Abstract
The increased intake of ultraprocessed foods (UPFs) in the pediatric age paralleled with the risen prevalence of childhood obesity. The Ultraprocessed Foods in Obesity (UFO) Project aimed at investigating the potential mechanisms for the effects of UPFs in facilitating pediatric obesity, focusing on the direct role of advanced glycation end-products (AGEs) on mitochondrial function, the key regulator of obesity pathophysiology. We comparatively investigated the daily dietary intake of UPFs, energy, nutrients, dietary AGEs [Nε -(carboxymethyl)lysine (CML), Nε -(1-carboxyethyl)lysine (CEL), and Nδ -(5-hydro-5- methyl-4-imidazolon-2-yl)-ornithine (MG-H1)] in 53 obese patients and in 100 healthy controls visiting the Tertiary Center for Pediatric Nutrition of the Department of Translational Medical Science at the University of Naples “Federico II”. AGEs skin accumulation and mitochondrial function in peripheral blood mononuclear cells (PBMCs) were also assessed. A higher intake of UPFs and AGEs, energy, protein, fat, and saturated fatty acids was observed in obese patients. Obese children presented significantly higher skin AGEs accumulation and alterations in mitochondrial metabolism. PBMCs from healthy controls exposed to AGEs showed the same mitochondrial alterations observed in patients. These findings support the UPFs role in pediatric obesity, and the need for dietary strategies limiting UPFs exposure for obesity prevention and treatment.
Similar content being viewed by others
Introduction
Pediatric obesity is one of the most common worldwide public health challenges, requiring great efforts of the clinical research into its prevention and management1. In the last decades, increased consumption of ultraprocessed foods (UPFs) paralleled with the risen prevalence of childhood obesity2. UPFs are industrial formulations of processed food that generally contain additives and other substances to make the final product palatable and more appealing, including advanced glycation end-products (AGEs), dominant compounds generated during late stages of the Maillard reactions occurring between carbonyl and amino groups3. Several published data have provided evidence regarding the detrimental effects of UPFs and their compounds AGEs on human health, and linked their dietary intake with the occurrence of non-communicable diseases including obesity4. Evidence have shown that AGEs can impair mitochondrial function resulting in abnormal reactive oxygen species (ROS) production and ATP level reduction5,6,7. Mitochondria play a crucial role in energy metabolism, whose dysfunctions can lead to obesity and metabolic alterations8.
The Ultraprocessed Food in Obesity (UFO) Project was launched to investigate the potential mechanisms of the UPFs effects in facilitating pediatric obesity, investigating the direct role of major components of UPFs (i.e., the AGEs).
We comparatively investigated the daily dietary intake of UPFs, energy, nutrients, major dietary AGEs [Nε -(carboxymethyl)lysine (CML), Nε -(1-carboxyethyl)lysine (CEL), and Nδ -(5-hydro-5- methyl-4-imidazolon-2-yl)-ornithine (MG-H1)] in obese pediatric patients and in healthy controls. AGEs skin accumulation and mitochondrial function in peripheral blood mononuclear cells (PBMCs) were also assessed.
Results
Higher dietary UPFs intake in obese patients
53 consecutive obese pediatric patients (52.8% male, mean age 10.7 years ± 3.5 SD) and 100 healthy controls (63% male, mean age 10.3 years ± 3.5 SD) were enrolled in the study.
The participants’ features are given in Table 1.
Subjects enrolled in the two study groups were comparable for all the demographic and anamnestic features, with the exception, as expected, for body weight and Body Mass Index values, that were significantly higher in obese patients if compared to healthy controls (67.72 kg ± 26.09 vs. 37.71 kg ± 13.12, p < 0.0001; 30.7 kg/m2 ± 6.79 vs. 18.25 kg/m2 ± 2.29, p < 0.0001).
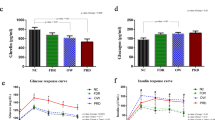
A significantly higher dietary intake of UPFs was observed in pediatric patients with obesity than controls, both in the proportion (%) of UPFs in the total weight of food and beverages consumed (g/day) [42.9% (95%CI 27–50) vs. 19.85% (95%CI 13.4–25.7), p = 0.0001], and in the daily energy (Kcal) deriving from UPFs [557.7 kcal (95% CI 433.4–717.8) vs. 356.5 kcal (95%CI 301.6–460.2), p = 0.0132] (Figs. 1 and 2).
Higher dietary UPFs intake in obese patients in %. The unpaired t-test found that patients with obesity reported a significantly higher dietary intake of UPFs than healthy controls in the proportion (%) of UPFs in the total weight of food and beverages consumed (g/day). Data are expressed as median 95% CI.
Obese patients reported a significantly higher daily dietary intake than healthy controls of energy (1741 kcal 95%CI 1506–1871, vs. 1470 kcal 95%CI 1362–1608, p = 0.01), protein (67.71 g 95%CI 64.88–73.45 vs. 60.92 g 95%CI 58.34–63.82, p = 0.02), fat (68.26 g 95%CI 57.14–79.78 vs. 49.49 g 95%CI 43.72–56.07, p = 0.01), and saturated fatty acids (16.38 g 95%CI 11.71–21.37 vs.10.80 g 95%CI 8.62–13.49, p = 0.0009).
We observed a trend toward higher daily dietary intake of CML, CEL, and MG-H1 in obese children than healthy controls [2.7 mg (95%CI 2.1–3.4) vs. 2.3 mg (95%CI 2.0–2.8), p = 0.26; 1.9 mg (95%CI 1.6–2.3) vs. 1.5 mg (95%CI 1.3–1.7), p = 0.99; 14.5 mg (95%CI 11.8–18.7) vs. 10.4 mg (95%CI 9.7–11.9), p = 0.09].
In addition, obese patients presented significantly higher skin AGEs accumulation compared to healthy controls (1.2 ± 0.21 vs. 1.1 ± 0.21, p = 0.03) (Fig. 3).
Effects of AGEs exposure on mitochondrial metabolism in PBMCs
We investigated the mitochondrial metabolism in PBMCs collected from obese children, and from healthy controls at baseline and after AGEs exposure. Representative graph of Cell Mito Stress assay performed by Seahorse XFp analyzer is reported in Fig. 4a. At the baseline, we observed a significant reduction in basal (− 46% vs. healthy controls) and maximal (− 64% vs. healthy controls) oxygen consumption rates, and in ATP synthesis (− 50% vs. healthy controls), in PBMCs from obese patients compared to healthy controls, suggesting an alteration of mitochondrial metabolism, leading to impaired oxidative capacity of substrates (Fig. 4b–d). The reduction of spare respiratory capacity (SRC), a functional parameter evaluating cell competence to respond to increased energy demand or stress, was also reduced in obese children (− 70% vs. healthy controls) (Fig. 4e). After the addition of rotenone and antimycin A, to inhibit complexes I and III, the non-mitochondrial respiration residual resulted lower (− 48% vs. healthy controls) in obese children (Fig. 4f).
Mitochondrial respiration-linked parameters in PBMCs. Representative graph of Cell Mito Stress assay performed by Seahorse XFp analyzer is reported (a). Basal respiration (b), maximal respiration (c), ATP production (d), spare respiratory capacity (e), and non mitochondrial respiration (f) are reported. Each point in the OCR time courses is the average of three technical replicates (n = 10/group). The values are expressed as mean ± SD. The unpaired t-test has been performed to compare the groups. ***p < 0.001; ****p < 0.0001.
Then, analyzing mitochondrial metabolism in PBMCs from healthy controls incubated with AGEs, we observed mitochondrial alterations similar to those observed in obese patients, characterized by a significant decrease of basal and maximal respiration, ATP production, SRC, and non mitochondrial respiration (Fig. 5a–f).
Mitochondrial respiration-linked parameters in PBMCs from healthy controls exposed to AGEs. Representative graph of Cell Mito Stress assay performed by Seahorse XFp analyzer is reported (a). Basal respiration (b), maximal respiration (c), ATP production (d), spare respiratory capacity (e), and non mitochondrial respiration (f) are reported. Each point in the OCR time courses is the average of three technical replicates (n = 7/group). The ANOVA and the Newman-Keuls post hoc test to correct for multiple comparisons have been performed to compare the groups. The values are expressed as mean ± SD. **p < 0.05;***p < 0.001; ****p < 0.0001.
Finally, we measured the ratio between glutathione (GSH) and reduced GSH/oxidized glutathione (GSSG), as plasmatic biomarkers of oxidative stress. We showed that the GSH/GSSG ratio in plasma of obese patients was significantly lower compared to healthy controls [mean ± standard error (0.21 ± 0.01 vs. 0.32 ± 0.01, p < 0.0001)], indicating higher oxidative stress level.
Discussion
In recent decades, changes in the global food system, particularly the substantial expansion of highly processed foods in the world’s food market, have been hypothesized as a contributor to the worldwide rise in childhood obesity9.
We confirmed a significantly higher UPFs consumption in obese pediatric patients. This data is in line with results deriving from cross-sectional10,11,12 and prospective studies conducted in pediatric populations13,14,15,16. These studies, mainly conducted in American countries, revealed that higher consumption of UPFs was related with obesity prevalence and increased fat mass10,11,12, and reported that the food processing has a role in body fat accumulation beyond its calorie content13. Furthermore, it has been showed that higher dietary intake of UPFs is associated with more rapid progression of excess weight into adolescence and early adulthood14,15,16.
Several mechanisms have been postulated for UPFs in facilitating the occurrence of obesity. Palatability and high energy density of UPFs may facilitate excessive energy intake17. Furthermore, high refined sugar and fat content may alter satiety signaling and may produce changes in neurocircuitry reward, leading to overconsumption and addictive-like eating behaviors18,19,20. In addition, UPFs can alter insulin response and promote the storage of excess nutrients in adipose tissue21.
UPFs provide high levels of the detrimental compounds AGEs, Maillard products formed during non-enzymatic reactions22. Animal protein, fat and saturated fatty acids foods, mostly cooked at high and dry heat, such as broiling, grilling, frying, and roasting, represent the main dietary sources of AGEs23. We observed a significantly higher dietary intake of energy, protein, fat, and saturated fatty acids in obese patients than controls. AGEs could be involved in the pathophysiology of obesity by altering directly protein structure and function, and by leading to an increased production of ROS and inflammatory cytokines, mainly by binding the AGEs receptor (RAGE), and ends up creating a vicious circle, exacerbating the typical chronic inflammation state of obesity24,25.
The AGEs–RAGE axis plays an important role in obesity, contributing to adipogenesis of preadipocytes26, and to adipocyte hypertrophy27. Preclinical studies have shown that high AGEs-diet induces obesity and related metabolic alterations compared to low AGEs-diet28,29. In turn, nutritional interventions aimed at reducing the dietary AGEs intake have been proved to be effective in reducing the risk of obesity and its complications in humans30.
We observed a trend toward higher daily dietary intake of AGEs in obese children than healthy controls. Similar results have been reported by others31. Furthermore, the higher consumption of dietary AGEs was associated with an increased risk to develop metabolic syndrome32. Unfortunately, the reference database used in these studies to quantify the dietary AGEs intake reported the content of only one AGEs (CML) in foods, determined by semi-quantitative enzyme-linked immunosorbent assay based on a monoclonal anti-CML antibody23.
To our knowledge, this is the first study evaluating the dietary AGEs intake in pediatric subjects with and without obesity using a reference database reporting the content of the most common AGEs (CML, CEL and MG-H1) quantified in the protein fractions of 190 foods using a highly sensitive, specific and rapid ultra-performance liquid chromatography tandem mass spectrometry method33.
Dietary AGEs may induce adverse health outcomes through their accumulation in human tissue34. We also observed a higher skin AGEs accumulation in obese pediatric patients. This result is in line with previous observations reported by others35.
Mitochondrial dysfunction could be involved in the pathogenesis of obesity36.
Another potential mechanism elicited by UPFs and their compounds AGEs in facilitating obesity, could be ascribed to their capacity to induce mitochondrial metabolism alterations37. AGEs could be able to increase the production of mitochondrial ROS and to decrease the ATP production5,38,39.
We observed alterations of mitochondrial metabolism in obese children, characterized by reduced basal and maximal respiration, ATP production, SRC, and non mitochondrial respiration. Similar mitochondrial metabolism alterations were observed in PBMCs from healthy controls incubated with AGEs, suggesting an additional mechanism elicited by dietary AGEs in facilitating pediatric obesity.
Potential limitations of our study could be related to the cross-sectional design, which do not address causality, and the relatively small sample size. Further studies, with prospective design evaluating younger and larger cohorts, could be necessary to confirm our results.
However, the present study has several strengths. Our results are consistent with previous evidence. The evaluation of the seven-day weighed food diaries using a high specific reference database provided an accurate evaluation of dietary UPFs and AGEs intake. The higher dietary AGEs exposure in children affected by obesity paralleled with a higher AGEs skin accumulation. In vitro experiments provided mechanistic data on the potential role of AGEs in facilitating the occurrence of pediatric obesity, through a negative impact on mitochondrial metabolism.
Conclusions
Our findings support the role of UPFs exposure in the pediatric obesity pandemic. The results of the UFO study provided data on potential mechanisms for the UPFs effects in facilitating pediatric obesity (i.e., mitochondrial alterations) involving the major components of UPFs (i.e., the AGEs). These results further support the potential importance of limiting dietary UPFs exposure as additional strategy for an integrated approach for pediatric obesity prevention and treatment.
Methods
Study design
The UFO (Ultraprocessed Foods in Obesity) Project was an observational, case-control, single-center, pilot study designed to investigate the potential mechanisms elicited by UPFs in facilitating pediatric obesity.
Ethics declarations
The study was approved by the Ethics Committee of the “University of Naples Federico II—A.O.R.N. Cardarelli” (Protocol n°00019173) and was performed in accordance with the Helsinki Declaration (Seoul revision, October 2008) and with the relevant European and Italian privacy regulations. Written informed consent to participate into the study was obtained by the subjects and their parents/legal guardian. The UFO Project was registered on https://clinicaltrials.gov/ with the identifier NCT05554016.
Participants
From September 2022 to December 2022, consecutive caucasian subjects of both sexes, aged ≥ 6 and ≤ 18 years, with a diagnosis of obesity (based on a Body Mass Index (BMI) > 97th percentile for age and sex) visiting for the first time the Tertiary Center for Pediatric Nutrition of the Department of Translational Medical Science at the University of Naples “Federico II”, and age- and sex-matched healthy controls with negative history for obesity and any form of malnutrition, visiting the Center because of minimal surgical procedures, were considered eligible for the study.
The exclusion criteria were: non-caucasian ethnicity; age < 6 or > 18 years; concomitant presence of chronic diseases, malignancies, immunodeficiencies, chronic infections, autoimmune diseases, chronic inflammatory bowel diseases, celiac disease, metabolic-genetic diseases, cystic fibrosis and other chronic lung diseases, cardiovascular/respiratory/gastrointestinal malformations, neuropsychiatric and neurological disorders; history of obesity surgery; presence of tattoos, scars, moles or lesions on both forearms.
Outcomes
The study outcomes were the comparative evaluation of the dietary UPFs intake in pediatric patients with obesity and in sex- and age- matched healthy controls, and of the potential mechanisms elicited by UPFs in facilitating obesity focusing on the daily intake of the three major dietary AGEs [Nε -(carboxymethyl)lysine (CML), Nε -(1-carboxyethyl)lysine (CEL), and Nδ -(5-hydro-5- methyl-4-imidazolon-2-yl)-ornithine (MG-H1)], the AGEs skin accumulation, and their effects on mitochondrial metabolism. The participants daily intake of energy and nutrients were also assessed.
Sample size calculation
Since “UFO” was a pilot study, sample size was not determined. An allocation ratio of 2:1 was used to adjust for case variability.
Clinical and biochemical assessment
After collection of the written informed consent by the participants and their parents/legal guardian, we collected data regarding anamnestic and clinical features, personal and anthropometric data, gestational age, mode of delivery, birth weight, breastfeeding, breastfeeding duration, weaning age, maternal smoking during pregnancy and lactation, living setting, parents/legal guardian educational level of all participants. Anthropometric measurements were performed by trained dieticians following a 12-h fasting. Body weight and height were measured with the participants dressed in light indoor clothing and without shoes using a calibrated mechanical column scale. BMI were calculated as weight (in kilograms) divided by height (in meters) squared. BMI percentile for sex and age were calculated using the World Health Organization (WHO) growth charts40.
Nutritional assessment
The 7-day (5 weekdays, 2 weekend days) weighed food diaries were used to investigate the UPFs intake and the daily intake of the three dietary AGEs (CML, CEL, MG-H1). Participants and their parents/legal guardian received complete oral and written instructions about how to weigh food and to record such data by experienced pediatric dietitians. They were requested to provide any appropriate additional information, for example, method of cooking and names of food brands. The food diaries section included the name of meals (breakfast, lunch, dinner, between-meal eating); mealtime; names of dishes (i.e., sushi); names of foods or ingredients in the dishes (i.e., rice, tuna, soya sauce); measured weight of each ingredient, food and/or meal; and place of meal consumption. Food diaries were revised by dietitians with parents/legal guardians and children to increase accuracy and completeness of reporting.
All foods and ingredients consumed by the participants were classified according to the NOVA classification41. The NOVA system considers groups of foods and drinks according their industrial processing and the use of additives. This system distinguishes four groups. All foods and ingredients consumed by the subjects were classified into one of the following four categories indicating levels of industrial food processing:
-
(1)
The first NOVA group (G1) is formed by “unprocessed or minimally processed foods”—fresh foods or foods that are minimally altered by industrial processes (drying, freezing, vacuum packaging, non-alcoholic fermentation), but free of added ingredients (i.e., fresh or frozen fruits and vegetables, eggs, pasteurized milk, meat, seeds, nuts, grains or plain yogurt);
-
(2)
The second group (G2) includes “processed culinary ingredients”—substances obtained directly from G1 foods or from nature, and used in preserving, cooking and seasoning (i.e., oils, fats, sugar and salt);
-
(3)
The third group (G3) is of “processed foods”—industrial products made by adding substance from G2 into foods from G1, using preservation methods such as canning and bottling, smoking, curing, or fermentation (i.e., canned vegetables, canned fish, fruits in syrup, cheeses, fresh bread, beer and wine);
-
(4)
The fourth group (G4) includes “ultra-processed food and drink products”, formulations manufactured using several ingredients, including little or no fresh foods, and a series of processes that greatly breakdown the food matrix. Processes and ingredients used in manufacturing are designed to create highly convenient, attractive and profitable products (i.e., soft drinks, sweet or savory packed snacks, processed meats, pre-prepared frozen dishes and ‘instant’ products).
The following foods were considered to be UPFs: instant and canned soups; reconstituted meat and fish products; ready-made sauces, gravies and dressings; French fries and other pre-made potato products such as chips; ready-to-eat and dry-mix desserts such as pudding; confectionery; sweet and savoury snack foods including granola bars and protein bars; sugar-sweetened or artificially sweetened beverages including soda, fruit drinks, pre-sweetened tea and coffee, energy drinks and dairy-based drinks; flavoured and/or sweetened yoghurt; industrially manufactured cakes, cookies and pies; dry cake and pancake mixes; industrially manufactured breads; sweet breakfast cereals; frozen and shelf-stable plate meals; ice cream, frozen yogurt and ice pops; meatless patties and fish sticks and infant formula.
Subjects’ UPFs consumption were calculated summing the amount consumed of food and beverage (g/day) included in the fourth category of the NOVA classification (G4), and then were calculated the proportion (%) of UPFs in the total weight of food and beverages consumed (in g/day) [daily intake of NOVA fourth group of food and beverages in grams/total daily food and beverages intake in grams *100], as previously reported42. In addition, the daily energy deriving from UPFs (in Kcal) was calculated using an ad hoc software (Winfood, Medimatica Srl, Italy).
Furthermore, specifically designed software (Winfood, Medimatica Srl, Italy) was also used to estimate the total daily intake of energy, macro- and micronutrients.
Finally, to estimate the daily dietary AGEs intake from the food diaries, a reference database of > 200 food products commonly consumed in a Western diet built at the Maastricht University (Maastricht, The Netherlands) was used33. The reference database can be found as Supplementary Table S1 online. This database reports the content of 3 major AGEs, CML, CEL and MG-H1, in mg per 100 g of food, quantified in the protein fractions of food products using highly specific ultra-performance liquid chromatography tandem mass spectrometry. All these data were recorded in a dedicated clinical chart.
Assessment of skin AGEs accumulation
Selected AGEs have structural properties that cause them to emit fluorescent light across a specific range of wave-lengths upon excitation by ultraviolet light43. This unique characteristic has been used to develop a non-invasive device (AGEs Reader®; DiagnOptics Technologies, Groningen, The Netherlands), that quantifies accumulated AGEs within the human skin through the evaluation of sAF as previously described44,45. For the measurement, the participants were asked to place the dominant forearm on the device for 60 s. The AGE Reader® illuminated a skin area of 4 cm2 with ultraviolet A light with a single peak excitation wavelength of 370 nm. Emission light (fluorescence in the wavelength of 420–600 nm) and reflected excitation light (with a wavelength of 300–420 nm) from the skin were measured using a spectrometer. Skin AGEs levels were calculated as the ratio between the emission light and reflected excitation light, multiplied by 100 and expressed in arbitrary units (AU). The intra- and inter-day coefficient was 2.6%. In all children, skin oils/ointments were not recently applied on the site of measurement before sAF measurement. Three measurements were performed on different sites of the skin on the volar side of the dominant forearm, as previously described46. The mean sAF value of the three measurements was reported in the participants’ clinical chart.
Isolation of peripheral mononuclear blood cells
Peripheral mononuclear blood cells (PBMCs) were collected from a randomly selected subgroup of obese pediatric patients and well sex- and age- matched healthy controls. PBMCs were obtained from 10 obese patients (50% male, mean age 11 years ± 2.8 SD) and 10 age- and sex-matched healthy controls (50% male, mean age 11 years ± 2.8 SD) and were isolated by Ficoll density gradient centrifugation (Ficoll-Histopaque − 1077, Sigma, St. Louis, Missouri, USA). Briefly, cells were stratified on 3 mL of Ficoll and centrifuged 15 min at 1000×g at room temperature. After centrifugation, the opaque interface containing mononuclear cells was carefully aspirated with a Pasteur pipette and cells were washed with 10 mL of PBS and centrifuged 10 min at 500×g at room temperature. After centrifugation, the upper layer was discarded and PBMCs (3.5 × 105 cells/well) were cultured in duplicates in 96-well plates in 200 µl culture medium (RPMI 1640, Gibco, ThermoFisher, Waltham, Massachusetts, USA) containing 10% FBS (Gibco), 1% non-essential amino acids (Gibco), 1% sodium pyruvate (Gibco), and 1% penicillin/streptomycin (Gibco).
PBMCs stimulation protocol
PBMCs (3.5 × 105cells/well) from healthy children were stimulated with 200 µg/mL of AGE-BSA (#ab51995, Abcam, Cambridge, UK; purity: > 98%; endotoxin level: < 0.100 Eu/µg) or BSA (Sigma-Aldrich, Missouri, USA), as control for 48 h. Timing and dosing were selected based on the results of previous time-course and dose response experiments performed at our laboratory. The cells with only medium were used as negative control. All the experiments were performed in triplicate.
Seahorse XFp analyses to assess mitochondrial metabolism
Mitochondrial metabolism in PBMCs were assessed by the Seahorse XFp analyzer (Seahorse Biosciences, North Billerica, MA, USA), by using the Cell Mito Stress Test kit (cat# 103,010–100). Before cell mito stress analyses, the cells were centrifuged at room temperature at 1200 rpm for 10 min, the medium was replaced with a buffered base medium (Agilent Seahorse-103193) supplemented with 2 mM glutamine, 1 mM pyruvate and 10 mM glucose at pH 7.4. In order to compare the mitochondrial metabolism of PBMCs from healthy controls and obese patients, PBMCs were seeded (3.5 × 105cells/well) in Seahorse mini-plates in enriched buffered base medium (Agilent Seahorse-103193). The plates were centrifuged at 200 g for 5 min at room temperature and equilibrated at 37 °C in a CO2 free incubator for at least 1 h. Basal oxygen consumption rate (OCR) was determined in the presence of glutamine (2 mM) and pyruvate (1 mM). The proton leak was determined after inhibition of mitochondrial ATP production by 1 µM oligomycin, as an inhibitor of the F0–F1 ATPase. The measurement of the ATP production in the basal state was obtained from the decrease in respiration by inhibition of the ATP synthase with oligomycin. The mitochondrial electron transport chain was stimulated maximally by the addition of the uncoupler FCCP (1 µM). Coupling efficiency is the proportion of the oxygen consumed to drive ATP synthesis compared with that driving proton leakage and was calculated as the fraction of basal mitochondrial OCR used for ATP synthesis (ATP-linked OCR/basal OCR). Spare respiratory capacity (SRC) is the capacity of the cell to respond to an energetic demand and was calculated as the difference between the maximal respiration and basal respiration. The mitochondrial respiration was expressed as the oxygen consumption rate per min normalized to the number of cells. The same cell number/well was plated before the OCR measurements; the cell count was obtained by using the Burker chamber. In addition, at the end of analyses, we determined the protein content/well by Bradford assay without observing significant differences.
Oxidative stress biomarkers assessment
Plasmatic concentrations of GSH and GSSG were measured with the dithionitrobenzoic acid-GSSG reductase recycling assay. The GSH/GSSG ratio has been performed.
Data quality assurance
All data were recorded anonymously. At the study Center, designated investigators were required to enter all collected data in the case report form (CRF). Two researchers performed separate checks of data completeness, clarity, consistency, and accuracy, and instructed site personnel to make any required corrections or additions. Using a single data-entry method, all data recorded in the CRF were entered in the study database by the same researcher. Then, the study dataset was reviewed and underwent data cleaning and verification according to standard procedures. Finally, the database was locked once it was declared complete and accurate, and the statistical analysis was performed.
Statistical analysis
Results were expressed as mean ± SD for continuous variables and as median and 95% cluster confidence intervals for variables with a non-Gaussian distribution. Discrete variables were reported as numbers and proportions. Differences between obese patients vs. healthy controls were tested by unpaired t-test. Data from mitochondrial metabolism experiments were evaluated by ANOVA followed by the Newman-Keuls post hoc test to correct for multiple comparisons. Differences were considered statistically significant at p < 0.05. All analyses were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
Data availability
The dataset used and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
Hampl, S. E. et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics 151, 2. https://doi.org/10.1542/peds.2022-060640 (2023).
Juul, F., Martinez-Steele, E., Parekh, N., Monteiro, C. A. & Chang, V. W. Ultra-processed food consumption and excess weight among US adults. Br. J. Nutr. 120, 90–100. https://doi.org/10.1017/S0007114518001046 (2018).
Monteiro, C. A. et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 22, 936–941. https://doi.org/10.1017/S1368980018003762 (2019).
Phuong-Nguyen, K., McNeill, B. A., Aston-Mourney, K. & Rivera, L. R. Advanced glycation end-products and their effects on gut health. Nutrients 15, 405. https://doi.org/10.3390/nu15020405 (2023).
Lo, M. C. et al. Glycoxidative stress-induced mitophagy modulates mitochondrial fates. Ann. N. Y. Acad. Sci. 1201, 1–7. https://doi.org/10.1111/j.1749-6632.2010.05935.x (2010).
Zhu, Y. et al. Inhibition of the Receptor for advanced glycation endproducts (RAGE) protects pancreatic b-cells. Biochem. Biophys. Res. Commun. 404, 159–165. https://doi.org/10.1016/j.bbrc.2010.11.085 (2011).
Bierhaus, A., Hofmann, M. A., Ziegler, R. & Nawroth, P. P. AGEs and their Interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc. Res. 37, 586–600. https://doi.org/10.1016/S0008-6363(97)00233-2) (1998).
Vakifahmetoglu-Norberg, H., Ouchida, A. T. & Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 15, 426–431. https://doi.org/10.1016/j.bbrc.2016.11.088 (2017).
Neri, D. et al. Ultraprocessed food consumption and dietary nutrient profiles associated with obesity: A multicountry study of children and adolescents. Obes. Rev. https://doi.org/10.1111/obr.13387 (2022).
Souza, S. F. et al. Degree of food processing and association with overweight and abdominal obesity in adolescents. Einstein (Sao Paulo) https://doi.org/10.31744/einstein_journal/2022AO6619 (2022).
Viola, P. C. A. F. et al. High consumption of ultra-processed foods is associated with lower muscle mass in Brazilian adolescents in the RPS birth cohort. Nutrition https://doi.org/10.1016/j.nut.2020.110983 (2020).
Louzada, M. L. et al. Consumption of ultra-processed foods and obesity in Brazilian adolescents and adults. Prev. Med. 81, 9–15. https://doi.org/10.1016/j.ypmed.2015.07.018 (2015).
Costa, C. D. S. et al. Role of ultra-processed food in fat mass index between 6 and 11 years of age: A cohort study. Int. J. Epidemiol. 50, 256–265. https://doi.org/10.1093/ije/dyaa141 (2021).
Chang, K. et al. Association between childhood consumption of ultraprocessed food and adiposity trajectories in the avon longitudinal study of parents and children birth cohort. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2021.1573 (2021).
Vedovato, G. M. et al. Ultra-processed food consumption, appetitive traits and BMI in children: A prospective study. Br. J. Nutr. 125, 1427–1436. https://doi.org/10.1017/s0007114520003712 (2021).
Costa, C. S. et al. Ultra-processed food consumption and its effects on anthropometric and glucose profile: A longitudinal study during childhood. Nutr. Metab. Cardiovasc. Dis. 29, 177–184. https://doi.org/10.1016/j.numecd.2018.11.003 (2019).
Crimarco, A., Landry, M. J. & Gardner, C. D. Ultra-processed foods, weight gain, and co-morbidity risk. Curr. Obes. Rep. 11, 80–92. https://doi.org/10.1007/s13679-021-00460-y (2022).
Carter, A. et al. The neurobiology of “food addiction” and its implications for obesity treatment and policy. Annu. Rev. Nutr. 36, 105–128. https://doi.org/10.1146/annurev-nutr-071715-050909 (2016).
Schulte, E. M., Avena, N. M. & Gearhardt, A. N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PloS One https://doi.org/10.1371/journal.pone.0117959 (2015).
Fardet, A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct. 7, 2338–2346. https://doi.org/10.1039/c6fo00107f (2016).
Hall, K. D. A review of the carbohydrate-insulin model of obesity. Eur. J. Clin. Nutr. 71, 323–326. https://doi.org/10.1038/ejcn.2016.260 (2017).
Twarda-Clapa, A., Olczak, A., Białkowska, A. M. & Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 11, 1312. https://doi.org/10.3390/cells11081312 (2022).
Uribarri, J. et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 110, 911–916. https://doi.org/10.1016/j.jada.2010.03.018 (2010).
Uribarri, J. et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 15, 461–473. https://doi.org/10.3945/an.115.008433 (2015).
Ribeiro, P. V. M., Tavares, J. F., Costa, M. A. C., Mattar, J. B. & Alfenas, R. C. G. Effect of reducing dietary advanced glycation end products on obesity-associated complications: A systematic review. Nutr. Rev. 77, 725–734. https://doi.org/10.1093/nutrit/nuz034 (2019).
Gaens, K. H. et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 34, 1199–1208. https://doi.org/10.1161/ATVBAHA.113.302281 (2014).
Monden, M. et al. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: Involvement of Toll-like receptor 2. Diabetes 62, 478–489. https://doi.org/10.2337/db11-1116 (2013).
Sayej, W. N. et al. Advanced glycation end products induce obesity and hepatosteatosis in CD-1 wild-type mice. Biomed. Res. Int. https://doi.org/10.1155/2016/7867852 (2016).
Hofmann, S. M. et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 51, 2082–2089. https://doi.org/10.2337/diabetes.51.7.2082 (2002).
Sohouli, M. H., Sharifi-Zahabi, E., Lari, A., Fatahi, S. & Shidfar, F. The impact of low advanced glycation end products diet on obesity and related hormones: A systematic review and meta-analysis. Sci. Rep. 10, 22194. https://doi.org/10.1038/s41598-020-79216-y (2020).
Rodríguez-Mortera, R. et al. Soluble receptor for advanced glycation end products and its correlation with vascular damage in adolescents with obesity. Horm. Res. Paediatr. 92, 28–35. https://doi.org/10.1159/000501718 (2019).
Saha, A. et al. Increased odds of metabolic syndrome with consumption of high dietary advanced glycation end products in adolescents. Diabetes Metab. 43, 469–471. https://doi.org/10.1016/j.diabet.2017.01.001 (2017).
Scheijen, J. L. J. M. et al. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 190, 1145–1150. https://doi.org/10.1016/j.foodchem.2015.06.049 (2016).
Guilbaud, A., Niquet-Leridon, C., Boulanger, E. & Tessier, F. J. How can diet affect the accumulation of advanced glycation end-products in the human body? Foods 5, 84. https://doi.org/10.3390/foods5040084 (2016).
Lentferink, Y. E., van Teeseling, L., Knibbe, C. A. J. & van der Vorst, M. M. J. Skin autofluorescence in children with and without obesity. J. Pediatr. Endocrinol. Metab. 32, 41–47. https://doi.org/10.1515/jpem-2018-0237 (2019).
Vakifahmetoglu-Norberg, H., Ouchida, A. T. & Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 482, 426–431. https://doi.org/10.1016/j.bbrc.2016.11.088 (2017).
Ward, M. S., Fortheringham, A. K., Cooper, M. E. & Forbes, J. M. Targeting advanced glycation endproducts and mitochondrial dysfunction in cardiovascular disease. Curr. Opin. Pharmacol. 13, 654–661. https://doi.org/10.1016/j.coph.2013.06.009 (2013).
Rodríguez-Cano, A. M. et al. Ultra-processed food consumption during pregnancy and its association with maternal oxidative stress markers. Antioxidants (Basel) 11, 1415. https://doi.org/10.3390/antiox11071415 (2022).
Coughlan, M. T. et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 20, 742–752. https://doi.org/10.1681/ASN.2008050514 (2009).
World Health Organization. Child growth standards. https://www.who.int/tools/child-growth-standards/standards. (2022).
Monteiro, C. A. et al. The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 21, 5–17. https://doi.org/10.1017/S1368980017000234 (2018).
Bonaccio, M. et al. Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani Study. Am. J. Clin. Nutr. 113, 446–455. https://doi.org/10.1093/ajcn/nqaa299 (2021).
Obayashi, H. et al. Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem. Biophys. Res. Commun. 226, 37–41. https://doi.org/10.1006/bbrc.1996.1308 (1996).
Mulder, D. J. et al. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: An overview of current clinical studies, evidence, and limitations. Diabetes Technol. Ther. 8, 523–535. https://doi.org/10.1089/dia.2006.8.523 (2006).
Meerwaldt, R. et al. Simple noninvasive measurement of skin autofluorescence. Ann. N. Y. Acad. Sci. 1043, 290–298. https://doi.org/10.1196/annals.1333.036 (2005).
Banser, A., Naafs, J. C., Hoorweg-Nijman, J. J., van de Garde, E. M. & van der Vorst, M. M. Advanced glycation end products, measured in skin, vs. HbA1c in children with type 1 diabetes mellitus. Pediatr. Diabetes 17, 426–32. https://doi.org/10.1111/pedi.12311 (2016).
Acknowledgements
The authors would like to thank all the participants of this study and their families for their time and effort. The study was supported by the Department of Translational Medical Science of the University of Naples “Federico II,” Naples, Italy, which received funding from the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.3 - Call for tender No. 341 of 15 March 2022 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Award Number: Project code PE00000003, Concession Decree No. 1550 of 11 October 2022 adopted by the Italian Ministry of University and Research, CUP D93C22000890001, Project title “ON Foods - Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods”.
Author information
Authors and Affiliations
Contributions
R.B.C., S.C. and M.P.M. conceived and designed the study; S.C., A.M.R., A.M., A.F.D.G.D.S.S., M.C., M.E., A.T. and C.E. acquired data; S.C., L.P., and G.T. elaborated the statistical analyses section; L.P. and G.T. elaborated the laboratories analysis and interpreted the data; S.C., L.P., G.T., M.P.M. and R.B.C. drafted the manuscript; and all authors critically revised the manuscript. All authors approved the submitted and final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coppola, S., Paparo, L., Trinchese, G. et al. Increased dietary intake of ultraprocessed foods and mitochondrial metabolism alterations in pediatric obesity. Sci Rep 13, 12609 (2023). https://doi.org/10.1038/s41598-023-39566-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39566-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.