing data demonstrated a significantly increased risk for mortality with longer aPPT (OR = 13.94, p = .038) and new or progressive hemorrhagic lesions after DC (OR = 3.03, p = .19). Notably, all but one hemorrhagic lesion occurred before discontinued anticoagulation and/or platelet inhibition was re-initiated. Despite perioperative discontinuation of anticoagulation and/or platelet inhibition, no coagulation-associated cardiovascular complications were noted. In conclusion, Cardiothoracic surgery patients suffering MHS will likely experience severe neurological disability after DC, which should remain a central aspect during counselling and decision-making. The complex coagulation situation after CTS, however, should not per se rule out the option of performing life-saving surgical decompression.
Similar content being viewed by others
Introduction
Ischemic stroke remains a frequent and devastating complication after cardiothoracic surgeries (CTS) leading to excess mortality and morbidity1,2. Analyses from databases and observational registers suggest an overall incidence between 0.98 and 5.2%2,3. In patients with type A acute aortic dissection (TAAAD), the rate of perioperative stroke can reach up to 32%4,5 and the in-hospital mortality rate in patients suffering from a perioperative stroke was significantly higher than in patients without stroke (32.8% vs. 4.9%1 or 14.93% vs. 2.15%6). The pathophysiological mechanisms of perioperative strokes primarily include thromboembolism and extension of an aortic dissection into the carotid artery and might cause a large vessel occlusion with subsequent malignant hemispheric stroke (MHS)7. This subtype of ischemic stroke may account for the majority of the morbidity and mortality associated with perioperative strokes. However, despite multiple studies on the incidence of perioperative cerebral stroke, there is only limited data on the number and outcome of patients suffering MHS following CTS3,8.
Neurosurgical management of patients with MHS after CTS poses a major dilemma, since CTS in the context of a life-threatening emergency predisposes patients to an increased risk for postoperative complications and morbidity9. This is highly relevant in the context of interdisciplinary decision making, since patients with such severe cardiovascular comorbidities were not included in previously published trials on MHS and the results of those randomized controlled trials cannot be generally translated to this patient population10,11,12,13,14. The expected functional outcome might be substantially worse, but so far, there is no systematic information addressing this issue and the lack of appropriate evidence hampers clinical decision-making and patient counselling. Further, decision-making in the setting of MHS after CTS is complicated by conflicting requirements for perioperative coagulation management, because antiplatelet and anticoagulant therapy is a central component of the patient management after CTS15. Therefore, we analyzed the clinical outcome, coagulation management and its impact on hemorrhagic and thromboembolic complications in patients undergoing DC for treatment of MHS following CTS in two of Europe’s highest-volume neurosurgical and cardiothoracic departments.
Methods
Study design
This observational cohort study was approved by the ethics committee of the Charité – Universitätsmedizin Berlin (EA4/019/22) and performed in compliance with Health Insurance Portability and Accountability Act regulations. For this study informed consent has been waived by the Charité – Universitätsmedizin Berlin ethics committee due to the anonymity and retrospective nature of the study. Data acquisition and presentation was done according to the STROBE guidelines for reporting observational studies16. All authors had full access to all the data in the study and take responsibility for its integrity and analysis.
Patient management
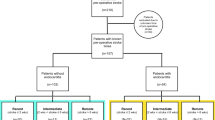
We identified 282 patients who underwent DC at the Department of Neurosurgery at the Charité Virchow Campus in Berlin, Germany, between June 2012 and November 2021. Of these, 123 underwent DC for MHS and 29/123 (24%) underwent DC for MHS following CTS in the German Heart Center Berlin, Germany (DHZB; Deutsches Herzzentrum Berlin). Malignant hemispheric stroke with a volume ≥ 140 cm3 was detected by computerized tomography (CT) due to delayed recovery or new neurological impairment after withdrawal of anesthesia following CTS. The decision to perform DC was primarily based on the uniform inclusion criteria of the four randomized controlled trials on hemicraniectomy, DECIMAL, DESTINY I/II and HAMLET10,11,12,13,14. Exclusion criteria, such as pre-morbid mRS > 1, pre-morbid Barthel-Index < 95, other concomitant severe disease that would confound with treatment, or life expectancy < 3 years were not applied. DC was performed as previously described17,18. All patients were anaesthetized with propofol and remifentanil and received a bodyweight-adapted dose of 50 mg/kg mannitol 30 min before skin incision. Mean arterial blood pressure was targeted at 80–90 mmHg. The end-expiratory carbon dioxide concentration during surgery was maintained at a level corresponding to an arterial partial pressure of carbon dioxide between 38 and 42 mmHg. Postoperatively, patients were transferred to our neurointensive care unit. Intracranial pressure (ICP) was continuously monitored and patients remained intubated and sedated until ICP was within normal ranges. A critical ICP threshold was defined as ICP > 20 mmHg for longer than 10 min and treated with cerebrospinal fluid drainage, osmotic therapy, deep sedation and cerebrospinal fluid drainage, if an EVD was inserted: According to our protocol, we always aim to insert an EVD instead of an ICP probe as a first line measure in patients that require ICP monitoring. In patients suffering MHS, however, placement of an EVD/ICP probe prior to the surgery is usually not performed, because the indication for DC in MHS is usually not based on an ICP threshold. Further, the majority of the patients in our cohort that required postoperative ICP monitoring primarily received an ICP probe instead of an EVD, because intraoperative placement of a parenchymal ICP probe is likely easier than attempting to correctly place an EVD due to the compression and lateralization of the ventricles.
A routine postoperative CT was performed within 24 h after surgery. Infarct volumetry was done on postoperative CT scans performed within 24 h after DC using Brainlab Cranial Planning SmartBrush Ver. 2.6.0.121 (Brainlab AG, Munich, Germany). Volume gain was calculated as ipsilateral volume minus contralateral volume. Swelling was determined as volume gain divided by contralateral volume. Volume of infarction corrected for hemispheric swelling was calculated as volume of infarction divided by swelling plus 1.
Outcome parameters
Demographic, clinical and radiographic patient data were retrospectively analyzed by a clinician who was not directly involved in the patients’ care. Primary endpoint was the functional status expressed on the modified Rankin Scale (mRS) score assessed at 1 and 3–6 months after DC. Since only one patient achieved a favorable functional outcome with an mRS score of ≤ 3, outcome was dichotomized into mRS 6 (death) versus mRS < 6 in the predictive models to assess variables associated with a higher risk for mortality. The secondary endpoint was postoperative occurrence of new hemorrhagic lesions. For this purpose, we analyzed all available postoperative CT scans regarding hemorrhagic lesions including hemorrhagic transformation within the established ischemic lesion, subgaleal hemorrhage, and intracerebral hemorrhage (ICH) beyond the established ischemic lesion.
Statistical analysis
Statistics were calculated using GraphPad Prism version 9 (GraphPad Software Inc, San Diego, CA, USA) and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria, 2022). Variables are reported as median with interquartile range (IQR). In order to evaluate a predictive association between clinical and radiological parameters and outcome, we used a multivariate logistic regression analysis that included the following variables: age, sex, infarct volume, type of surgery [(TA(B)AAD or valve surgery], whether CTS was an emergency or elective surgery, left affected hemisphere, midline shift, maximum DC diameter, time to decompression, anisocoria or bilaterally dilated pupils before DC, INR, aPTT, platelet count, whether the patients received anticoagulant, antiplatelet drugs, or both after CTS and before DC, and the occurrence of a new or progressive hemorrhagic lesion after DC. The predictor analysis used the data of 26 patients that had a reported mRS at 3–6 months. Since the predictor variables also exhibited some missing data (17 patients with complete data; 7.7% of all data was missing) we used multiple imputation to be able to use data from cases with incomplete reports (k = 30 imputations, R package “mice”)19. Due to the small sample size and many dichotomous predictors, we ran Bayesian logistic regression in the package: “brms”20. Twenty Markov Chain Monte Carlo samples with 10,000 iterations each were applied. A normal prior with mean 0 and standard deviation of 10 was used for all regression coefficients.
Results
Patient characteristics
Overall, twenty-nine patients were identified (Fig. 1). Detailed information on patient characteristics and co-morbidities are shown in Tables 1 and 2. Most patients suffered type A or B acute aortic dissection (14/29, 48%) or underwent surgery for aortic or mitral valve repair or replacement (10/29, 34%). After CTS, 21/29 patients (72%) presented with inadequate recovery from anesthesia and 12/29 patients (41%) with hemiparesis or hemiplegia. Further, 2/29 (7%) had an additional generalized seizure and 12/29 (41%) a unilateral or bilateral mydriasis. The median time from diagnosis of MHS to DC was 2.6 (2.0–3.7) hours.
Outcome
The in-hospital mortality rate was 31% and all 9/29 patients died within 4 (2–7) days after DC. Two patients died due to a cardiogenic shock with consecutive multiorgan dysfunction and 1 patient died due to a relapsing LVAD thrombosis. The remaining 6 patients died after withdrawal of care, following consultation with their family or next of kin and under consideration of the patient’s living will: In 1/6 patients, the decision to discontinue treatment was made after the clinical situation was further aggravated by septic shock. The other 5/6 patients were hemodynamically stable. One out of five had a patient decree regarding prolonged intubation/ventilation and refusing further treatment under consideration of the expected long-term dependency. In the remaining 4 patients, treatment was discontinued due to extensive postoperative hemorrhage, which further limited the already unfavorable prognosis.
Overall, 25/29 patients (86%) suffered from non-surgical and 7/29 patients (24%) from surgical complications after DC, which except for 3 patients that died from a cardiogenic shock or LVAD thrombosis likely had no effect on outcome (Table 3). All patients were transferred to a neurointensive care unit and back to a cardiac ICU or to neurorehabilitation after 26 (13–35) days. Among the 20 patients who survived the acute phase, tracheostomy was performed in 15/20 patients (75%) and 15/20 patients (75%) received cranioplasty after 98 (59–102) days. Three out of 20 patients (15%) required a ventriculoperitoneal shunt 121 (53–185) days after DC.
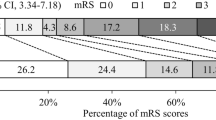
At 1-month, the median mRS of 29 patients was 5 (5–6), remained stable at 3–6 months with an mRS of 5 (4–6). At this time point, the overall mortality rate was 42% (11/26 patients). Three out of 29 patients (10%) were lost to follow-up after 3–6 months (Fig. 2).
Anticoagulation management
Before DC, 23/29 patients (79%) had received anticoagulation and/or antiplatelet therapy (Fig. 3A): 12/29 patients (41%) received heparin and 2/29 (7%) argatroban only, 1/29 patients (3%) received phenprocoumon, 4/29 patients (14%) received heparin and acetylsalicylic acid (ASA), 1/29 patients (3%) received heparin and clopidogrel, 1/29 patients (3%) received ASA and ticagrelor and 2/29 patients (7%) received ASA alone. For the other 6/29 patients (21%), documentation on preoperative anticoagulation and antiplatelet therapy was not available. Overall, only 6/29 patients (21%) reached therapeutic anticoagulation within an aPTT of 50–70 s or INR > 2 before DC and uncorrected coagulation parameters after CTS and before DC were within normal ranges [Quick (prothrombin time) 69 (56–76) %; INR 1.3 (1.2–1.5); aPTT 39 (37–47) seconds]. The median platelet count was 135 (106–181)/nl.
Perioperative anticoagulation/platelet inhibition and substitution. (A) Pre- and postoperative anticoagulation and platelet inhibition before and after decompressive hemicraniectomy. (B) Perioperative substitution of coagulations factors and platelets during decompressive hemicraniectomy. The numbers inside the bar graphs represent the corresponding absolute number of events. ASA acetylsalicylic acid, DAPT dual antiplatelet therapy, FFP fresh frozen plasma, PC platelet concentrate, PCC prothrombin complex concentrate.
Pre- or intraoperatively, 24/29 patients (83%) received substitution with platelets, fresh frozen plasma, tranexamic acid, fibrinogen or prothrombin complex (Fig. 3B). Twenty out of 29 patients (69%) received red blood cell concentrates.
Postoperatively, 11/29 patients (38%) received no or only prophylactic anticoagulation with heparin or argatroban, 3/29 patients (10%) received therapeutic anticoagulation, 12/29 patients (42%) received antiplatelet therapy with ASA and/or Clopidogrel, and 3/29 patients (10%) received both therapeutic anticoagulation and antiplatelet therapy (Fig. 3A). In 18/29 patients (62%), therapeutic anticoagulation and/or antiplatelet therapy was re-initiated 6 (4–11) days after DC.
Postoperative thrombotic cardiovascular and hemorrhagic intracranial complications
Fifteen out of 29 patients (52%) presented a postoperative intracranial hemorrhage on postoperative day 1 (1–5). Four of these 15 patients (27%) suffered a massive ICH on postoperative day 1 (1–2) despite perioperative substitution. Of note, all but one new hemorrhagic lesion occurred before the discontinued anticoagulation and/or platelet inhibition were re-initiated. Bayesian logistic regression yielded a longer preoperative aPPT and new or progressive postoperative hemorrhagic lesions on CT imaging as statistically significant predictors of mortality (p < 0.05; Table 4). In complete data sets, both variables displayed strongly increased risks when analyzed bi-variately: If aPPT was prolonged by ten seconds, the risk of mortality increased 14-fold (OR = 13.94, p = 0.038). If a new or progressive lesion after DC was noted, the risk of mortality was tripled (OR = 3.03, p = 0.19).
Despite perioperative discontinuation of anticoagulation and/or platelet inhibition, no coagulation-associated cardiovascular complications were noted, except for a recurrent LVAD thrombosis in a patient who already had suffered an initial pump thrombosis immediately after LVAD implantation and underwent an endovascular thrombectomy.
Missing patient data
Documentation of the preoperative anticoagulation and/or antiplatelet therapy before DC was missing in 6/29 patients (21%). Preoperative coagulation parameters before DC were not available in 1/29 patients (3%). Documentation of perioperative coagulation factor and platelet substitution was missing in 1/29 patients (3%). Three out of 29 patients (10%) were lost for follow-up data at 3–6 months. In 1/29 patients (3%) no postoperative CT within 24 h was available for infarct volumetry.
Discussion
In this study, we found that DC in patients with MHS following CTS may help reduce mortality compared to the natural course of MHS. Specifically, a prolonged aPTT and new or progressive postoperative hemorrhagic lesions were found to be associated with a significantly greater risk for mortality at 3–6 months. This highlights the need to optimize preoperative coagulation, because even after discontinuation and correction of anticoagulation and/or platelet inhibition, the risk for cardiovascular complications remained low. Nevertheless, the benefit of reducing mortality through decompressive surgery needs to be carefully balanced against a high likelihood of unfavorable neurological outcome.
Malignant hemispheric stroke is a life-threatening condition with a mortality of up to 80% in intensive care-based series21. Randomized controlled trials (RCTs) performed in MHS patients without significant comorbidities have evidenced that early DC undertaken within 48 h of stroke onset significantly reduces mortality10,12,13,14. Unfortunately, early identification of patients with neurological deterioration after CTS remains challenging due to the prolonged recovery phase from anesthesia. Although extended neuromonitoring might allow early identification of patients with space-occupying perioperative stroke following CTS22,23, in clinical practice, monitoring is often limited to neurological examination and affected patients are mainly identified by prolonged impaired consciousness, hemiplegia, seizures or dilated pupils due to an already manifest and life-threatening space-occupying brain edema. Apart from this delayed detection, another problem regarding decision-making and patient counselling is that CTS patients suffer serious cardiovascular comorbidities. Therefore, the known randomized controlled trials on MHS cannot be generally translated to this patient population14.
A natural conflict exists between coagulation requirements following CTS on one side, and after DC for MHS on the other, where the main goal is to limit the risk of postoperative hemorrhage and secondary lesion progression by strict anticoagulation control and hemodynamic management22. To the best of our knowledge, only few case reports exist regarding the feasibility but not functional outcome of performing DC in the context of acute aortic repair24,25,26,27 or LVAD thrombosis28. Against this background, the present study represents the first and largest series to date and aims to provide comprehensive information regarding outcome and coagulation management in patients undergoing DC after CTS.
Mortality and functional outcome
In the present study, the overall mortality rate of 42% after 3–6 months appears to be lower than the mortality rate of up to 80% that has been reported for the natural course of MHS in patients without severe cardiothoracic comorbidity21. On the other hand, mortality was also higher than the 29–33% described in the known RCTs on MHS (DESTINY, DECIMAL and HAMLET)14 but these trials had excluded patients with a pre-morbid mRS > 1, pre-morbid Barthel-Index < 95, and with other concomitant severe diseases that would likely have affected treatment or reduced life expectancy below 3 years. In contrast, patients in our study population suffered severe pre-existing cardiovascular co-morbidities and underwent a major CTS procedure, which may likely affect morbidity and mortality. This is mirrored by the fact that in our cohort only 7% reached an mRS score of 3 and none had an mRS ≤ 2 after 3–6 months, compared to previously published RCTs where favorable outcome (mRS 0–3) was reached in 25–47% after 6 months10,11,12,13,14. Therefore, our present results rather compare to MHS patients with a median age of 70, where DC may still reduce mortality (33% vs. 70% without surgery), but with a much lower likelihood of merely 6% that experience favorable outcome (mRS 0–3) at 6 months11.
Another prognostic factor is the time between stroke onset and DC. Latest studies suggest that DC performed after 48 h after stroke onset might still have a beneficial effect on mortality and outcome and that herniation could be the more decisive factor, rather than the mere temporal aspect29. Due to the delayed recovery from anesthesia, however, the stroke onset in patients after CTS is difficult to determine. In fact, in 41% of our patients the decision to perform CT imaging was triggered by uni- or bilaterally dilated pupils due to an already existing, space-occupying brain edema, which highlights that patients in our cohort were more severely affected than patients included in previous RCTs and this could at least partially explain why the benefit of survival that we observed was associated with a strikingly high proportion of patients that experienced severe neurological disability. Next to other factors22,30,31, this risk of severe disability must be taken into account when counselling patients and caregivers on what to expect from decompressive surgery after CTS The delayed identification of patients with postoperative stroke following CTS further emphasizes the need for continuously improving interdisciplinary standard operating procedures for stroke detection, for example by implementing routine CT perfusion imaging after certain high-risk CTS procedures as a tool for earlier stroke diagnosis.
Postoperative anticoagulation management and complications
Approximately 79% of the patients in our study had received anticoagulant and/or antiplatelet therapy before DC but only 21% had therapeutic aPTT levels. This discrepancy is partially explained by the fact that the effect of antiplatelet medication is not reflected by anticoagulation parameters. Further, the short interval between CTS and DC may have additionally hampered an establishment of stable heparin levels32. Nevertheless, we observed a significant association between a prolonged aPTT and the development of new or progressive hemorrhagic lesions and the overall mortality after DC, which suggests an increased risk related to anticoagulation after CTS and highlights the importance of optimizing coagulation parameters before DC. Under perioperative substitution, the risk of severe space-occupying ICH was still 14% but this appears to be in line with the approximately 10% rate of new ipsilateral hemorrhagic complications reported in the literature across all indications for DC33,34. Thus, the risk for severe postoperative hemorrhagic complications after DC may be considered to be acceptable, given the severity of the disease and complex anticoagulation management requirements in CTS patients. This is further supported by the fact that the vast majority of postoperative hemorrhagic lesions were noted before a re-initiation of anticoagulation and/or platelet inhibition was begun. Most importantly, despite perioperative substitution and discontinuation of anticoagulation and/or platelet inhibition, no thromboembolic cardiovascular complications occurred, except for one recurrent pump thrombosis in a patient who had already suffered an initial pump thrombosis immediately after LVAD implantation. The high rate of cranioplasty in our study despite the high proportion of patients with unfavorable outcome remains debatable and the management of anticoagulant/antiplatelet therapy adds another layer of complexity to the cranioplasty decision-making process. However, several reports have shown clinical improvement after cranioplasty, suggesting that the craniectomy defect itself may prolong recovery, especially in patients with a sunken skin flap and syndrome of the trephined35,36. Thus, cranioplasty might facilitate neurological rehabilitation regardless of the degree of disability, next to providing protection of the underlying brain during mobilization and nursing activities37. Together with the low rate of thromboembolic complications that we experienced, we believe that the consideration of performing cranioplasty is also justified in a high-risk CTS population.
Limitations
This study bears well known limitations due to its retrospective design and limited patient number and our database only provided data on functional outcome until 3–6 months after the event. On the other hand, DC in CTS remains rare the short follow-up period appears reasonable, considering the severe co-morbidities within our cohort. The major limitation of our study is the missing control group of CTS patients with MHS that were managed without DC. This lacking control most likely mirrors our own selection bias following the ethical consideration that in situations of MHS after CTS where survival is already judged to be highly unlikely at the time-point of diagnosis, the reasonable interdisciplinary decision was made to rather offer supportive care than surgical decompression.
Conclusion
Cardiothoracic surgery patients suffering MHS will likely suffer severe neurological disability after DC, since the majority of patients in our study experienced either mortality or significant long-term disability. This should remain a central aspect during counselling and decision-making and given the poor prognosis, surgeons need to carefully consider whether DC should be performed at all. Nevertheless, the fact that we observed no thromboembolic complication due to discontinuation of anticoagulation and/or antiplatelet therapy argues that the complex coagulation situation after CTS should not per se rule out the option of performing life-saving surgical decompression.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Anyanwu, A. C., Filsoufi, F., Salzberg, S. P., Bronster, D. J. & Adams, D. H. Epidemiology of stroke after cardiac surgery in the current era. J. Thorac. Cardiovasc. Surg. 134(5), 1121–1127 (2007).
Bucerius, J. et al. Stroke after cardiac surgery: A risk factor analysis of 16,184 consecutive adult patients. Ann. Thorac. Surg. 75(2), 472–478 (2003).
Sheriff, F. et al. Large-vessel occlusion stroke after cardiothoracic surgery: Expanding time windows offer new salvage opportunities. J. Thorac. Cardiovasc. Surg. 158(1), 186–196 (2019).
Bossone, E. et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation 128(11_suppl_1), S175–S179 (2013).
Estrera, A. L. et al. Acute type A aortic dissection complicated by stroke: Can immediate repair be performed safely?. J. Thorac. Cardiovasc. Surg. 132(6), 1404–1408 (2006).
Sultan, I. et al. Predictors and outcomes of ischemic stroke after cardiac surgery. Ann. Thorac. Surg. 110(2), 448–456 (2020).
Gaudino, M. et al. Early versus delayed stroke after cardiac surgery: A systematic review and meta-analysis. J. Am. Heart Assoc. 8(13), e012447 (2019).
Wilkinson, D. A. et al. Mechanical thrombectomy improves outcome for large vessel occlusion stroke after cardiac surgery. J. Stroke Cerebrovasc. Dis. 30(8), 105851 (2021).
Hagan, P. G. et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. JAMA 283(7), 897–903 (2000).
Jüttler, E. et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY) a randomized, controlled trial. Stroke 38(9), 2518–2525 (2007).
Jüttler, E. et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N. Engl. J. Med. 370(12), 1091–1100 (2014).
Hofmeijer, J. et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): A multicentre, open, randomised trial. Lancet Neurol. 8(4), 326–333 (2009).
Vahedi, K. et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 38(9), 2506–2517 (2007).
Vahedi, K. et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 6(3), 215–222 (2007).
Dunning, J. et al. Guideline on antiplatelet and anticoagulation management in cardiac surgery. Eur. J. Cardiothorac. Surg. 34(1), 73–92 (2008).
Vandenbroucke, J. P. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 4(10), e297 (2007).
Neugebauer, H. et al. Large size hemicraniectomy reduces early herniation in malignant middle cerebral artery infarction. Cerebrovasc. Dis. 41(5–6), 283–290 (2016).
Güresir, E. et al. Rapid closure technique in decompressive craniectomy. J. Neurosurg. 114(4), 954–960 (2011).
Van Buuren, S. & Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Bürkner, P.-C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1–28 (2017).
Hacke, W. et al. “Malignant” middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch. Neurol. 53(4), 309–315 (1996).
Hecht, N. et al. Perfusion-dependent cerebral autoregulation impairment in hemispheric stroke. Ann. Neurol. 89(2), 358–368 (2021).
Woitzik, J. et al. Excitotoxicity and metabolic changes in association with infarct progression. Stroke 45(4), 1183–1185 (2014).
Mohamed, W., Zaheer, A. & Deshpande, R. Early aortic repair and decompressive hemicraniectomy in aortic dissection with ischaemic stroke. Perfusion 36(2), 113–117 (2021).
Biancari, F. et al. Decompressive hemicraniectomy for treatment of space occupying ischemic stroke after repair of type-A aortic dissection. Ann. Ital. Chir. 86(3), 258–260 (2015).
Kano, M., Iwahori, A. & Ogino, H. Aortic repair following initial decompressive craniectomy for acute type A aortic dissection complicated with extensive hemorrhagic cerebral infarction: A case report. Surg. Case Rep. 8(1), 1–5 (2022).
Iliescu, V. A. et al. Combined cardiac-neurosurgical treatment of acute aortic dissection, stroke, and coma. Tex. Heart Inst. J. 35(2), 200 (2008).
Oulehri, W. et al. Decompressive hemicraniectomy for acute ischemic stroke in a patient implanted with a left ventricular assist device: a case report. BMC Cardiovasc. Disord. 20(1), 1–4 (2020).
Goedemans, T. et al. Outcome after decompressive craniectomy for middle cerebral artery infarction: Timing of the intervention. Neurosurgery 86(3), E318–E325 (2020).
Hecht, N. et al. Infarct prediction by intraoperative laser speckle imaging in patients with malignant hemispheric stroke. J. Cereb. Blood Flow Metab. 36(6), 1022–1032 (2016).
Hecht, N. et al. Infarct volume predicts outcome after decompressive hemicraniectomy for malignant hemispheric stroke. J. Cereb. Blood Flow Metab. 38(6), 1096–1103 (2018).
Guervil, D. J. et al. Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion. Ann. Pharmacother. 45(7–8), 861–868 (2011).
Kurland, D. B. et al. Complications associated with decompressive craniectomy: A systematic review. Neurocrit. Care 23(2), 292–304 (2015).
Lee, M.-H. et al. Hydrocephalus following decompressive craniectomy for malignant middle cerebral artery infarction. Clin. Neurol. Neurosurg. 114(6), 555–559 (2012).
Ashayeri, K., Jackson, E. M., Huang, J., Brem, H. & Gordon, C. R. Syndrome of the trephined: A systematic review. Neurosurgery 79(4), 525–534 (2016).
Eaton, J. C. et al. Complications associated with early cranioplasty for patients with traumatic brain injury: A 25-year single-center analysis. J. Neurosurg. 137(3), 776–781 (2022).
Sveikata, L. et al. Syndrome of the trephined: clinical spectrum, risk factors, and impact of cranioplasty on neurologic recovery in a prospective cohort. Neurosurg. Rev. 45(2), 1431–1443. https://doi.org/10.1007/s10143-021-01655-6 (2022).
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors confirm that the manuscript complies with all instructions to authors. The authorship requirements have been met and the final manuscript was approved by all authors. This manuscript has not been published elsewhere and is not under consideration by another journal. The authors further confirm adherence to ethical guidelines and the manuscript was approved by the ethics committee of the Charité – Universitätsmedizin Berlin (EA4/019/22). For this type of study, formal consent is not required. Data acquisition and presentation was done according to the STROBE guidelines for reporting observational studies. This research received no specific grant or funding. NH is Berlin Institute of Health Clinical Fellow, funded by Stiftung Charité.
Author information
Authors and Affiliations
Contributions
Conception and design: N.H., V.P. Acquisition of data: P.T., J.F., R.A. Analysis and interpretation of data: P.T., N.H., V.P. Drafting the article: P.T. Critically revising the article: All authors. Reviewed submitted version of manuscript: N.H., V.P., P.T. Approved the final version of the manuscript on behalf of all authors: N.H. Statistical analysis: D.S., P.T., A.F. Administrative/technical/material support: A.F., P.V., S.J. Study supervision: N.H., V.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Truckenmueller, P., Fritzsching, J., Schulze, D. et al. Outcome and management of decompressive hemicraniectomy in malignant hemispheric stroke following cardiothoracic surgery. Sci Rep 13, 12994 (2023). https://doi.org/10.1038/s41598-023-40202-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40202-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.