Abstract
The foodborne bacterium Staphylococcus equorum strain KS1030 harbours plasmid pSELNU1, which encodes a lincomycin resistance gene. pSELNU1 undergoes horizontal transfer between bacterial strains, thus spreading antibiotic resistance. However, the genes required for horizontal plasmid transfer are not encoded in pSELNU1. Interestingly, a relaxase gene, a type of gene related to horizontal plasmid transfer, is encoded in another plasmid of S. equorum KS1030, pKS1030-3. The complete genome of pKS1030-3 is 13,583 bp long and encodes genes for plasmid replication, biofilm formation (the ica operon), and horizontal gene transfer. The replication system of pKS1030-3 possesses the replication protein-encoding gene repB, a double-stranded origin of replication, and two single-stranded origins of replication. The ica operon, relaxase gene, and a mobilization protein-encoding gene were detected in pKS1030-3 strain-specifically. When expressed in S. aureus RN4220, the ica operon and relaxase operon of pKS1030-3 conferred biofilm formation ability and horizontal gene transfer ability, respectively. The results of our analyses show that the horizontal transfer of pSELNU1 of S. equorum strain KS1030 depends on the relaxase encoded by pKS1030-3, which is therefore trans-acting. Genes encoded in pKS1030-3 contribute to important strain-specific properties of S. equorum KS1030. These results could contribute to preventing the horizontal transfer of antibiotic resistance genes in food.
Similar content being viewed by others
Introduction
Antibiotic resistance (AR) among microbes has become one of the biggest threats to global human health. In 2013, the Centers for Disease Control and Prevention of the United States published a report that warned about the spread among environments of foodborne pathogenic bacteria with mobile AR genes, including from foods to humans1. The World Health Organization has also recognised the threat of AR and published a global priority list of antibiotic-resistant bacteria in 20172.
Bacteria can acquire new genetically determined traits from other microbes in their ecosystem via horizontal gene transfer (HGT)3,4. HGT plays an important role in the spread of AR among bacterial taxa. There are three major/canonical mechanisms of HGT: transformation, via extracellular DNA; transduction, via bacteriophage; and conjugation, via mobile genetic elements such as plasmid. Conjugation is the most common mechanism of HGT, and conjugative transfer of plasmids is the most efficient way of horizontally spreading AR genes5,6. Conjugation via plasmids involves the enzyme relaxase, which recognises an origin of transfer (oriT) on the plasmid and forms a “relaxosome” complex for gene exchange through binding with oriT, the type IV coupling protein, and mating channel components such as the type IV secretion system (T4SS)7.
In our previous analysis of cultivable bacteria in high-salt fermented seafood, Staphylococcus equorum was identified as a predominant bacterial species8. During subsequent safety and functional assessments of S. equorum isolates to identify safe and efficient candidate starter strains, a lincomycin resistance gene (encoding lincosamide O-nucleotidyltransferase, lnuA) was amplified from 4 of 126 isolates9. The lnuA gene was located on small plasmids, named pSELNU1–3, with only two different nucleotide sequences among these three plasmids10. Notably, although pSELNU1 is not a conjugative plasmid, pSELNU1 in S. equorum strain KS1030 was transferred to other Staphylococcus species, Enterococcus faecalis, and Tetragenococcus halophilus in vitro10, and was also transferred to S. saprophyticus during soybean fermentation (in situ) and passage through murine intestines (in vivo) under lincomycin pressure11. Recent complete genome sequencing revealed that S. equorum KS1030 also harbours plasmid pKS1030-3, which encodes elements facilitating gene mobility such as a relaxase12.
In the current study, we characterised pKS1030-3 from S. equorum KS1030 on the genomic level and illuminate its involvement in plasmid transfer between species involved in food fermentation, which demonstrates the possibility of horizontal AR gene transfer within food matrices. We show that pKS1030-3 can serve as an auxiliary trans-acting factor for horizontal transfer of pSELNU1 (containing the lincomycin resistance gene lnuA), and also that pKS1030-3 may be involved in biofilm formation.
Materials and methods
Bacterial strains and culture conditions
Staphylococcus equorum strain KS1030, originally isolated from a Korean high-salt fermented seafood, was subjected to genomic and experimental analyses9. Here, plasmid pKS1030-3 from S. equorum KS1030 was used in assessment of gene transferability and biofilm formation. In gene transfer experiments, S. saprophyticus KM1053 was used as the recipient strain because it is resistant to tetracycline11. S. aureus RN4220 was used as an expression host for constructed plasmids. Staphylococcus strains were cultured in tryptic soy broth (TSB; Becton, Dickinson and Co., Franklin Lakes, NJ, USA) at 37 °C for 12 h.
Escherichia coli DH5α was used as the cloning host and was cultured in Luria–Bertani broth (Becton, Dickinson and Co.) at 37 °C for 12 h.
Genomic analyses
The complete genome sequence of S. equorum strain KS1030 was published previously (GenBank accession: CP068576–CP068580)12. In the current study, the nucleotide sequence of plasmid pKS1030-3 (CP068579) from S. equorum KS1030 was analysed. CLgenomics™ software v.1.55 (CJ Bioscience, Inc, Seoul, Korea) and the web-hosted BLAST programs of NCBI were used to find genes and gene products with sequence identity. Rapid Annotation using Subsystem Technology (RAST)13 was used to determine gene contents based on functional subsystem classifications. MEGA11 software was used for sequence analyses of DNA and proteins14. The sequence identities of deduced amino acid were determined by MegAlign module of Lasergene DNAStar15. Protein secondary and tertiary structures were predicted using Predict Secondary Structure (PSIPRED)16 and AlphaFold17.
Comparative genomics
For comparative genomic analysis within the species S. equorum, genome sequence data for strains KS1030 (GenBank accession: CP068576–CP068580), KM1031 (CP013980–CP013983), C2014 (CP013714–CP013719), KS1039 (CP013114), Mu2 (CAJL01000001–CAJL01000030), and UMC-CNS-924 (AVBD01000001–AVBD01000039) were obtained from the NCBI database (http://ncbi.nlm.nih.gov/genomes). Genes were predicted using the RAST server for SEED-based automated annotation13. The predicted genes of strains were confirmed using the iPath (v.3) module18, and CLgenomics™ v.1.55 software.
DNA cloning and transformation
Approximately 3.7-kb fragments containing biofilm formation genes (the ica operon) were amplified from pKS1030-3 of S. equorum KS1030 using primer set, ica-F and ica-R with restriction enzyme site XhoI, (Supplementary Table 1) and then digested by XhoI. For construction of plasmids containing the relaxase (rlx) gene, the fragment containing mobilization relaxosome protein (mobC), rlx, and a hypothetical protein (HP) genes was amplified from pKS1030-3 with primers Rlx-F and Rlx-R with EcoRV and XhoI restriction enzyme site, respectively (Supplementary Table 1). Digested fragments were inserted into the same sites of pYJ33519, respectively. The resulting plasmids were named pYJ335-ica and pYJ335-rlx. PCR amplifications of the ica operon and fragments containing the rlx gene were performed using a T3000 thermocycler (Analytik Jena, Jena, Germany) using an Inclone Taq polymerase kit (Inclone Biotech, Seongnam, South Korea) according to the manufacturer’s manual. All PCRs were performed using 30 cycles of denaturing at 95 °C for 1 min, annealing at 60 °C for 2 min, and elongation at 72 °C for 1 min. Constructed plasmid DNA was introduced into E. coli DH5α by the method of Hanahan and Meselson20, and into S. aureus RN4220 by electroporation21 with a gene pulser (BioRad, Hercules, CA, USA).
Biofilm formation analysis
An overnight culture of S. aureus RN4220 containing pYJ335-ica in TSB was diluted 200-fold with fresh TSB containing 0.5% glucose. Culture (200 μl) was added to each well of a 96-well microtiter plate and incubated for 24 h at 37 °C without shaking. After the supernatant was discarded, the plates were dried, and the cells were stained with 0.1% crystal violet22. For quantitative analysis of biofilm production, stained cells were released by adding 150 μl of 50% dimethylsulfoxide, 100 μl aliquots were transferred to a new microtiter plate, and the optical density at 595 nm was measured23. The experiment was conducted three times, independently.
Plasmid transfer experiments
To determine horizontal plasmid transferability, recipient strain S. saprophyticus KM105311 was mated with donor strain S. aureus RN4220 (carrying pSELNU1 and pYJ335-rlx) using the broth mating method24. S. aureus RN4220 (pSELNU1) and S. aureus RN4220 (pYJ335-rlx) were used as control donor strains. Donor strains containing pSELNU1 were lincomycin-resistant, and the recipient strain was lincomycin-sensitive and tetracycline-resistant. Therefore, transfer of pSELNU1 confers resistance to lincomycin, which facilitates transconjugant selection. Donor cells in the logarithmic growth phase in Mueller–Hinton (MH) broth (Becton, Dickinson and Co.) were mixed with recipient cells in the logarithmic growth phase (also in MH broth) at a 1:10 ratio and incubated at 30 °C for 3 h. The mixture was spread onto the surface of TSA plates supplemented with 30 mg/l lincomycin and 10 mg/l tetracycline. Transconjugants were selected after incubation at 30 °C for 24 h, and were confirmed by colony PCR with primers for amplification of lnuA9,25. Recipient traits of transconjugants were confirmed S. saprophyticus, not S. equorum by 16S rRNA gene sequence analysis.
Statistical analysis
Duncan’s multiple range test following one-way analysis of variance was applied to evaluate significant differences between the average values obtained in biofilm formation analyses. All statistical analysis was performed using the SPSS software package (v.27.0; IBM SPSS Statistics, Armonk, NY, USA).
Results and discussion
Overall features of pKS1030-3
S. equorum strain KS1030 harbours four plasmids: pKS1030-1, pKS1030-2, pKS1030-3, and pSELNU112. pKS1030-3 (13,583 bp) contains 14 open reading frames (ORFs). Eleven of these ORFs were assigned putative functions via in silico sequence analysis (Fig. 1A and Table 1). The other three ORFs show high similarity to proteins of unknown function, or have no relatives in public databases (Table 1). The overall G + C content of the pKS1030-3 nucleotide sequence (29.1 mol%) is lower than the values for chromosomal DNAs and typical plasmids in S. equorum strains (30.9–34.3 mol%). Comparative sequence analysis identified three representative putative gene systems within the pKS1030-3 genome: the plasmid replication system, a biofilm formation system, and an HGT system.
Circular representation of plasmid pKS1030-3 from Staphylococcus equorum strain KS1030 (A), three dimensional structure, (B) and domain architecture (C) of putative Rep, and gene structure of dso (D), and sso (E) in the plasmid. In (A), genes including open reading frames and genetic elements for replications are represented by the coloured arrows on the inner circle and bar on the outer circle, respectively. In (B), helix–turn–helix motif was presented by fold of α-helix and β-sheet. In (D), the nick site of dso was indicated by black arrows. In (E), inverted repeats and their stem-loops are displayed as arms and the CS-6 is shown using a line. Abbreviations: DDR, distal direct repeat.
Replication system of pKS1030-3
The typical replication system of a plasmid via the rolling-circle mechanism in Gram-positive bacteria contains a replication protein-encoding gene (repB), a double-stranded origin of replication (dso), a single-stranded origin of replication (sso), and an origin of transfer (oriT)26. pKS1030-3 contains these genetic elements (Fig. 1).
The putative RepB protein encoded within pKS1030-3 comprises 286 amino acids and exhibits 96.5% and 95.1% sequence identity with the RepB family plasmid replication initiator proteins encoded by S. equorum 876_5 (WP_129651037) and Companilactobacillus halodurans TMW 1.2172 (WP_153386870), respectively. In silico analysis revealed that pKS1030-3 RepB contains a helix–turn–helix (HTH) motif in the central part of the protein, which is expected in replication proteins for rolling-circle replication27. The putative HTH motif resembles the winged HTH motif that binds with DNA (Fig. 1B). Additionally, pKS1030-3 RepB contains the conserved tyrosine residue (residues 99 in RepB) which commonly attacks the phosphodiester bond of the DNA for nicking (Fig. 1B)28. However, three conserved motifs (G, T, and H-U (hydrophobic amino acid)-H), which are commonly detected in replication proteins for rolling-circle replication, were not detected in the RepB of pKS1030-329,30. A putative promoter, a − 35 region (5′-TTGCCA-3′, nucleotides 5619–5624) and a − 10 region (5′-TATTTA-3′, nucleotides 5644–5649) were detected upstream of repB in pKS1030-3, as was a Shine–Dalgarno sequence (5′-AGGAG-3′, nucleotides 5763–5767).
The Rep protein recognises the dso-containing nick site to generate single strand for rolling circle replication31,32. The dso sequence had a stem-loop structure containing the nick site that matches a consensus pattern (tACTAC gac-x-cccc-x(3)-GTg) and several copies of direct repeats31,32. The pKS1030-3 dso site (nucleotides 5417–5438) is located upstream of the start codon in the repB gene and contains the characteristic four distal direct repeat sequences, inverted repeat sequence, and nick site (Fig. 1D).
Additionally, pKS1030-3 possesses two ssos (nucleotides 3515–3657 and 7957–8080, respectively) (Fig. 1E), which are the initiation sites of lagging-strand synthesis and have a 6-bp consensus sequence (CS-6; 5′-TAGCGt/a-3′)33. Generally, sso sites contain extensive palindromic sequences that can form a folded structure34,35—we detected these palindromic sequences in pKS1030-3 sso when an RNA secondary structure prediction program (RNAstructure, v.6.4) was used (http://rna.urmc.rochester.edu).
Biofilm formation system of pKS1030-3
Four ica genes (icaA, icaB, icaC, and icaD, constituting the ica operon) contribute to the production of exopolysaccharide (partially N-acetylated linear β-1–6-linked glycosaminoglycan), which is involved in biofilm formation by staphylococcal speceis36. The ica operon was detected in pKS1030-3 (nucleotides 9116–12579) and strain KS1030 forms biofilms (Fig. 1A). Comparative genomic analysis with two other S. equorum strains showed that the ica operon was present in strain KS1030, but absent from strains C2014 and KM1031(Supplementary Table 2).
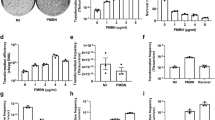
S. equorum strain KS1030 showed about three times higher biofilm formation capacity of strain KM1031 (Fig. 2). These results suggested that the ica operon of pKS1030-3 contributes to the biofilm ability of strain KS1030. Thus, the ica operon of pKS1030-3 was inserted into pYJ335 to construct pYJ335-ica, which was introduced into S. aureus RN4220. The biofilm formation ability of S. aureus RN4220 containing pYJ335-ica was confirmed to be about 2.8 and 7.6 times higher that of the control strain RN4220 and RN4220 containing pYJ335, respectively (Fig. 2). These results suggest that the ica genes in pKS1030-3 contribute to the biofilm formation ability of S. equorum strain KS1030.
Effect of the ica operon from pKS1030-3 on biofilm formation. S. equorum strain KS1030 served as the positive control and donated the ica operon for construction of pYJ335-ica, which was expressed in S. aureus RN4220. S. equorum strain KM1031, which does not possesses the ica operon, served as a negative control. The means optical density values of the stained biofilms were calculated from three biological replicates run in duplicate. Statistical relevance was analysed using Duncan’s multiple range test; *, ** and ***indicate p < 0.05, p < 0.001 and p < 0.0001, respectively.
In generally recognised as safe (GRAS) lactic acid bacteria, exopolysaccharides contribute to beneficial effects such as improvement of the texture of food, antitumour activity, and survival of probiotic strains in the gut37. However, in clinical isolates, exopolysaccharides contribute to biofilm formation, which is an important virulence factor via protection of bacteria from innate host defenses36,38. Biofilms contribute to the enhancement of AR and bacterial pathogenicity36,39. Genomic analysis of S. equorum KS1030 revealed that this strain does not possess virulence factors such as α-hemolysin, β-hemolysin, or enterotoxin genes, which have been detected in pathogenic S. aureus40,41. ica operon non-possessor strain KM1031 had a higher minimum inhibitory concentration of lincomycin than strain KS103010, indicating that the ica operon did not contribute to the enhancement of lincomycin resistance in strain KS1030. Strain KS1030 is avirulent, so the ica operon by itself appears not to be an important virulence factor.
HGT system of pKS1030-3
HGT in Gram-positive bacteria can occur via conjugation. Commonly, DNA containing an oriT site, a relaxase (rlx), and a secretion system (such as the T4SS) is needed for conjugation42. The rlx gene of strain KS1030 is encoded on plasmid pKS1030-3. Comparative genomic analysis showed that the rlx gene in pKS1030-3 is a strain-specific gene, i.e., it was present in strains KS1030, C2014, UMC-CNS-924, and Mu2 but absent from strains KM1031 and KS1039 (Fig. 3). In addition, strain-specific putative mobilization protein genes, mobC and orf2 (hypothetical protein), were located in flanking regions of the rlx gene (Fig. 1A). Interestingly, those genes were identified on the plasmid: pKS1030-3 in strain KS1030, pC2014-5 in strain C2014 and pSEQU2 in strain UMC-CNS-924, except strain Mu2. Genome of Mu2 is at the draft level, not at the complete genome, so it was not clear the position of those genes.
Relaxase is key enzyme of plasmid transfer initiation. Relaxase binds covalently to the recognition site of oriT and then cleaves the nick site of oriT; this complex is subsequently guided to the DNA transport machinery (such as the T4SS)41. The putative relaxase protein encoded by pKS1030-3 comprises 319 amino acids and exhibits 97.5% sequence identity with the relaxase (RIL47000.1) encoded on S. equorum SNUC 115 (Table 1). The deduced relaxase of pKS1030-3 has three conserved motifs that are identified in the MOBp family among several mobilization families43. The relaxase in pKS1030-3 displayed the catalytic tyrosine residues (Y18) in motif I, a serine residue (S61) in motif II, and a histidine triad (H94, H101, and H103) in motif III (Fig. 4A). A conserved Y18 in motif I has been presumed to initiate the cleavage reaction via attack on the scissile DNA phosphodiester bond, and the conserved serine in motif II might be implicated in the interaction of the relaxase with the 3′-end of the nick DNA44. The histidine triad is involved in coordination a metallic cofactor such as a Mg2+ ion, essential in the cleavage mechanism30. The relaxase predicted using AlphaFold had a structure in which N-terminal and C-terminal were distinguished (Fig. 4B). And conserved motifs were located on the N-terminal, and it was able to predict the structure of binding with DNA (Fig. 4B). Relaxase recognises and binds to a specific DNA binding sequence, oriT, on the plasmid, while Rep recognises the dso. The oriT in Gram-positive bacteria has inverted sequence containing nick site45, and/or consensus sequence 5′-NcgtNtaAgtGCGCcCTta-3′46. Putative oriT sequence was detected in pKS1030-3 (Fig. 4C), these sequences was contained inverted sequences containing nick site, GT, and non-perfect consensus sequence.
Relaxase and putative oriT sites of S. equorum. Alignment of sequence of rlx in pKS1030-3 with relevant regions of sequence from several plasmids (A), Three dimensional structure of putative Rlx (B), and putative oriT sequences of pKS1030-3 and pSELNU1 from S. equorum strain KS1030 (C). In (A), key conserved amino acids in the three conserved motifs of Rlx are indicated by white on red. Black on yellow: conserved residue; black on green: similar amino acid residues. In (B), key conserved amino acids are indicated by pink colour. In (C), inverted sequences are indicated by arrows of the same colour and the same as the consensus sequence is marked with white letters on a black background.
Interestingly, a putative oriT site in pSELNU1 was more perfect matched with consensus sequences, even though this is not a conjugative plasmid (Fig. 4C). In addition, it was confirmed that it has three inverted sequences. These results suggest that the relaxase from pKS1030-3 might recognise the oriT sequence of pSELNU1, and then be trans-acting in the transfer of pSELNU1 into other bacteria. Although studies on the horizontal gene transfer of non-conjugation plasmids are not clear yet, results have been reported that they are transferred through trans-acting of relaxase from conjugal plasmids or sequences that mimic the sequence of bonding plasmids47,48. Accordingly, we assumed that the relaxase of pKS1030-3 might be involved the HGT of pSELNU1 via trans-acting and tried to prove this. A mobC gene was identified upstream of the rlx gene in plasmid pKS1030-3 (Fig. 1A). MobC helps to determine the specificity of oriT recognition by relaxase, enhances its nicking activity, stimulates the ATPase activity of the coupling protein, and acts as a transcriptional regulator for conjugative transfer operons49. MobC is homologous to prokaryotic transcription factors of the ribbon–helix–helix (RHH) superfamily, and putative MobCs have a RHH motif according to primary sequence analysis50. The putative MobC of pKS1030-3 showed the RHH structure (Supplementary Fig. 1). Consequently, genomic analysis suggests that the rlx, mobC, and oriT of pKS1030-3 might contribute to HGT. Therefore, an approximately 2.3-kb fragment of pKS1030-3 containing mobC, rlx, and ORF2 was amplified and cloned into the staphylococcal shuttle vector pYJ335. ORF2 was included because it showed 93% similarity with the mobilization protein (WP_012817945) of Staphylococcus sp. 693.2 (although its highest similarity was with a hypothetical protein) (Table 1). The resulting plasmid was named pYJ335-rlx. pSELNU1, containing the lnuA gene, was used to confirm the horizontal transfer via trans-acting mobilization. Plasmids pYJ335-rlx and pSELNU1 were co-introduced into S. aureus RN4220, which does not normally possesses the relaxosome and is sensitive to lincomycin. S. saprophyticus KM1053, which is resistant to tetracycline, was used as the recipient strain11. Transconjugants via S. aureus RN4220 (pSELNU1 and pYJ335-rlx) showing lincomycin and tetracycline resistance were detected at a frequency of 3.5 × 10−6, while transconjugants via S. aureus RN4220 (pSELNU1) were detected at a frequency of just 8.3 × 10−9 (Table 2). No transconjugants via S. aureus RN4220 (pYJ335-rlx) were detected. These results indicate that the relaxase encoded by pKS1030-3 increased the horizontal transfer of pSELNU1 in a trans-acting manner. Nevertheless, there are still some issues to be resolved, such as the need for experimental proof of the binding of the relaxase with the oriT site, determination of the role(s) of MobC and ORF2 in the process, identification of the inducer for horizontal transfer, and identification of the secretion system for pSELNU1.
In conclusion, S. equorum KS1030 shows strain-specific lincomycin resistance and biofilm formation properties9. Previously, we determined that the lincomycin resistance derives from acquired plasmid pSELNU1, which encodes the lincomycin resistance gene lunA without mobile elements such as relaxase, from S. equorum KS103010. In the current study, we identified that the relaxase and ica operon of plasmid pKS1030-3 and those genes involved the HGT of pSELNU1 and biofilm formation by S. equorum KS1030, respectively. Notably, in the transfer, the HGT elements encoded in pKS1030-3 are trans-acting (Fig. 5). Recently, there have been many concerns that strains exhibiting acquired antibiotic resistance are unsafe1,51,52. However, current results showed that even if strain has acquired antibiotic resistance gene, it cannot be transfer horizontally into other species without a mobile element such as relaxase. These results were also confirmed in other experimental results. S. equorum strain KM1031 has plasmid pSELNU3 encoding the lnuA gene that is resistant to lincomycin10. However, S. equorum strain KM1031 did not encoded the relaxase gene and did not showed the horizontal gene transfer of pSELNU3 into other strains and/or species. These results can be assumed that horizontal gene transfer did not occurred without mobile elements even if the acquired antibiotic resistance gene is present. Therefore, to select the safe starter candidates in horizontal gene transfer of antibiotic resistance, it is necessary to check whether you have mobile elements at the same time as checking whether you have acquired antibiotic resistance genes. Fermented foods act as reservoirs and vehicles for large populations of living bacteria and have been proposed as possible sources of antibiotic-resistant bacteria53,54,55. It is not yet known if pSELNU1 could transfer between S. equorum strains, or into other species, if S. equorum KS1030 containing pSELNU1 were used as a starter strain. However, if such horizontal plasmid transfer can occur during fermentation, there is a need to control it.
Working model for horizontal transfer of pSELNU1 by S. equorum strain KS1030, using the relaxase encoded in pKS1030-3 acting in trans. pKS1030-3 carries an origin of transfer (oriT; blue line) and encodes a DNA relaxase (rlx; green box) as well as a coupling protein (mobC; pink box) and products for formation of the mating pore. Rlx and MobC were indicated by green and pink circle, respectively. HP and secretion systems were grayed out because their genes or functions were not inferred. Brown was the cell membrane, beige meant peptidoglycan. pSELNU1 also carries an oriT that is recognised by the relaxase of pKS1030-3 and forms the mating pore.
Data availability
The genome sequences used during the current study are available in in NCBI data base (http://ncbi.nlm.nih.gov/genomes): CP068576–CP068580 for S. equorum strain KS1030; CP013980–CP013983 for strain KM1031; CP013714–CP013719 for strain C2014; CP013114 for strain KS1039; CAJL01000001–CAJL01000030 for strain Mu2; and AVBD01000001–AVBD01000039 for strain UMC-CNS-924. All relevant data are within the manuscript and its supporting information files.
References
CDC. Antibiotic Resistance Threats in the United States. (U.S. Department of Health and Human Services, Atlanta, GA, CDC, 2019).
Tacconelli, E. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018).
Sommer, M. O. A., Dantas, G. & Church, G. M. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 325, 1128–1131 (2009).
Sørensen, S. J., Bailey, M., Hansen, L. H., Kroer, N. & Wuertz, S. Studying plasmid horizontal transfer in situ: a critical review. Nat. Rev. Microbiol. 3, 700–710 (2005).
Alekshun, M. N. & Levy, S. B. Molecular mechanisms of antibacterial multidrug resistance. Cell 128, 1037–1050 (2007).
Millan, A. S. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 26, 978–985 (2018).
Garcillan-Barcia, M. P., Pluta, R., Lorenzo-Diaz, F., Bravo, A. & Espinosa, M. The facts and family secrets of plasmids that replicate via the rolling-circle mechanism. Microbiol. Mol. Biol. Rev. 86, e0022220 (2022).
Guan, L., Cho, K. H. & Lee, J.-H. Analysis of the cultivable bacterial community in jeotgal, a Korean salted and fermented seafood, and identification of its dominant bacteria. Food Microbiol. 28, 101–113 (2011).
Jeong, D.-W., Han, S. & Lee, J.-H. Safety and technological characterization of Staphylococcus equorum isolates from jeotgal, a Korean high-salt-fermented seafood, for starter development. Int. J. Food Microbiol. 188, 108–115 (2014).
Lee, J.-H. & Jeong, D.-W. Characterization of mobile Staphylococcus equorum plasmids isolated from fermented seafood that confer lincomycin resistance. PLoS ONE 10, e0140190 (2015).
Heo, S., Bae, T., Lee, J.-H. & Jeong, D.-W. Transfer of a lincomycin-resistant plasmid between coagulase-negative staphylococci during soybean fermentation and mouse intestine passage. FEMS Microbiol. Lett. 366, fnz113 (2019).
Kim, T., Heo, S., Lee, J.-H. & Jeong, D.-W. Complete genome sequence of Staphylococcus equorum KS1030 exhibiting acquired lincomycin resistance. Kor. J. Microbiol. 57, 210–212 (2021).
Aziz, R. K. et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 9, 75 (2008).
Kumar, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucletic. Acids Res. 22, 4673–4680 (1994).
McGuffin, L. J., Bryson, K. & Jones, D. T. The PSIPRED protein structure prediction server. Bioinformatics 16, 404–405 (2000).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Darzi, Y., Letunic, I., Bork, P. & Yamada, T. iPath3.0: Interactive pathways explorer v3. Nucl. Acids Res. 46, W510–W513 (2018).
Ji, Y., Marra, A., Rosenberg, M. & Woodnutt, G. Regulated antisense RNA eliminates alpha-toxin virulence in Staphylococcus aureus infection. J. Bacteriol. 181, 6585–6590 (1999).
Hanahan, D. & Meselson, M. Plasmid screening at high colony density. Methods Enzymol. 100, 333–342 (1983).
Kraemer, G. R. & Iandolo, J. J. High-frequency transformation of Staphylococcus aureus by electroporation. Curr. Microbiol. 21, 373–376 (1990).
Heilmann, C. et al. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20, 1083–1091 (1996).
Djordjevic, D., Wiedmann, M. & McLandsborough, L. A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68, 2950–2958 (2002).
Dunny, G. M., Craig, R. A., Carron, R. L. & Clewell, D. B. Plasmid transfer in Streptococcus faecalis: Production of multiple sex pheromones by recipients. Plasmid 2, 454–465 (1979).
Lina, G. et al. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 43, 1062–1066 (1999).
Kwong, S. M., Ramsay, J. P., Jensen, S. O. & Firth, N. Replication of staphylococcal resistance plasmids. Front. Microbiol. 8, 2279 (2017).
García de Viedma, D., Serrano-López, A. & Díaz-Orejas, R. Specific binding of the replication protein of plasmid pPS10 to direct and inverted repeats is mediated by an HTH motif. Nucl. Acids Res. 23, 5048–5054 (1995).
Moscoso, M., Eritja, R. & Espinosa, M. Initiation of replication of plasmid pMV158: Mechanisms of DNA strand-transfer reactions mediated by the initiator RepB protein. J. Mol. Biol. 268, 840–856 (1997).
Khan, S. A. Plasmid rolling-circle replication: Highlights of two decades of research. Plasmid 53, 126–136 (2005).
Chandler, M. et al. Breaking and joining single-stranded DNA: The HUH endonuclease superfamily. Nat. Rev. Microbiol. 11, 525–538 (2013).
Marsin, S. & Forterre, P. A rolling circle replication initiator protein with a nucleotidyl-transferase activity encoded by the plasmid pGT5 from the hyperthermophilic archaeon Pyrococcus abyssi. Mol. Microbiol. 27, 1183–1192 (1998).
Ruiz-Masó, J. A., Lurz, R., Espinosa, M. & del Solar, G. Interactions between the RepB initiator protein of plasmid pMV158 and two distant DNA regions within the origin of replication. Nucl. Acids Res. 35, 1230–1244 (2007).
Kramer, M. G., Khan, S. A. & Espinosa, M. Lagging-strand replication from the ssoA origin of plasmid pMV158 in Streptococcus pneumoniae: in vivo and in vitro influences of mutations in two conserved ssoA regions. J. Bacteriol. 180, 83–89 (1998).
Li, M. S. et al. Cloning and molecular characterization of a novel rolling-circle replicating plasmid, p K1S–1, from Bacillus thuringiensis subsp. kurstaki K1. J. Microbiol. 47, 466–472 (2009).
Kramer, M. G., Espinosa, M., Misra, T. K. & Khan, S. A. Lagging strand replication of rolling-circle plasmids: specific recognition of the ssoA-type origins in different Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 95, 10505–10510 (1998).
Nguyen, H. T. T., Nguyen, T. H. & Otto, M. The staphylococcal exopolysaccharide PIA - biosynthesis and role in biofilm formation, colonization, and infection. Comput. Struct. Biotechnol. J. 18, 3324–3334 (2020).
Patel, S., Majumder, A. & Goyal, A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J. Microbiol. 52, 3–12 (2012).
Kropec, A. et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 73, 6868–6876 (2005).
de Silva, G. D. I. et al. The ica operon and biofilm production in coagulase-negative staphylococci associated with carriage and disease in a neonatal intensive care unit. J. Clin. Microbiol. 40, 382–388 (2002).
Kane, T. L., Carothers, K. E. & Lee, S. W. Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr. Drug Targets 19, 111–127 (2018).
Pluta, R. et al. Structural basis of a histidine-DNA nicking/joining mechanisms for gene transfer and promiscuous spread of antibiotic resistance. Proc. Natl. Acad. Sci. USA 114, E6526–E6535 (2017).
Kiss, J. et al. Identification and characterization of oriT and two mobilization genes required for conjugative transfer of Salmonella genomic island 1. Front. Microbiol. 10, 457 (2019).
Ramachandran, G. et al. Discovery of a new family of relaxases in Firmicutes bacteria. PLoS Genet. 13, e1006586 (2017).
Pansegrau, W., Schroder, W. & Lanka, E. Concerted action of three distinct domains in the DNA cleaving-joining reaction catalysed by relaxase (TraI) of conjugative plasmid RP4. J. Biol. Chem. 269, 2782–2789 (1994).
Hegyi, A., Szabo, M., Olasz, F. & Kiss, J. Identification of oriT and a recombinantion hot spot in the IncA/C plasmid backbone. Sci. Rep. 7, 10595 (2017).
Grohmann, E., Muth, G. & Espinosa, M. Conjugative plasmid transfer in Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67, 277–301 (2003).
Chang, A. C. & Cohen, S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134, 1141–1156 (1978).
Ramsay, J. & Firth, N. Diverse mobilization strategies facilitate transfer of non-conjugative mobile genetic elements. Curr. Opin. Microbiol. 38, 1–9 (2017).
Godziszewska, J. et al. Concerted action of NIC relaxase and auxiliary protein MobC in RA3 plasmid conjugation. Mol. Microbiol. 101, 439–456 (2016).
Drozdetskiy, A., Cole, C., Procter, J. & Barton, G. J. JPred4: a protein secondary structure prediction server. Nucl. Acids Res. 43, W389–W394 (2015).
EFSA Panel on Additives and Products or Substance used Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10, 2740–2749 (2012).
Perreten, V. et al. Antibiotic resistance spread in food. Nature 389, 801–802 (1997).
Cocconcelli, P. S., Cattivelli, D. & Gazzola, S. Gene transfer of vancomycin and tetracycline resistances among Enterococcus faecalis during cheese and sausage fermentations. Int. J. Food Microbiol. 88, 315–323 (2003).
Rizzotti, L. et al. Contribution of enterococci to the spread of antibiotic resistance in the production chain of swine meat commodities. J. Food Prot. 68, 955–965 (2005).
Nawaz, M. et al. Characterization and transfer of antibiotic resistance in lactic acid bacteria from fermented food products. Curr. Microbiol. 62, 1081–1089 (2011).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) [NRF-2019R1A2C1003639]. We thank Edanz (www.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
D.W.J. designed and supervised the study. S.H., S.E.O., G.L., J.L., N.C.H., C.O.J., K.J., and J.H.L. collected and analysed the data. S.H., and D.W.J. drafted the manuscript. S.H., J.H.L., and D.W.J finalised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heo, S., Oh, SE., Lee, G. et al. Staphylococcus equorum plasmid pKS1030-3 encodes auxiliary biofilm formation and trans-acting gene mobilization systems. Sci Rep 13, 11108 (2023). https://doi.org/10.1038/s41598-023-38274-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38274-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.