Abstract
Elective single-embryo transfers of euploid or low-level mosaic blastocysts were analyzed in this retrospective study to determine the correlations of live birth (LB) probability with embryonic developmental features of implanted day 5 (D5, n = 245) or day 6 (D6, n = 73) blastocysts using time-lapse (TL) monitoring. According to the logistic regression analyses (adjusted odds ratio [OR] = 0.341, 95% confidence interval [CI] = 0.169–0.685, P < 0.05), the LB probability was negatively associated with the D6 group. The LB rate of the D5 group was higher than the D6 group (88.2% vs. 75.3%; P < 0.05). Compared with the D5 blastocysts, the D6 blastocysts exhibited comparable dysmorphisms except for the multinucleation at the 4-cell stage (10.9% vs. 2.9%, P < 0.05). Moreover, D6 blastocysts had considerably slower developmental kinetics and poorer blastocyst morphologies. Further analysis confirmed that the LB rate was not associated with developmental kinetics or dysmorphisms but rather with blastocyst morphology (inner cell mass [ICM] grade ≤ C vs. ICM grade A, adjusted OR = 0.155, 95% CI = 0.04–0.596, P < 0.05; trophectoderm [TE] grade ≤ C vs. TE grade A, adjusted OR = 0.157, 95% CI = 0.032–0.760, P < 0.05). In conclusion, D6 implanted blastocysts have a considerably lower LB rate than D5 implanted blastocysts. As determined by TL monitoring, the diminished blastocyst morphology can be one of the primary reasons underlying the decreased likelihood of LB.
Similar content being viewed by others
Introduction
Although randomized controlled trials have revealed no difference in pregnancy outcomes between frozen and fresh embryos1,2, advances in embryonic cryopreservation technology, specifically the development of vitrification techniques, have not only greatly increased the safety and usefulness of embryo freezing but also helped women seeking to preserve their fertility by promoting the widespread use of blastocyst frozen embryo transfer (FET)3. Research has also indicated that endometrial receptivity is adversely affected by ovarian stimulation during in vitro fertilization (IVF) procedures, which have a negative effect on endometrial normality and influence endometrial development4,5. These findings indicate that FET may be advantageous for endometrial–embryonic synchronization during IVF cycles.
High-resolution next-generation sequencing (hr-NGS) has been increasingly used worldwide in preimplantation genetic testing for aneuploidy (PGT-A) because of its high precision, effectiveness, and throughput6,7. When combined with the FET strategy, embryo selection through PGT-A can substantially improve the implantation outcomes of single-embryo transfer (SET) cycles8. Cytogenetic studies have indicated that approximately 50% of miscarriage samples contain chromosomal abnormalities, which are regarded as the most critical cause of spontaneous miscarriages9,10. Specifically, advanced maternal age has been associated with a sharp increase in miscarriages11. In IVF cycles, the application of PGT-A has been reported to effectively mitigate the risk of miscarriage12,13,14. However, miscarriages are still maintained at a low level after the implantation of euploid embryos, and their underlying causes remain unclear15.
Recent studies on embryo–endometrium interactions have indicated that women with a risk of miscarriage may be less selective than their counterparts in terms of embryo implantation. During peri-implantation, embryonic signals reach the decidualized endometrium and stimulate endometrial cell migration, and the biosensor of these endometrial cells selects a qualified blastocyst for implantation16,17,18,19. Therefore, selecting the most viable embryo before embryo transfer (ET) is essential to mitigate the miscarriage risk of successfully implanted embryos in PGT-A cycles.
Accordingly, selecting qualified frozen blastocysts is critical for optimizing the pregnancy outcomes of FET. Numerous studies have indicated that fast-growing blastocysts are associated with better clinical outcomes than those of slow-growing blastocysts. They have also indicated that the differences in the embryonic factors between fast-growing and slow-growing blastocysts are a topic that warrants further study20,21,22,23. Time-lapse (TL) monitoring has been used in IVF to provide a detailed and dynamic evaluation of the kinetics, dysmorphisms, and morphology of fertilized embryos, all of which have been proposed as being able to predict embryo growth, ploidy status, and pregnancy success24,25,26,27. The primary objective of the present study was to evaluate the TL data of individual implanted embryos in PGT-A cycles to determine whether developmental kinetics, cleavage anomalies, and blastocyst morphologies are related to the likelihood of fetal loss (FL) or live birth (LB). These TL features can be used in noninvasive analyses to improve the selection of competent embryos that may have high LB potential after implantation. This may be particularly useful for IVF patients who have a risk of miscarriage.
Materials and methods
Study design
This retrospective cohort study was performed in accordance with relevant guidelines and regulations. The Institutional Review Board of Chung Shan Medical University (approval number CS1-21156) provided a waiver of written informed consent for this study. Data on pregnant women undergoing ET of frozen–thawed blastocysts after PGT-A cycles were gathered from Lee Women’s Hospital from January 2018 to December 2021. Patients with an endometrial thickness of 7 mm or less, severe uterine abnormalities, transfers of more than one embryo, and a transferred embryo with a mosaic level of 50% or higher were excluded. A total of 318 FET cycles, in which intrauterine pregnancies were confirmed by visualizing at least one gestational sac, from 304 patients were included. The baseline characteristics and FET cycle parameters were collected, including the number of previous IVF cycles, age, anti-Müllerian hormone (AMH) level, body mass index (BMI), serum estradiol (E2), and progesterone (P4) levels on the day of ET, oocyte sources, sperm quality, endometrial preparation protocols, embryo ploidy status, and blastocyst vitrification day.
Laboratory procedures
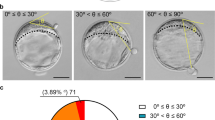
All laboratory procedures were conducted in accordance with the standard protocols described in our previous reports25,26. Briefly, a gonadotrophin-releasing hormone (GnRH) agonist long protocol (Lupron; Takeda Chemical Industries, Osaka, Japan) or a GnRH antagonist protocol (Cetrotide; Merck Serono, Geneva, Switzerland) was used for controlled ovarian stimulation. Follicle development was stimulated by the administration of exogenous gonadotropin (GONAL-f, Merck Serono; Menopur; Ferring Pharmaceuticals, São Paulo, Brazil) until the size of the leading follicle reached or exceeded 18 mm. Human chorionic gonadotropin (hCG, 250 μg, Ovidrel; Merck Serono, Modugno, Italy) was then used to stimulate oocyte maturation, and ultrasound-guided ovum retrieval was conducted 36 h after the administration of hCG. The derived oocytes inseminated by the method of intracytoplasmic sperm injection or conventional insemination were cultured in a TL culture system (EmbryoScope + ; Vitrolife, Kungsbacka, Sweden) with sequential media (SAGE Biopharma, Bedminster, NJ, USA) in an environment containing 5% O2, 5% CO2, and 90% N2 at 37 °C. TL assessments of individual embryos for morphokinetics, cleavage dysmorphisms, and morphology were subsequently performed at 118 h post insemination (hpi) by using all of the recorded TL images in accordance with the process outlined in our previous report26. Briefly, the blastocyst expansion levels were annotated to highlight the specific developmental features, including a blastocoel cavity beginning to form (level 1), to expand (level 2), and to herniate (level 3). Additionally, the evaluations of ICM and TE quality were conducted following the manufacturer's guidelines, which were applied to generalize the Gardner blastocyst grading system to time-lapse imaging. Detailed information is provided in Supplementary Table 1.
Qualified blastocysts on day 5 (D5) or day 6 (D6) expanded blastocysts (embryo diameter ≥ 150 µm) with an inner cell mass (ICM) grade of at least B or a trophectoderm (TE) grade of at least B were selected for embryo biopsy. Micromanipulation with inverted microscopy and a laser system was applied to carefully separate 5 to 8 trophectoderm (TE) cells from a blastocyst. The separated TE cells were rinsed with phosphate-buffered saline thoroughly and then cautiously placed on the bottom of an RNAse–DNAse-free polymerase chain reaction tube for the following tests. The remaining blastocysts were incubated in vitro for at least 3 h prior to cryopreservation. An hr-NGS platform (Illumina, San Diego, CA, USA) was used to determine the mosaic levels of biopsied blastocysts as per the manufacturer’s instructions.
Embryo cyropreservation and FET
Vitrification and warming of the biopsied blastocysts were accomplished using the Cryotech method (Cryotech, Tokyo, Japan). Women undergoing FETs of a single blastocyst selected on the basis of hr-NGS results and blastocyst morphology were subjected to a natural (NC), modified natural (mNC), or artificial (AC) cycle of endometrial preparation. The ovulation of the dominant follicle in a NC was monitored by transvaginal ultrasound detection. A mNC was defined by triggering the ovulation of the leading follicle (≥ 18 mm) using hCG injection (250 μg, Ovidrel; Merck Serono, Modugno, Italy). Luteal-phase support (LPS) for NC and mNC was offered from the first day after ovulation to the day of the pregnancy test, including oral dydrogesterone 10 mg three times a day (Duphaston, Abbott Biologicals B.V., the Netherlands), vaginal micronized progesterone 90 mg two times a day (Crinone 8%, Merck Serono, Darmstadt, Germany), and oral estradiol valerate 6 mg daily (Estrade, Synmosa, Taipei, Taiwan). In the AC, the patients were administered with an estradiol valerate supplementation regimen of endometrial preparation as follows: (1) 4 mg daily on days 3–4 of their natural menstrual cycle; (2) 8 mg daily on days 5–7 of their natural menstrual cycle; (3) 12 mg daily on days 8–12 of their natural menstrual cycle. From day 13 of the menstrual cycle to the day of the pregnancy test, LPS for an AC was offered for patients with sufficient endometrial thickness, including oral dydrogesterone 10 mg three times a day, vaginal micronized progesterone 90 mg three times a day, and oral estradiol valerate 6 mg daily. The embryo transfer was performed on day 5 after ovulation in the NC and mNC or after progesterone administration in the AC for the patients with endometrial thickness of at least 8 mm. If the endometrial thickness was less than 8 mm, the transfer was canceled and shifted to the next cycle. The LPS with oral dydrogesterone and vaginal micronized progesterone continued up to 10 weeks of gestation for pregnant patients.
On the ET day, the endometrial thickness and serum E2 and P4 levels were measured for each patient, and then the clinical outcomes of pregnant patients with a visualized intrauterine gestational sac were evaluated. A LB was defined as a baby born alive at 24 weeks of gestation or more. A FL was defined as a pregnancy characterized by the occurrence of a blighted ovum, absence of a fetal heartbeat, intrauterine fetal death or growth restriction, or stillbirth (fetal death at 20 weeks of gestation or more).
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 20.0 (IBM, Armonk, NY, USA) or GraphPad Prism version 6.0 h (GraphPad Software, San Diego, CA, USA). Proportions were used to summarize categorical variables, and means with standard deviations were used to summarize continuous variables. Generalized estimating equation (GEE) analysis with logistic regression settings was used to assess the correlations between the LB probability and independent variables in unadjusted (univariate) and adjusted (multivariate) models. The confounders were determined by the backward elimination procedure until the remaining variables in the multivariate regression model had a P value < 0.2. The differences between groups were assessed using the Kolmogorov–Smirnov test, Fisher’s exact test, or chi-square test, as applicable. Statistical significance was set at P < 0.05 in all analyses.
Ethics approval and consent to participate
This retrospective cohort study was reviewed and approved by the Institutional Review Board of Chung Sun Medical University, Taichung, Taiwan (Approval Number CS1-21156).
Results
Potential factors influencing the LB probability of implanted blastocysts
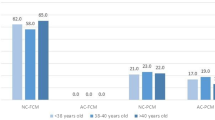
Women (n = 304) who underwent FETs with single euploid or low-level mosaic blastocysts and who exhibited a positive sign of pregnancy through gestational sac visualization 5 weeks after their last menstrual period were included in this study. Of the cohort of 318 SETs, 245 women successfully gave birth. Univariate logistic analysis with the GEE model revealed that none of the patient characteristics were correlated with the probability of LB, i.e., the number of previous IVF cycles, age, AMH level, BMI, E2, and P4 levels on the day of ET, oocyte sources, sperm quality, endometrial preparation protocols, and embryo ploidy status, with the exception of the blastocyst vitrification day. Compared with ETs with a D5 blastocyst (i.e., the D5 group), ETs with a D6 blastocyst (i.e., the D6 group) were negatively correlated with the probability of LB (odds ratio [OR] = 0.41, 95% confidence interval [CI] = 0.207–0.813; P = 0.011; Table 1). When taking consideration of the confounders (i.e., AMH, E2 levels, oocyte sources, and ploidy status) determined by the backward elimination procedure, ETs with a D6 blastocyst were still negatively correlated with the probability of LB (adjusted OR = 0.341, 95% CI = 0.169–0.685; P = 0.003; Table 1). In addition, the D5 group had lower rates of FL at ≤ 12 weeks (10.6% versus [vs.] 17.8%), > 20 to ≤ 20 weeks (0% vs. 2.7%), and > 20 weeks (1.2% vs. 4.1%) than those of the D6 group, which resulted in a significant increase in the LB rate in the D5 group (88.2% vs. 75.3%; P < 0.05; Fig. 1).
The postimplantation outcomes of day 5 vs. day 6 blastocysts. Following embryo transfer and implantation, Fisher’s exact test was used to compare the fetal loss and live birth rates between day 5 and day 6 groups. Abbreviations “wks”, “D5”, and “D6” denoted the weeks of gestation, day 5, and day 6, respectively.
Baseline characteristics of transfer cycles with D5 and D6 implanted blastocysts
Table 2 presented the differences in the patient characteristics between the D5 and D6 groups. The Kolmogorov–Smirnov test revealed that the female age (oocytes and recipients), AMH level, BMI, E2, and P4 levels on the FET day, number of previous IVF cycles, and infertility periods did not differ between the two groups. The chi-square test or Fixher’s exact test revealed no differences in infertility status, oocyte sources, sperm quality, endometrial preparation, or ploidy status (Table 2).
Embryological characteristics of D5 and D6 implanted blastocysts
TL monitoring was used to evaluate the differences in the embryological characteristics between the D5 and D6 groups. Fisher’s exact test or chi-square test was used to determine the differences in embryonic dysmorphism between the groups. The results indicated that the incidence of embryonic dysmorphism in the implanted embryos was typically less than 10%. However, TL monitoring revealed an increased frequency of uneven cleavage at the four-cell stage (13.7% to 15.9%), multinucleation at the two-cell stage (21.6% to 24.7%), noncentral juxtaposition (74% to 76.3%), uneven pronuclear size (39.7% to 42%), and vacuolization (9% to 10.9%). A comparison of the D5 and D6 blastocysts revealed that the proportions of embryonic dysmorphisms (i.e., uneven cleavage at the two-cell stage, uneven cleavage at the four-cell stage, multinucleation at the two-cell stage, noncentral juxtaposition of pronuclei, no pronuclear contact, uneven pronuclear size, unsynchronized pronuclear fading, twist-and-crumble division, incomplete chaotic division, direct unequal cleavage, reverse cleavage, delayed cleavage, vacuolization, and premature compaction) were not significantly different between the groups (Table 3). However, the rate of multinucleation at the 4-cell stage (10.9% vs. 2.9%) was significantly higher in the D6 group than that in the D5 group.
After pronuclear fading, the embryonic kinetics of the D5 group were faster than those of the D6 group. Specifically, significant differences were observed between the groups in t3 (13.9 ± 1.3 h vs. 15.5 ± 1.5 h), t4 (14.8 ± 2.3 h vs. 16.5 ± 1.9 h), t5 (27.6 ± 2.8 h vs. 31.7 ± 4.7 h), t8 (32.7 ± 6.5 h vs. 37.6 ± 6.9 h), tM (62.8 ± 6.9 h vs. 70.9 ± 6.9 h), tSB (71.6 ± 4.7 h vs. 79.6 ± 6.5 h), and tB (80.2 ± 5.0 h vs. 90.3 ± 7.2 h). In addition, blastocyst formation took longer in the D6 group (tSB–tB, 10.7 ± 4.2 h) than in the D5 group (tSB–tB, 8.7 ± 2.9 h; P < 0.05). When the KIDScore™ D5 algorithm was used, these differences in embryonic kinetics resulted in considerably lower scores in the D6 group (2.9 ± 1.6) than in the D5 group (4.8 ± 1.3; Table 4).
The components of the blastocyst morphology were redefined through a detailed evaluation completed using TL monitoring at a specific time window (118 hpi). Substantial differences were noted between the D5 and D6 groups in terms of expansion level ≥ 2 (99.2% vs. 78.1%), ICM level ≥ B (98.4% vs. 74.0%), and TE level ≥ B (92.7% vs. 46.6%; Table 5).
Embryological factors influencing the LB probability of implanted blastocysts
Logistic analysis with the GEE model was used to determine the correlation between the probability of LB and embryological factors, which varied between the D5 and D6 groups. The results revealed that multinucleation at the 4-cell stage, embryonic developmental kinetics, KIDScore™ D5 scores, and expansion levels were not significantly associated with the probability of LB (Tables 6 and 7). However, compared with ICM level A, ICM levels ≤ C were negatively correlated with LB in univariate (OR = 0.179, 95% CI = 0.049–0.656, P = 0.009) and multivariate (adjusted OR = 0.155, 95% CI = 0.04–0.596, P = 0.007) logistic regression models. Compared with TE level A, TE levels ≤ C were negatively correlated with LB in univariate (OR = 0.226, 95% CI = 0.061–0.844, P = 0.027) and multivariate (adjusted OR = 0.157, 95% CI = 0.032–0.760, P = 0.021) logistic regression models (Table 7). A blastocyst morphology ≥ 2BB was defined as a favorable morphology. As indicated in Fig. 2, the LB rate was considerably higher in blastocysts with a favorable morphology (≥ 2BB, 88.1%) than in those with a poor morphology (< 2BB, 72.4%). However, a comparison of D5 and D6 blastocysts with the same embryonic morphology indicated that the LB rate did not differ between the poor-morphology (84.2% vs. 66.7%) and favorable-morphology (88.5% vs. 85.3%) blastocyst groups (P > 0.05).
The live birth rates of implanted blastocysts stratified by embryo morphology. Following embryo transfer and implantation, Fisher exact test was used to compare the live birth rates of implanted blastocysts with morphology < 2BB or ≥ 2BB. The abbreviation “2BB” denoted blastocysts with the level 2 of expansion, the grade B of inner cell mass, and the grade B of trophectoderm. The abbreviations “D5” and “D6” denoted day 5, and day 6, respectively.
Discussion
In our previous study28, in which a hr-NGS platform was used for PGT-A, we reported that FET groups of euploid and low-level mosaic blastocysts have comparable abortion rates. This means that the transfer of low-level mosaic embryos results in the birth of healthy, viable children. In the present study, we investigated the factors that likely influence the postimplantation development of euploid or low-level mosaic blastocysts in FET cycles. Logistic regression analysis with and without controlling for confounders revealed a correlation between the LB probability of implanted frozen–thawed embryos and blastocysts developing on D5 or D6 (Table 1). According to array comparative genomic hybridization and single-nucleotide polymorphism data, the LB rate per FET cycle is considerably higher in D5 euploid blastocysts than in D6 euploid blastocysts29,30,31. Even when confounders are adjusted for, the associations between the blastocyst development rate and clinical outcomes remain notable29. However, given the similar FL rates, the differences in the LB rates of successfully implanted embryos are nonsignificant between D5 and D6 euploid transfers29,30,32. In our clinical setting, we discovered that the D6 blastocysts were associated with an increased risk of FL in pregnant patients who received euploid or low-level mosaic blastocysts, which resulted in a considerably lower LB rate of D6 blastocyst transfers compared with D5 blastocyst transfers (Fig. 1). Generally, delayed blastocyst development is associated with an increased risk of mosaicism26, indicating that the incidence of aberrant ploidy in D6 blastocysts may increase and thereby lead to an increased risk of FL in non-PGT-A cycles33,34,35. However, according to our findings, the difference in ploidy status between the D5 and D6 blastocysts may not be the only intrinsic factor influencing the postimplantation development of embryos. Therefore, further understanding the differences between blastocysts developing on D5 and D6 through TL monitoring is essential. Embryonic factors may also serve as biomarkers for embryo selection that can be used to mitigate the risk of FL after implantation.
Several in vitro coculture studies have supported the concept of embryo–endometrium interactions in the selection of potent embryos for implantation. According to these studies, the production of implantation regulators of decidualized endometrial cells, such as the cytokines of interleukin (IL)-1β, heparin-binding epidermal growth factor-like growth factor, IL-6, and IL-10, is substantially inhibited in developmentally impaired human embryos16. In cocultures of embryos with a poor morphology, the migratory response of decidualized endometrial cells derived from normally fertile women is diminished17. However, for women with a risk of FL, the migratory response of decidualized endometrial cells is not inhibited in embryos with a poor morphology. According to molecular evidence, in women with recurrent pregnancy loss (RPL), endometrial cells downregulate the expression of mucin-1, which is a regulator of embryonic implantation, and thereby prevent the attachment of embryos with a poor morphology to the endometrium18,19. In addition, aberrant expression of decidual markers, such as prolactin and prokineticin-1, indicates impaired decidualization, which extends the receptivity window for the implantation of low-viability embryos10,36. These findings suggest that selecting blastocysts with a favorable quality for ET not only influences implantation and pregnancy outcomes but also mitigates the risk of FL after implantation34,37. Our results indicate that the cycle and patient characteristics were similar between the D5 and D6 implanted blastocyst groups (Table 2). Under these conditions, TL analysis indicated a low frequency of embryonic dysmorphisms in implanted embryos selected by PGT-A and no substantial differences between the D5 and D6 groups, with the exception of the multinucleation at the 4-cell stage (Table 3). Moreover, a comparison of the embryonic kinetics in the groups with implanted D5 vs. D6 blastocysts revealed considerably delayed cell division during both the early cleavage and blastocyst stages, prolonged intervals of blastocyst formation, and lower scores of KIDScore™ D5 model in the D6 group (Table 4). Nevertheless, logistic regression analysis revealed that the probability of LB was not associated with any dysmorphisms or developmental kinetics (Table 6). In a previous study, a mouse model demonstrated that asynchronous cell division during the early cleavage stage affects embryonic compaction and cell lineage formation through the aberrant nuclear translocation of yes-associated protein 1, resulting in a reduced cell number with ICM and an increased risk of abortion38. However, a large body of evidence has indicated that the morphokinetic profiles of FL and LB embryos are indistinguishable39. McQueen et al. used TL imaging to compare the morphokinetics between euploid embryos resulting in clinical FL, biochemical FL, and LB in patients undergoing IVF. Similar to our findings, they reported that the embryonic morphokinetic parameters cannot predict FL in PGT-A cycles40.
In the present study, using uniform time point assessments along with TL monitoring, we discovered substantial differences in the blastocyst morphological components between the D5 and D6 implanted blastocyst groups. In contrast to the developmental kinetics, positive correlations between the probability of LB and ICM or TE grading were observed in successfully implanted embryos (Table 7). Shi et al. reported considerably different FL rates between blastocysts classified by TE grading (grade A vs. grade B vs. grade C, 15.3% vs. 11.8% vs. 9.8%) in young IVF patients who received embryos without PGT-A and had a positive intrauterine pregnancy34. Moreover, in accordance with the findings of the present study, several reports have indicated that the grade of ICM or TE morphology in euploid embryos with FL is inferior to that in embryos with LB, indicating that blastocyst morphology is a critical biomarker associated with FL in euploid transfer cycles40,41. Recent reports have revealed better clinical outcomes in blastocysts with equal morphological quality on D5 than on D6, regardless of the PGT-A status22,23,29. The refined evaluation of blastocyst morphology based on TL monitoring at a uniform time point in the present study also demonstrated that blastocysts with a favorable morphology had a higher LB rate than that of blastocysts with a poor morphology. However, the clinical outcomes were similar between D5 and D6 blastocysts with an indistinguishable quality of morphology.
The primary limitation of the present single-center study was its retrospective nature. The absence of randomization may have resulted in selection bias, and 14 of the 304 enrolled patients had undergone multiple cycles of ET. Therefore, in this study, we analyzed repeated measurements by introducing the GEE model, which can be used to address the problem of potential correlations within the same subjects and is a marginal model that is widely adopted for longitudinal data. Although no major confounding variables related to LB were detected in the data set, a multivariate logistic regression model was used to confirm the correlation between LB and the dysmorphisms, development speed, and morphology of blastocysts by adjusting the candidate variables (i.e., AMH, serum estradiol levels, oocyte sources, and ploidy status; Table 1).
Despite the promising clinical improvement offered by PGT-A, FL may still occur in patients who undergo successful IVF. Even if patients experience RPL, research has indicated that embryonic quality may play an essential role in postimplantation development, indicating the necessity of extensive exploration of the embryonic factors that can predict FL in PGT-A cycles. In conclusion, using TL monitoring, we discover that D6 blastocysts are of a lower quality, which may affect the LB rate of implanted embryos, than that of D5 blastocysts. We also demonstrate that intrinsic embryonic factors are more likely to be the morphology of ICM and TE rather than dysmorphisms or morphokinetics.
Data availability
The datasets generated and/or analysed during the current study are available in the NCBI SRA repository (PRJNA937335, https://www.ncbi.nlm.nih.gov/sra). The reviewer link: https://dataview.ncbi.nlm.nih.gov/object/PRJNA937335?reviewer=vi5umrj0t9mqb2unrdti17q7rd.
Abbreviations
- PGT-A:
-
Preimplantation genetic testing for aneuploidy
- D5:
-
Day 5
- D6:
-
Day 6
- LB:
-
Live birth
- TL:
-
Time-lapse
- SET:
-
Single-embryo transfer
- ET:
-
Embryo transfer
- ICM:
-
Inner cell mass
- TE:
-
Trophectoderm
- IVF:
-
In vitro fertilization
- FET:
-
Frozen embryo transfer
- hr-NGS:
-
High-resolution next-generation sequencing
- FL:
-
Fetal loss
- AMH:
-
Anti-Müllerian hormone
- BMI:
-
Body mass index
- E2:
-
Estradiol
- P4:
-
Progesterone
- GnRH:
-
Gonadotrophin-releasing hormone
- hCG:
-
Human chorionic gonadotropin
- GEE:
-
Generalized estimating equation
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- IL:
-
Interleukin
- RPL:
-
Recurrent pregnancy loss
- vs.:
-
Versus
References
Shi, Y. et al. Transfer of fresh versus frozen embryos in ovulatory women. N. Engl. J. Med. 378, 126–136 (2018).
Stormlund, S. et al. Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilisation in women with regular menstrual cycles: multicentre randomised controlled trial. BMJ 370 (2020).
Rienzi, L. et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 23, 139–155 (2017).
Kolibianakis, E. M. et al. Abnormal endometrial development occurs during the luteal phase of nonsupplemented donor cycles treated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil. Steril. 80, 464–466 (2003).
Horcajadas, J. A. et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J. Clin. Endocrinol. Metab. 93, 4500–4510 (2008).
Fiorentino, F. et al. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum. Reprod. 29, 2802–2813 (2014).
Yang, Z. et al. Randomized comparison of next-generation sequencing and array comparative genomic hybridization for preimplantation genetic screening: a pilot study. BMC Med. Genom. 8, 30 (2015).
Friedenthal, J. et al. Next generation sequencing for preimplantation genetic screening improves pregnancy outcomes compared with array comparative genomic hybridization in single thawed euploid embryo transfer cycles. Fertil. Steril. 109, 627–632 (2018).
van den Berg, M. M., van Maarle, M. C., van Wely, M. & Goddijn, M. Genetics of early miscarriage. Biochim. Biophys. Acta 1822, 1951–1959 (2012).
Larsen, E. C., Christiansen, O. B., Kolte, A. M. & Macklon, N. New insights into mechanisms behind miscarriage. BMC Med. 11, 154 (2013).
Nybo Andersen, A. M., Wohlfahrt, J., Christens, P., Olsen, J. & Melbye, M. Maternal age and fetal loss: Population based register linkage study. BMJ 320, 1708–1712 (2000).
Grifo, J. A. et al. Single thawed euploid embryo transfer improves IVF pregnancy, miscarriage, and multiple gestation outcomes and has similar implantation rates as egg donation. J. Assist. Reprod. Genet. 30, 259–264 (2013).
Verpoest, W. et al. Preimplantation genetic testing for aneuploidy by microarray analysis of polar bodies in advanced maternal age: A randomized clinical trial. Hum. Reprod. 33, 1767–1776 (2018).
Murugappan, G., Shahine, L. K., Perfetto, C. O., Hickok, L. R. & Lathi, R. B. Intent to treat analysis of in vitro fertilization and preimplantation genetic screening versus expectant management in patients with recurrent pregnancy loss. Hum. Reprod. 31, 1668–1674 (2016).
Ying, L. Y., Sanchez, M. D., Baron, J. & Ying, Y. Preimplantation genetic testing and frozen embryo transfer synergistically decrease very pre-term birth in patients undergoing in vitro fertilization with elective single embryo transfer. J. Assist. Reprod. Genet. 38, 2333–2339 (2021).
Teklenburg, G. et al. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS ONE 5, e10258 (2010).
Weimar, C. H. et al. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS ONE 7, e41424 (2012).
Aplin, J. D., Hey, N. A. & Li, T. C. MUC1 as a cell surface and secretory component of endometrial epithelium: Reduced levels in recurrent miscarriage. Am. J. Reprod. Immunol. 35, 261–266 (1996).
Meseguer, M. et al. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol. Reprod. 64, 590–601 (2001).
Desai, N. et al. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil. Steril. 106, 1370–1378 (2016).
Haas, J. et al. Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J. Assist. Reprod. Genet. 33, 1553–1557 (2016).
Ferreux, L. et al. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expanded on Day 5 than on Day 6. Hum. Reprod. 33, 390–398 (2018).
Yerushalmi, G. M. et al. Day 5 vitrified blastocyst transfer versus day 6 vitrified blastocyst transfer in oocyte donation program. Sci. Rep. 11, 10715 (2021).
Meseguer, M. et al. The use of morphokinetics as a predictor of embryo implantation. Hum. Reprod. 26, 2658–2671 (2011).
Lee, C. I. et al. Embryo morphokinetics is potentially associated with clinical outcomes of single-embryo transfers in preimplantation genetic testing for aneuploidy cycles. Reprod. Biomed. Online 39, 569–579 (2019).
Chen, C. H. et al. Blastocyst morphology based on uniform time-point assessments is correlated with mosaic levels in embryos. Front. Genet. 12, 783826 (2021).
Desai, N., Goldberg, J. M., Austin, C. & Falcone, T. Are cleavage anomalies, multinucleation, or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy?. Fertil. Steril. 109, 665–674 (2018).
Lee, C. I. et al. Healthy live births from transfer of low-mosaicism embryos after preimplantation genetic testing for aneuploidy. J. Assist. Reprod. Genet. 37, 2305–2313 (2020).
Irani, M. et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil. Steril. 110, 95–102 (2018).
Barash, O. O. et al. Association between growth dynamics, morphological parameters, the chromosomal status of the blastocysts, and clinical outcomes in IVF PGS cycles with single embryo transfer. J. Assist. Reprod. Genet. 34, 1007–1016 (2017).
Li, N. et al. Effect of blastocyst morphology and developmental rate on euploidy and live birth rates in preimplantation genetic testing for aneuploidy cycles with single-embryo transfer. Front. Endocrinol. 13, 858042 (2022).
Abdala, A. et al. Day 5 vs day 6 single euploid blastocyst frozen embryo transfers: Which variables do have an impact on the clinical pregnancy rates?. J. Assist. Reprod. Genet. 39, 379–388 (2022).
Kaing, A. et al. Earlier day of blastocyst development is predictive of embryonic euploidy across all ages: Essential data for physician decision-making and counseling patients. J. Assist. Reprod. Genet. 35, 119–125 (2018).
Shi, D. et al. Association between the quality of inner cell mass and first trimester miscarriage after single blastocyst transfer. Reprod. Biol. Endocrinol. 18, 43 (2020).
Park, D. S. et al. Obstetric, neonatal, and clinical outcomes of day 6 vs. day 5 vitrified-warmed blastocyst transfers: Retrospective cohort study with propensity score matching. Front Endocrinol 11, 499 (2020).
Salker, M. et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS ONE 5, e10287 (2010).
Gardner, D. K., Lane, M., Stevens, J., Schlenker, T. & Schoolcraft, W. B. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil. Steril. 73, 1155–1158 (2000).
Mashiko, D. et al. Asynchronous division at 4-8-cell stage of preimplantation embryos affects live birth through ICM/TE differentiation. Sci. Rep. 12, 9411 (2022).
Amitai, T. et al. Embryo classification beyond pregnancy: early prediction of first trimester miscarriage using machine learning. J. Assist. Reprod. Genet. (2022).
McQueen, D. B. et al. Can embryo morphokinetic parameters predict euploid pregnancy loss?. Fertil. Steril. 115, 382–388 (2021).
Irani, M. et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil. Steril. 107, 664–670 (2017).
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This work was supported by Grants (111-2218-E-040-001 and 10-2314-B-040-005) from the Ministry of Science and Technology, Executive Yuan, Taiwan, Republic of China.
Author information
Authors and Affiliations
Contributions
L.M.S., L.T.H., & C.C.H. formulated this study. C.C.H., L.C.I., H.C.C., C.H.H., C.C.Y., C.E.H., C.C.I., and L.P.Y. collected and processed the data. C.C.H., L.C.I., and C.H.H. carried out analyses. C.C.H. wrote the manuscript. All authors reviewed the manuscript and provided editorial feedback.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, CH., Lee, CI., Huang, CC. et al. Increased incidence of live births in implanted day 5 versus day 6 blastocysts following single embryo transfers with PGT-A. Sci Rep 13, 12725 (2023). https://doi.org/10.1038/s41598-023-40052-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40052-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.