Abstract
Renal dysfunction is a long-term complication associated with an increased mortality after lung transplantation (LT). We investigated the association of single-nucleotide polymorphisms (SNPs) with the development of renal dysfunction after LT using a Japanese-specific SNP array. First, eligible samples of 34 LT recipients were genotyped using the SNP array and divided into two groups, according to the presence of homozygous and heterozygous combinations of mutant alleles of the 126 renal-related SNPs. To identify candidate SNPs, the renal function tests were compared between the two groups for each SNP. Next, we investigated the association between the candidate SNPs and the time course of changes of the estimated glomerular filtration rate (eGFR) in the 99 recipients until 10 years after the LT. ΔeGFR was defined as the difference between the postoperative and preoperative eGFR values. Eight SNPs were identified as the candidate SNPs in the 34 recipients. Validation analysis of these 8 candidate SNPs in all the 99 recipients showed that three SNPs, namely, rs10277115, rs4690095, and rs792064, were associated with significant changes of the ΔeGFR. Pre-transplant identification of high-risk patients for the development of renal dysfunction after LT based on the presence of these SNPs might contribute to providing personalized medicine.
Similar content being viewed by others
Introduction
Lung transplantation (LT) is an established therapeutic option for selected patients with end-stage pulmonary disease. Although advances in immunosuppressive drug therapy have improved the outcomes after solid organ transplantation, the survival rate after LT has been lower than that after other organ transplantation1,2. Life-long immunosuppressive therapy, including with calcineurin inhibitors (CNI), mycophenolate mofetil and glucocorticoids is required after LT to prevent chronic lung allograft dysfunction (CLAD), this latter condition being the main hindrance to long-term survival3,4,5. However, long-term immunosuppression is also associated with the risk of development of chronic kidney disease (CKD), potentially necessitating initiation of chronic dialysis or renal transplantation after LT6,7,8,9,10. According to the registry report from the International Society for Heart and Lung Transplantation (ISHLT), severe renal dysfunction, defined by a serum creatinine level of 2.5 mg/dL or higher and the need for chronic dialysis or renal transplantation, is estimated to occur in 5.6% of patients at 1 year, 16.0% of patients at 5 years, and 24.6% of patients at 10 years after LT1. Importantly, severe renal dysfunction is associated with increased mortality7. Fluid retention caused by CKD has a negative effect on the lung allograft, leading to the development of infection and CLAD. In addition, therapeutic options for late complications after LT, including CLAD, infection, and malignancy, is definitely limited due to poor kidney function in patients with CKD. Therefore, preventing the development of CKD over the long term is necessary to improve the prognosis after LT.
Genome-wide association studies have been performed to identify novel genetic associations for many diseases. Recently, a meta-analysis of genome-wide association studies identified multiple genetic loci associated with kidney function-related traits in east Asian populations11. In relation to LT, various kinds of single-nucleotide polymorphisms (SNPs) have been shown to be associated with the rates of utilization of donor lung allografts12, risk of development of bronchiolitis obliterans syndrome13, risk of development of primary graft dysfunction (PGD)14, rate of postoperative decline in the total lung capacity15, and infection free-survival after discharge from the intensive care unit in recipients of living-donor lobar LT (LDLLT)16. However, the associations between SNPs and the risk of development of renal dysfunction over the long term after LT have not yet been elucidated. Identification of the SNPs might contribute to distinguishing high-risk patients for the development of renal dysfunction and offering personalized medicine to improve prognosis of these patients after LT. The purpose of this study is to explore SNPs associated with the risk of development of renal dysfunction over the long term after LT.
In this study, we investigated the SNPs that might be associated with the risk of development of renal dysfunction over the long term after LT using an ethnic-specific SNP array for the Japanese population, the Japonica Array NEO (Toshiba, Japan), which comprises a total of 66,883 markers17.
Results
Exploratory cohort analysis
The characteristics of the 34 recipients in the exploratory cohort are shown in Table 1. The patients had normal renal function, with a median eGFR of 95.8 (46.0–174.0) mL/min/1.73 m2 prior to LT. The prevalence rates of posttransplant diabetes mellitus18, hypertension18,19, and dyslipidemia18, which are known as risk factors for posttransplant renal dysfunction, were 8.8%, 79.4%, and 26.5%, respectively. Two patients (5.9%) required hemodialysis after LT. The median follow-up period was 8.40 (2.20–21.9) years after LT.
Of the total of 126 renal-related SNPs, 8 SNPs, including rs102275 (P = 1.2 × 10–4, MAF = 0.33), rs10277115 (P = 4.2 × 10–5, MAF = 0.25), rs174549 (P = 1.2 × 10–4, MAF = 0.33), rs2980098 (P = 8.4 × 10–5, MAF = 0.33), rs4690095 (P = 3.3 × 10–9, MAF = 0.47), rs72719193 (P = 1.2 × 10–4, MAF = 0.49), rs792064 (P = 3.4 × 10–4, MAF = 0.40), and rs7956634 (P = 1.2 × 10–4, MAF = 0.34), were identified as candidate SNPs associated with significant differences in the postoperative changes of the ΔeGFR over a 10-year period after LT (Fig. 1). Detailed information on the chromosome, reference allele, alternation allele, gene, location, and MAF is presented in Table 2.
In the exploratory cohort, 8 single-nucleotide polymorphisms (SNPs), including rs102275 (P = 1.2 × 10–4, MAF = 0.33), rs10277115 (P = 4.2 × 10–5, MAF = 0.25), rs174549 (P = 1.2 × 10–4, MAF = 0.33), rs2980098 (P = 8.4 × 10–5, MAF = 0.33), rs4690095 (P = 3.3 × 10–9, MAF = 0.47), rs72719193 (P = 1.2 × 10–4, MAF = 0.49), rs792064 (P = 3.4 × 10–4, MAF = 0.40), and rs7956634 (P = 1.2 × 10–4, MAF = 0.34), were identified as candidate SNPs associated with significant differences in the ΔeGFR (postoperative eGFR − preoperative eGFR) during the first 10 years after lung transplantation.
Validation cohort analysis
The characteristics of the 99 recipients in the validation cohort are shown in Table 1. The pre-transplant median eGFR value was normal in all the recipients, at 99.0 (46.0–322.6) mL/min/1.73 m2. The prevalence rates of posttransplant diabetes mellitus, hypertension, and dyslipidemia were 14.1%, 81.8%, and 19.2%, respectively. Six patients (6.1%) eventually required hemodialysis after LT. The median follow-up period was 7.90 (0.8–23.4) years after LT.
Validation analysis of the 8 candidate SNPs showed that three of the SNPs (rs10277115, rs4690095, and rs792064) were associated with significant differences in the postoperative changes of the ΔeGFR over a 10-year period after LT (Fig. 2). The postoperative changes of the ΔeGFR were significantly lower in the AA group for rs10277115, CC group for rs460095, and CC group for rs792064 (P = 0.02, 0.04, and 0.03, respectively). Figure 3 shows the mean trough level of CNIs during the first 10 years after LT in the three identified SNPs, and there were no significant differences between the two groups for the three SNPs. The characteristics of the patients with each of the 3 SNPs are shown in the Supplementary data. In the patients with rs10277115, there was a significant difference in the distribution of the underlying disease between the two groups (P = 0.034) (Supplementary Table S1). Interestingly, the pretransplant creatinine was significantly lower (P = 0.023) and pretransplant eGFR was significantly higher (P = 0.008) in the CC group than in the CT/TT group for rs4690095 (Supplementary Table S2). Although the renal function before the LT was worse in the CT/TT group, it was better preserved over time in this group as compared with that in the CC group. The patient characteristics did not differ between the two groups classified according to homozygosity/heterozygosity for rs792064 (Supplementary Table S3).
In the validation cohort of 99 recipients, there were no significant differences in the trough level of cyclosporine or tacrolimus during the first 10 years after lung transplantation between the two groups for the three identified single-nucleotide polymorphisms (SNPs) (rs10277115; cyclosporine (P = 0.96), tacrolimus (P = 0.54); rs4690095, cyclosporine (P = 0.47), tacrolimus (P = 0.96); rs792064; cyclosporine (P = 0.70), tacrolimus (P = 0.10)).
Additional analysis of the 8 candidate SNPs in the 65 recipients except the 34 recipients in the exploratory cohort showed that rs4690095 was associated with significant difference in the postoperative changes of the ΔeGFR over a 10-year period after LT (P = 0.048) (Supplementary Fig. S1). The characteristics of the 65 recipients in the validation cohort are shown in Supplementary Table S4 and the patient characteristics did not differ between the two groups classified according to homozygosity/heterozygosity for rs4690095 (Supplementary Table S5).
Discussion
In the present study, we found from our analysis using a Japanese-specific SNP array that three SNPs, namely, rs10277115, rs4690095, and rs792064, among the 8 candidate SNPs were associated with significant differences in the postoperative changes of the ΔeGFR over a 10-year period after LT. Presence of these three SNPs was associated with the development of renal dysfunction in the long term after LT, suggesting that these SNPs might represent unique predictive risk factors for the development of CKD over the long term after LT. Furthermore, genotypic assessment for the presence of these SNPs prior to LT may contribute to improvement in personalized medicine, such as provision of individualized immunosuppressive therapy to protect the kidney function over the long term after LT. To the best of our knowledge, this study is the first to explore SNPs associated with the risk of development of renal dysfunction over the long term after LT.
We demonstrated that the T alleles of rs10277115, rs4690095, and rs792064 might be nephroprotective in patients receiving long-term immunosuppressive therapy after LT. The first SNP, rs10277115 in the UNCX gene, which encodes a paired-type homeobox transcription factor and has essential roles in skeleton formation and kidney development20, has been shown to be associated with an increased risk of development of CKD in East Asian populations, including Japanese11. The second SNP, rs4690095, that has been mapped to the RGS gene involved in transcription factors and tumorigenesis, which was identified in a genome-wide association analysis conducted using 58 clinical tests in 160,000 Japanese subjects, was reported to be associated with the serum creatinine levels, eGFR values, and serum albumin levels21. The third SNP, rs790264, which is located in an intergenic region, was identified from a genome-wide association analysis conducted for a European pediatric CKD cohort as being associated with proteinuria22. As previously reported, Asian populations are reported as being at a lower risk of developing CKD after non-renal solid organ transplantation7. The risk of CKD after transplantation might be influenced by racial differences represented by SNPs associated with the risk of renal dysfunction developing over the long term after transplantation, as shown in the present study.
The results of this study revealed that the nephroprotective effect of each SNP was marked from 3 to 6 months, reached a plateau between 6 months and 1 year, and was maintained over the long-term after LT. In fact, the decline of renal function after LT is known to be usually biphasic, with a rapid decline in the first post-transplant year and a slower decline thereafter9. After LT, acute kidney injury in the perioperative period results from the administration of multiple drugs, including immunosuppressive and prophylactic therapies for viral and fungal infections, and CKD is attributed primarily to CNI therapy7. The recipients of LT with the T allele of the three identified SNPs in our study may show a slower progression of renal dysfunction after the baseline immunosuppressive therapy is tapered. Notably, whereas the pre-transplant renal function did not differ between the homozygous and heterozygous groups for rs10277115 and rs792064, the CT/TT genotypic group for rs4690095 showed worse renal function prior to the LT. Our results indicate that the post-transplant decline in renal function might be independent of the pre-transplant renal function.
Based on the information about the SNPs identified in the present study, pretransplant identification of patients at a high risk for developing renal dysfunction after LT might enable providers to minimize the doses of or discontinue nephrotoxic agents, including of immunosuppressive agents, even from the early phase after LT. Since CKD after LT is caused mainly by CNIs7, tapering or withdrawal of CNIs and initiation of treatment with a mammalian target of rapamycin (m-TOR) inhibitor may potentially improve or slow the decline of renal function after LT23,24. The switch from CNI to mTOR inhibitor in the early phase after LT might alleviate deterioration of the renal function in recipients who are identified as being at a high risk for developing renal dysfunction after LT. In addition, investigation of SNPs related to drug metabolism of CNIs in the future might contribute to identification of further SNPs associated with the risk of development of renal dysfunction after LT.
In regard to the patient characteristics, the prevalences of known risk factors for the development of renal dysfunction after LT, such as age18,25, hypertension18,19, dyslipidemia18, and diabetes mellitus18, were similar between the two groups for each of the three identified SNPs in the present study. Presence of these risk factors/comorbidities did not appear to affect the differences in the renal function changes associated with the identified SNPs. To assess the renal function change over the long term after LT in the present study, we used the modified eGFR equation for the Japanese population26, which is independent of the muscle mass, rather than the serum creatinine, because the estimated eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation has been shown to be a better predictor of the risk of CKD and may allow a better assessment of the differences in the renal function over time than that possible using serum creatinine alone in patients undergoing LT27,28.
This study had several limitations. First, it was a retrospective and single-center study, and the sample size was small. Second, a Japanese-specific SNP array and modified eGFR values tailored to the Japanese population were used, so that our results were ethnicity-specific. Third, for financial reasons, the Japanese-specific SNP array was used for the genotyping of SNPs only in the exploratory cohort. Fourth, many long-term survivors were included, while patients who had died prior to the start of the blood sample collection were not included, leading to a selection bias. However, this study may provide valuable information about SNPs associated with the development of renal dysfunction in the long term after LT.
In conclusion, our analysis using an ethnic-specific SNP array revealed that three SNPs, namely, rs10277115, rs4690095, and rs792064, were associated with the development of renal dysfunction in the long term after LT. Accordingly, pretransplant identification of high-risk patients for the development of renal dysfunction after LT based on the presence of these SNPs might contribute to providing personalized/precision medicine, such as individualized immunosuppression, in patients requiring lung transplantation.
Methods
Patients
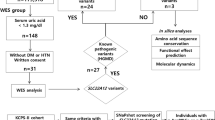
This retrospective cohort study initially included 204 patients who had undergone LT at Okayama University Hospital between October 1998 and March 2020. Among these, 43 pediatric-age patients (age < 18 years) were excluded to eliminate the effect of physical growth on the renal function; in addition, 62 patients who had died or failed to visit our outpatient clinic were also excluded. Blood samples were prospectively collected from the remaining 99 patients who were ≥ 18 years of age, including 65 patients who had undergone cadaveric LT (CLT) and 34 patients who had undergone LDLLT (Fig. 4A). No prisoner organs were procured for this study. The recipients of LT were prescribed life-long triple immunosuppressive therapy, consisting of a CNI, including tacrolimus or cyclosporine, mycophenolate mofetil or azathioprine, and a glucocorticoid. The pre-, intra-, and postoperative characteristics of the recipients were retrospectively collected from the institutional database and medical records. The Lung Allocation Score (LAS) of each patient was calculated at registration with the LT waiting list using the LAS calculator published on the OPTN website (https://optn.transplant.hrsa.gov/resources/allocation-calculators/las-calculator/) in July 2020 to assess the preoperative severity of the recipients. The severity grades of primary graft dysfunction (PGD) were assigned based on the definition of PGD proposed by the consensus report from the ISHLT29. CLAD was diagnosed using the classification system proposed by the ISHLT30.
(A) Among 204 patients who underwent lung transplantation, including cadaveric lung transplantation (CLT) (N = 110) and living-donor lobar lung transplantation (LDLLT) (N = 94), 105 patients were excluded as they were < 18 years old (N = 43) or had died/not visited our outpatient clinic (N = 62). Blood samples were collected from the remaining 99 patients, including 65 patients who had undergone CLT and 34 patients who had undergone LDLLT. (B) In the exploratory cohort, 34 patients were divided into two groups according to the presence of homozygous and heterozygous combinations. The association between 126 renal-related single-nucleotide polymorphisms (SNPs) out of 666,883 variants and the postoperative changes of renal function were evaluated to explore candidate SNPs. Among SNPs with minor allele frequencies (MAF) of between 0.2 and 0.5 in the Japanese population, SNPs with P values of ≤ 0.0004 were identified as candidate SNPs. In the validation cohort, 99 patients were divided into two groups according to the presence of homozygous and heterozygous combinations of each candidate SNP. The associations between the candidate SNPs and the postoperative changes of renal function were evaluated. SNPs associated with renal function changes at P values of < 0.05 were identified as valid SNPs.
The study protocol (No.1610-037) was approved by the institutional review board of Okayama University Hospital. Written informed consent for the study was obtained from each of the patients. All methods adopted for the study were in compliance with the relevant guidelines and regulations.
Assessment of renal function
The estimated glomerular filtration rate (eGFR) value was used as an index of global kidney function and calculated using a revised equation for serum creatinine-based estimation of the eGFR in mL/min/1.73 m2 for Japanese, as follows: 194 × serum creatinine−1.094 × age−0.287 × 0.739 (if female)26. The preoperative eGFR values of the recipients were determined at the time of hospital admission immediately before the LT. The postoperative eGFR values of the were measured at 7 days, 1, 3, 6, and 12 months, and then every year until 10 years after the LT. Delta eGFR (ΔeGFR) was defined as the difference between the postoperative and preoperative eGFR values: ΔeGFR = postoperative eGFR value—preoperative eGFR value.
Sample collection and preparation
Blood samples were collected from the 99 recipients for SNP genotyping between September 2016 and July 2020. The blood samples were centrifuged at 3500×g for 10 min, and the sedimented red blood cells were stored at − 80 °C. Genomic DNA was extracted from the buffy coat with a QIAamp DNA Mini Kit (QIAGEN K.K., Tokyo, Japan) and stored at − 80 °C.
Genotyping of SNPs in the exploratory cohort
Among the blood samples collected from the 99 LT recipients, genomic DNA extracted from the buffy coats of only 34 samples fulfilled the criteria (genomic DNA concentration ≥ 50 ng/µL) for genotyping using a SNP array explicitly designed for the Japanese population, the Japonica array NEO. The array contains 666,883 SNPs, including 654,246 tag SNPs of autosomes and the X chromosome, and 28,298 previously identified disease-related markers. The full marker list and detailed list of disease-related SNPs are available at the jMorp website (https://jmorp.megabank.tohoku.ac.jp/downloads/#jpa). Genome-wide SNP genotyping of the samples from 34 patients were performed using this array17.
According to the presence of homozygous or heterozygous combinations, the 34 recipients were divided into two groups. The associations between 126 renal-related SNPs out of the 666,883 variants and the postoperative changes of renal function were evaluated to explore candidate SNPs. Among the SNPs with minor allele frequencies (MAF) of between 0.2 and 0.5 in the Japanese population, SNPs with P values of < 0.0004 were identified as candidate SNPs to counteract the problem of multiple comparisons (Fig. 4B).
Genotyping of SNPs in the validation cohort
A genotype analysis for functional polymorphisms was also conducted in the samples of the remaining 65 recipients. Genomic DNA was extracted from the red blood cells with a TaqMan® Sample-to-SNP™ kit (Applied Biosystems, Foster City, CA, USA). Samples were analyzed by TaqMan genotyping assay using the StepOne™ real-time polymerase chain reaction (PCR) system (Applied Biosystems) in a 96-well array plate that included 2 blank wells as negative controls. The PCR profile consisted of an initial denaturation step at 95 °C for 20 s, 40 cycles at 95 °C for 3 s, and at 60 °C for 20 s. The PCR products were analyzed using the StepOne™ Software Ver. 2.3 (Applied Biosystems). To assess the quality of the genotyping, repeat genotyping was conducted in a randomly selected 5% of the samples, and 100% agreement was confirmed.
All of the 99 recipients, consisting of the 65 recipients of the validation cohort and 34 recipients of the exploratory cohort, were divided into two groups according to the presence of homozygous or heterozygous combinations of each candidate SNP. The associations between the candidate SNPs and the postoperative changes of renal function were evaluated. SNPs with P values of < 0.05 were identified as valid SNPs. The same analysis was performed only in the 65 recipients except the 34 recipients of the exploratory cohort. In addition, the postoperative changes of trough level of CNIs during the first 10 years after LT were compared between the two groups for each identified SNP.
Statistical analysis
Categorical variables were expressed as number of cases with percentages, and continuous variables were expressed in median values with ranges. Categorical variables were compared using Fisher’s exact probability test, as appropriate, and continuous variables were compared using the Mann–Whitney U test. The postoperative changes in the ΔeGFR were compared between the two groups classified according to the presence of homozygous or heterozygous combinations of each SNP using 2-way repeated measures analysis of variance (ANOVA). All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria)31. More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics. P < 0.05 was considered as being indicative of statistical significance.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Change history
04 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-43995-x
References
Chambers, D. C. et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 38, 1042–1055 (2019).
Lodhi, S. A., Lamb, K. E. & Meier-Kriesche, H. U. Solid organ allograft survival improvement in the United States: The long-term does not mirror the dramatic short-term success. Am. J. Transplant. 11, 1226–1235 (2011).
Korom, S., Boehler, A. & Weder, W. Immunosuppressive therapy in lung transplantation: State of the art. Eur. J. Cardiothorac. Surg. 35, 1045–1055 (2009).
Scheffert, J. L. & Raza, K. Immunosuppression in lung transplantation. J. Thorac. Dis. 6, 1039–1053 (2014).
Sugimoto, S. et al. Impact of chronic lung allograft dysfunction, especially restrictive allograft syndrome, on the survival after living-donor lobar lung transplantation compared with cadaveric lung transplantation in adults: A single-center experience. Surg. Today 49, 686–693 (2019).
Fisher, N. C. N. P., Gunson, B. K., Lipkin, G. W. & Neuberger, J. M. Chronic renal failure following liver transplantation: A retrospective analysis. Transplantation 66, 59–66 (1998).
Ojo, A. O. et al. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 20, 20 (2003).
Pattison, J. M. et al. The incidence of renal failure in one hundred consecutive heart-lung transplant recipients. Am. J. Kidney Dis. 26, 643–648 (1995).
Wilkinson, A. H. & Cohen, D. J. Renal failure in the recipients of nonrenal solid organ transplants. J. Am. Soc. Nephrol. 10, 1136–1144 (1999).
Sugimoto, S. et al. Long-term outcomes of living-donor lobar lung transplantation. J. Thorac. Cardiovasc. Surg. 164, 440–448 (2022).
Okada, Y. et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 44, 904–909 (2012).
Sapru, A. et al. Single-nucleotide polymorphisms in the beta-adrenergic receptor genes are associated with lung allograft utilization. J. Heart Lung Transplant. 30, 211–217 (2011).
Budding, K. et al. A Promoter polymorphism in the CD59 complement regulatory protein gene in donor lungs correlates with a higher risk for chronic rejection after lung transplantation. Am. J. Transplant. 16, 987–998 (2016).
Diamond, J. M. et al. Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 186, 546–552 (2012).
Yamamoto, H. et al. A single-nucleotide polymorphism in a gene modulating glucocorticoid sensitivity is associated with the decline in total lung capacity after lung transplantation. Surg. Today 49, 268–274 (2019).
Kayawake, H. et al. Impacts of single nucleotide polymorphisms in Fc gamma receptor IIA (rs1801274) on lung transplant outcomes among Japanese lung transplant recipients. Transpl. Int. 34, 2192–2204 (2021).
Sakurai-Yageta, M. et al. Japonica Array NEO with increased genome-wide coverage and abundant disease risk SNPs. J. Biochem. 170, 399–410 (2021).
Bloom, R. D. & Doyle, A. M. Kidney disease after heart and lung transplantation. Am. J. Transplant. 6, 671–679 (2006).
Kunst, H., Thompson, D. & Hodson, M. Hypertension as a marker for later development of end-stage renal failure after lung and heart-lung transplantation: A cohort study. J. Heart Lung Transplant. 23, 1182–1188 (2004).
Mansouri, A. et al. Paired-related murine homeobox gene expressed in the developing sclerotome, kidney, and nervous system. Dev. Dyn. 210, 53–65 (1997).
Kanai, M. et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 50, 390–400 (2018).
Wuttke, M. et al. Genetic loci associated with renal function measures and chronic kidney disease in children: The Pediatric Investigation for Genetic Factors Linked with Renal Progression Consortium. Nephrol. Dial. Transplant. 31, 262–269 (2016).
Groetzner, J. et al. Conversion to sirolimus and mycophenolate can attenuate the progression of bronchiolitis obliterans syndrome and improves renal function after lung transplantation. Transplantation 81, 355–360 (2006).
Otani, S. et al. Long-term successful outcomes from kidney transplantation after lung and heart-lung transplantation. Ann. Thorac. Surg. 99, 1032–1038 (2015).
Barraclough, K., Menahem, S. A., Bailey, M. & Thomson, N. M. Predictors of decline in renal function after lung transplantation. J. Heart Lung Transplant. 25, 1431–1435 (2006).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992 (2009).
Osho, A. A. et al. The Chronic Kidney Disease Epidemiology Collaboration (CKDEPI) equation best characterizes kidney function in patients being considered for lung transplantation. J. Heart Lung Transplant. 33, 1248–1254 (2014).
Shaffi, K. et al. Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am. J. Kidney Dis. 63, 1007–1018 (2014).
Snell, G. I. et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 36, 1097–1103 (2017).
Verleden, G. M. et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment—a consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 38, 493–503 (2019).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Acknowledgements
We thank all this study participants for their cooperation and International Medical Information Center (www.imic.or.jp) for their assistance with English-language editing. This work was supported by a Grant-in-Aid for Scientific Research (Grant nos. 19K09305, 22K08974 and 23K08294) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
Y.T., S.S., Haruchika Y. and S.Tomida designed the study. Y.T. and S.Tomida analyzed the data. S.Tomida performed the statistical analysis. Y.T. and S.S. drafted and revised the article. All authors critically edited the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Abstract. Full information regarding the correction made can be found in the Correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomioka, Y., Sugimoto, S., Yamamoto, H. et al. Identification of genetic loci associated with renal dysfunction after lung transplantation using an ethnic-specific single-nucleotide polymorphism array. Sci Rep 13, 8912 (2023). https://doi.org/10.1038/s41598-023-36143-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36143-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.