Abstract
Head and Neck Cancer (HNC) is a globally rare cancer that includes a variety of tumors affecting the upper aerodigestive tract. It presents with difficulty breathing or swallowing and is mainly treated with radiation therapy, chemotherapy, or surgery for tumors that have spread locally or throughout the body. Alternatively, exercise can be used during cancer treatment to improve function, including pain relief, increase range of motion and muscle strength, and reduce cancer-related fatigue, thereby enhancing quality of life. Although existing evidence suggests the adjunctive use of exercise in other cancer types, no previous studies have examined the effects on HNC survivors. The aim of this meta-analysis was to quantify the effect of exercise-based rehabilitation on functionality and quality of life in HNC survivors who underwent surgery and/or chemoradiotherapy. A systematic review and meta-analysis were carried out following PRISMA statement and registered in PROSPERO (CRD42023390300). The search was performed in MEDLINE (PubMED), Cochrane Library, CINAHL and Web of Science (WOS) databases from inception to 31st December 2022 using the terms “cancer”, “head and neck neoplasms”, “exercise”, “rehabilitation”, “complications”, “muscle contraction”, “muscle stretching exercises” combining with booleans “AND”/“OR”. PEDro scale, Cochrane Risk of Bias Tool and GRADE were used to assess methodological quality, risk of bias and grade of recommendation of included studies respectively. 18 studies (n = 1322) were finally included which 1039 (78.6%) were men and 283 (21.4%) were women. In patients who underwent radio-chemotherapy, overall pain [SMD = − 0.62 [− 4.07, 2.83] CI 95%, Z = 0.35, p = 0.72] and OP [SMD = − 0.07 [− 0.62, 0.48] CI 95%, Z = 0.25, p = 0.81] were slightly reduced with exercise in comparison to controls. Besides, lower limb muscle strength [SMD = − 0.10 [− 1.52, 1.32] CI 95%, Z = 0.14, p = 0.89] and fatigue [SMD = − 0.51 [− 0.97, − 0.057] CI 95%, Z = 2.15, p < 0.01] were also improved in those who receive radio-chemoradiation. In HNC survivors treated with neck dissection surgery, exercise was superior to controls in overall pain [SMD = − 1.04 [− 3.31, 1.23] CI 95%, Z = 0.90, p = 0.37] and, in mid-term, on shoulder pain SMD = − 2.81 [− 7.06, 1.43] CI 95%, Z = 1.76, p = 0.08]. No differences in quality of life were found at any of the follow-up periods. There is evidence of fair to good methodological quality, low to moderate risk of bias, and weak recommendations supporting the use of exercise-based rehabilitation to increase functionality. However, no evidence was found in favor of the use of this modality for improving the quality of life of HNC survivors who underwent chemoradiotherapy or surgery.
Similar content being viewed by others
Introduction
Head and neck tumors comprise a heterogeneous group of lesions arising from different structures of the head and neck, with the exception of intracranial, skin, and ocular tumors1,2. They present certain characteristics that make them similar, such as being squamous cell carcinomas (HNcSCC) in most cases (95%) and being in easily accessible areas for inspection, which means that they can be detected in early stages3. In addition, malignant head and neck cancers (HNC) can histologically present as lymphoepithelioma4,5 spindle cell carcinoma6, verrucous carcinoma7, and undifferentiated carcinoma8. HNC accounted for approximately 3% of all cancers in the United States in 2018 and 1.8% of all cancer deaths in the United States during 20209. Particularly, HNcSCC is considered a fatal disease within the first 3.5 years of follow-up, with a relapse mortality rate of 2.3% per year10. Depending on anatomical location, they can be located in the lips, pharynx, larynx, paranasal sinus, nasal and oral cavities11,12.
These tumors are clearly associated with tobacco and alcohol abuse13. Other risk factors include poor oral hygiene, infection of oncogenic viruses as papillomavirus and Epstein-Barr virus and finally Plummer-Vinson syndrome14,15,16.
Treatment for these cancers includes surgery, radiation therapy (RDT), and chemotherapy (CHT)17,18. In general, phase I and II outcomes are similar in patients undergone RDT or surgery19. In some cases, such as the base of the tongue, stages I and II may require the combination of surgery and RDT, or alternatively, the use of external tele-radiotherapy and brachytherapy20,21,22. Furthermore, advanced stages III and IV are usually treated with a combination of surgery and almost always postoperative RDT19. Instead, patients within stages II and IV may be offered the possibility of being treated within clinical trials that purchase CT and RDT and/or radiosensitizers23,24.
Rehabilitation with physical activity during and after treatment is an important aspect for cancer survivors, as sequelae are often identified25,26,27,28. Exercise is known to have positive effects on physical recovery (i.e. body composition, nutritional status, etc.), physical function (i.e. pain, muscle strength, range of motion, fatigue, etc.), psychological outcomes (i.e. depression, anxiety, etc.) and quality of life, such as after breast cancer treatment29. Among the many motivations for exercising in the setting of cancer setting include avoiding muscle weakness and atrophy, cardiorespiratory fitness decline and improving energy metabolism efficiency at the cellular level30,31,32. Immunological effects in cancer survivors have also been reported to improve with exercise, including increased cytolytic activity of natural killer cells, the monocyte-functional fraction of circulating granulocytes, and reduced duration of neutropenia33,34,35. Another important reason for cancer patients to exercise is to lose weight, because body mass index is directly proportional to tumor recurrence rate and all-cause mortality36,37,38. Furthermore, obesity has also been described as a risk factor for postoperative lymphedema, assuming loss of function and quality of life39,40.
Although it has been studied in other types of cancer, such as breast and prostate cancer, the effectiveness of exercise on disease and treatment related sequelae in HNC have not been thoroughly studied41,42. Furthermore, the reviews conducted to date have not been systematic and have provided only partial and, in some cases, circumstantial evidence43,44. For all the above reasons, the aim of this meta-analysis was to quantify the effect of exercise-based rehabilitation on functionality and quality of life in HNC survivors who underwent surgery and/or chemoradiotherapy.
Materials and methods
Data source and search strategy
A systematic literature review and meta-analysis were carried out regarding the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA)45. The protocol for this systematic review was previously registered on PROSPERO (International database for prospectively registered systematic reviews; CRD42023390300).
Study selection
The selection criteria were: (1) randomized or non-randomized clinical trials, (2) published from the start of the database until December 31, 2022, (3) published in English, Spanish and Portuguese, (4) available in full text, (5) recruiting adults with HNC survivors who have undergone radio-chemotherapy or surgery, and (6) exercise-based program alone or combined with other educational or psychological support that measured (7) functionality and quality of life.
Search strategy
A literature search was conducted January 10, 2023 to February, 2, 2023 to identify all available studies on the effectiveness exercise-based rehabilitation on functionality and quality of life in HNC survivors in MEDLINE (PubMED), Cochrane Library, CINAHL complete and Web of Science (WOS) databases. In MEDLINE, the search string was “Head and Neck Neoplasms” [Mesh] OR “Exercise” [Mesh] OR “rehabilitation” [Mesh] OR “complications” [Mesh] OR “Muscle Contraction” [Mesh] OR “Muscle Stretching Exercises” [Mesh] OR “cancer” [tw] OR exercis*[tw] OR stretch*[tw] OR plyometric*[tw] OR resist* [tw] OR eccentric [tw] OR concentric [tw] OR isometric*[tw] OR isotonic*[tw] OR activat*[tw] OR contract*[tw] OR conditioning [tw] OR training [tw] and “Head and Neck Neoplasms” [Mesh] OR “Exercise” [Mesh] OR “rehabilitation” [Mesh] OR “Muscle Contraction” [Mesh] OR “Muscle Stretching Exercises” [Mesh] OR “lymphedema” [Mesh] OR “quality of life” [Mesh] OR “pain” [Mesh] OR “cancer” [tw] OR function*[tw] OR exercis*[tw] OR stretch*[tw] OR plyometric*[tw] OR resist* [tw] OR eccentric [tw] OR concentric [tw] OR isometric*[tw] OR isotonic*[tw] OR activat*[tw] OR contract*[tw] OR conditioning [tw] OR training [tw]. Similar research equations are used to consult the Cochrane Library, CINAHL and Web of Science (WOS). Two independent researchers (RPG and IMMP) performed the search and a blinded researcher, CRG, scored all retrieved articles by title and abstract, and then scored full-text publications to determine their eligibility. In case of discrepancies, a fourth author served as decision judge (FHR) (Table 1).
Selection and data extraction
Data extraction was performed independently by two authors (DZL and GBM), and in case of disagreement, a third author (NKY) was the responsible of resolving discrepancies. A standardized work template based on PICO question was used to extract and detail all the information related to authors, year and country of publication, study design, aim of the study, outcomes, participants (characteristic of disease, medical intervention, sample size, gender distribution, etc.), intervention and control details, results of measured outcomes and conclusions. The Cochrane Handbook for Systematic Reviews of Interventions—v.5.1.0 was used to develop these sections. The reliability of the table was tested using a representative sample of the studies to be reviewed.
Methodological quality analysis
The PEDro scale were used to assess the methodological quality of clinical trials and were ultimately included in the assessment for this review46. It consists of 11 items, each worth one point, and can be used to assess whether a randomized clinical trial has sufficient internal validity (criteria 2–9) and sufficient statistical information to make its results interpretable (criteria 10–9). Studies scoring 9–10 on the PEDro scale were considered to be of excellent methodological quality. Studies with a score between 6 and 8 were of good methodological quality, and studies with a score of less than 4 were of poor methodological quality.
Risk of bias analysis
Risk of bias analyzes of randomized clinical trials were independently performed by SMP using the Cochrane Risk of Bias Tool for Randomized Trials (RoB 2.0)47. The tool evaluates the methods researchers use in clinical trial design and individually rates the presence of the following biases: (1) randomization process, (2) deviations from the intended interventions, (3) missing outcome data, (4) measurement of the outcome and (5) selection of the reported result. Interpretation of the scores obtained considers the fact that a low risk of bias means that the bias committed is unlikely to significantly alter the results, whereas a high risk of bias indicates lower confidence in the results receive. Any disagreement of the authors was resolved by discussion, and in case of conflicting scores, the third reviewer (FRH) resolved to make the decision.
Grade of recommendation (GRADE)
The certainty of the evidence analysis was established by the different levels of evidence according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework which is based on five domains: study design, imprecision, indirectness, inconsistency, and publication bias48. The evidence was classified into the following four levels: high quality (all domains satisfied), moderate (one domain not satisfied), low quality (two domains not satisfied), or very low quality (three or more domains not satisfied).
Data synthesis
Meta-analyses were undertaken using Review Manager (RevMan v.5.3; Cochrane Collaboration, Oxford, UK) when more than two studies reported on the same outcome. In the pooled analysis of studies by duration, outcome data were organized into short-term (≤ 6 weeks), medium-term (7–23 weeks) and long-term (≥ 24 weeks) according to previous studies49. In cases where it is not possible to convert the units of measurement, the standardized mean difference is used. Data are presented as standard mean differences (SMD) and 95% CIs. The I2 statistic is used to quantify statistical heterogeneity as follows: 0–40%, probably not important; 30–60%, moderate heterogeneity; 50–90%, significant heterogeneity; 75–100%, significant heterogeneity. Analysis was performed using a fixed-effects model, however, when statistical heterogeneity (I2 > 40%) was found, a random-effects model was used for meta-analysis.
Results
Study selection
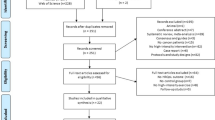
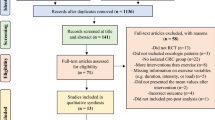
A total of 5,672 studies were detected and analyzed by performing the agreed searches in the detailed databases MEDLINE (PubMed) (n = 1875), Cochrane Library (n = 986), CINAHL (n = 1050) and Web of Science (WOS) (n = 1761). After removing duplicates (n = 812) and analyzing the titles and abstracts of the remaining articles (n = 4036), 824 full-text articles were potentially relevant studies according to the search strategies. Finally, 806 of these manuscripts were excluded because the studies did not meet our eligibility criteria as they had a different study design (n = 671), published in different languages (n = 5), carried out another intervention (n = 90) and have measured other outcomes of little interest for our research question (n = 40). Therefore, 18 studies were ultimately selected for this review for qualitative synthesis and meta-analysis (Fig. 1).
Study characteristics
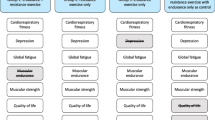
The 18 studies were clinical trials which 10 were prospective, parallel, single-blinded, randomized, controlled trials, 3 pilot-controlled trials, 1 randomized, controlled, double-blind, 3-arm, parallel-group, prevention clinical trial, 1 prospective clinical cohort study, 1 single-blinded multicenter, 1 Phase III randomized trial and 1 uncontrolled pre–posttest design. All the included studies were carried out within a population diagnosed with HNC in oropharynx, hypopharynx, oral cavity, larynx, or unknown primary (UPT) undergone to RDT, CHT, brachytherapy or modified radical neck dissection reaching a total sample of 1322 patients (n = 1039 men (78.6%) and n = 283 women (21.4%) with a mean age of 53.2. Most of the studies included exercise interventions based on supervised multimodal exercise programs. For example, most studies included aerobic (AET)50,51,52,53, anaerobic resistance (RET)50,51,52,53,54,55,56,57,58,59 or endurance60,61, stretching52,57,59,62,63,64, relaxation65 and postural control exercises59 and the goal of increasing joint range in shoulder59,61,63 or in cervical spine57 in their interventions to improve functionality and quality of life. Most of these developed a protocol of exercise in combination with biomedical education52,55, psycho-behavioral couching30, diet carried out during CHT50,56,62,66 or RDT53,54,58,62 alone or combinate radio-chemotherapy51,67 or surgery52,57,59,60,63,65 with follow-up ranging from 2 weeks to 12 months. The 18 studies included were conducted in Sweden64,67, Denmark54,58, The Netherlands60,66, United States53,56, Canada55,61, Brazil62, India51,63, Taiwan50,52,65 and Australia57,59 (Table 2).
Methodological Quality assessment (PEDro Scale)
The methodological quality of the studies included in our review was good (PEDro score = 6.38 out of 10, SD = 1.09). During methodological quality assessment, 14 studies of good quality50,51,52,53,55,56,57,58,59,60,62,63,65,67 and 4 studies of acceptable methodological quality were identified54,61,64,66. Most of the studies made systematic errors in relation to the blinding of the participants, since of the total that were included, only 1 carried out the blinding of the participants50. Also, the blinding of the therapists who applied treatment was only carried out in a study54 (Table 3).
Risk of bias assessment (ROB 2.0)
The risk of bias of the included randomized clinical trials, as measured by the ROB 2.0 tool, can be marked as low to moderate risk of bias. When analyzing each bias, we found a high risk of bias related to the deviations from intended interventions in most of the included studies50,51,52,53,55,56,57,58,59,60,61,62,63,64,65,66,67, mainly due to lack of blinding of participants and personnel. In relation to the selection of the reported outcomes51,58,61,63,64,65,66,67, we detected a high risk of bias since the researchers did not report the outcome in the study variables in detail. With regard to the randomization process, there is a high risk of bias due to the lack of concealment-related errors in the random assignment sequence52,54,56,58,61,66 (Table 4).
Grade of recommendation (GRADE)
The Grade of recommendation is weak in favor of exercise to improve functionality and quality of life in survivors with HNC (Table 5).
Data synthesis
Effectiveness of exercise-based rehabilitation in HNC survivors undergone chemo-radiotherapy
Functionality related to pain
According to our results, there was evidence of acceptable to good methodological quality, low to moderate risk of bias and significant heterogeneity [Tau2 = 6.14; I2 = 99%] that showed little size effect of exercise to reduce overall pain in HNC patients underwent chemo-radiotherapy [SMD = − 0.62 [− 4.07, 2.83] CI 95%, Z = 0.35, p = 0.72]54,62 (Fig. 2).
Forest plot and Funnel Plot of the effect of the exercise on functionality related to pain in HNC undergone radio-chemotherapy. (A) Forest plot of the effect of the exercise on functionality related to pain in HNC undergone radio-chemotherapy. (B) Funnel plot of comparison: Exercise vs Control n HNC survivors undergone Chemo-radiotherapy, outcome: Overall Pain. (C) Funnel plot of comparison: Exercise vs Control n HNC survivors undergone Chemo-radiotherapy, outcome: Orofacial Pain.
Functionality related to muscle strength
There was evidence of good to moderate methodological quality, low to moderate risk of bias, and significantly heterogeneity [Tau2 = 0.79; I2 = 86%], suggesting no positive treatment effect on upper limb isometric strength in the exercise group compared with controls at medium term [SMD = 0.80 [0.14, 1.46] CI 95%, Z = 0.45, p = 0.66]50,53. A little improvement has been reported on long-term [SMD = − 0.57 [− 1.09, − 0.05] CI 95%, Z = 2.16, p = 0.03]30 being the overall effect not in favor of exercise [SMD = 0.25 [− 0.484, 1.34] CI 95%, Z = 0.45, p = 0.66] in patients that received radiation or concurrent chemoradiation. In contrast, there were positive results, despite the very small effect size, favoring the use of isometric lower body strength exercises [SMD = − 0.10 [− 1.52, 1.32] CI 95%, Z = 0.14, p = 0.89] in HNC for managing patients scheduled to receive radio-chemotherapy50,53,56,58 (Fig. 3).
Forest plot and Funnel Plot of the effect of the exercise on functionality related to muscle strength in HNC undergone radio-chemotherapy. (A) Forest plot of the effect of the exercise on functionality related to muscle strength in HNC undergone radio-chemotherapy. (B) Funnel plot of comparison: Exercise vs Control n HNC survivors undergone Chemo-radiotherapy, outcome: Lower Limb Muscle strength.
Functionality related to fatigue
There was evidence of good methodological quality, moderate risk of bias, and moderate heterogeneity [Tau2 = 0.19; I2 = 73%] that showed a significant efficacy of exercise in cancer-related fatigue in HNC who were treated with RDT in all terms of following-up [SMD = − 0.51 [− 0.97, − 0.057] CI 95%, Z = 2.15, p < 0.01]. In the short term, exercise was minimally superior to controls in reducing fatigue perceived by HNC patients who were treated with chemo-radiotherapy [SMD = − 0.78 [− 1.15, − 0.41] CI 95%, Z = 4.10, p < 0.01]51. In the medium term, these clinical differences in favor of exercise remain the same [SMD = − 0.35 [− 1.30, 0.60] CI 95% Z = 0.72, p = 0.47]51,53,58 as in the long term [SMD = − 0.41 [− 1.03, 0.21] CI 95% Z = 1.29 p = 0.20]58 (Fig. 4).
Forest plot and Funnel Plot of the effect of the exercise on functionality related to fatigue in HNC undergone radio-chemotherapy. (A) Forest plot of the effect of the exercise on functionality related to fatigue in HNC undergone radio-chemotherapy. (B) Funnel plot of comparison: Exercise vs Control n HNC survivors undergone Chemo-radiotherapy, outcome: Fatigue.
Functionality related to range of motion
Evidence of acceptable methodological quality, moderate risk of bias and significantly heterogeneity [Tau2 = 0.57; I2 = 92%] showed exercise was not superior over controls regarding the range of motion of mouth opening [SMD = 0.65 [− 0.44; 1.74] CI 95%, Z = 1.16, p = 0.24] after performing joint mobility and RET combined with stretching in patients curatively intended RDT treatment54,64.
Quality of life
There was evidence of acceptable to good methodological quality, moderate risk of bias, and high heterogeneity [Tau2 = 0.19; I2 = 83%] that showed nor efficacy of exercise in quality of life in comparison to controls [SMD = 1.06 [0.51, 1.60] CI 95% Z = 3.80, p < 0.01]. If we perform an analysis by time, no effects were found in favor of exercise in any of the study terms, short term [SMD = 1.29 [0.68, 1.89] CI 95%, Z = 4.15, p < 0.01]51,54 and long term [SMD = 0.51 [− 0.01, 1.03] CI 95%, Z = 1.94, p = 0.05]55 in patients with HNC underwent chemoradiation (Fig. 5).
Forest plot and Funnel Plot of the effect of the exercise on functionality related to quality of life in HNC undergone radio-chemotherapy. (A) Forest plot of the effect of the exercise on functionality related to quality of life in HNC undergone radio-chemotherapy. (B) Funnel plot of comparison: Exercise vs Control n HNC survivors undergone Chemo-radiotherapy, outcome: Quality of life.
Effectiveness of exercise-based rehabilitation in HNC survivors undergone surgery
Functionality related to pain
Evidence of good methodological quality, moderate risk of bias, and high heterogeneity [Tau2 = 2.59; I2 = 97%] detected improvements but not statistically significant on overall pain when HNC patients participated in a RET, joint mobility and stretching program after neck dissection surgery [SMD = − 1.04 [− 3.31, 1.23] CI 95%, Z = 0.90, p = 0.37]. Moreover, most effects in favor exercise of RET, joint mobility and relaxation exercise have been showed concerning shoulder pain in the short [SMD = − 0.48 [− 1.13, 0.18] CI 95%, Z = 1.43, p = 0.15]52 and long term [SMD = − 2.81 [− 7.06, 1.43] CI 95%, Z = 1.76, p = 0.08] underwent HNC surgery management57,59 (Figs. 6, 7).
Forest plot and Funnel Plot of the effect of the exercise functionality related to pain (overall pain) in HNC undergone surgery. (A) Forest plot of the effect of the exercise on functionality related to pain (overall pain) in HNC undergone surgery. (B) Funnel plot of comparison:Exercise vs Control in HNC survivors undergone surgery, outcome: Overall Pain.
Forest plot and Funnel Plot of the effect of the exercise functionality related to pain (shoulder pain) in HNC undergone surgery. (A) Forest plot of the effect of the exercise on functionality related to pain (shoulder pain) in HNC undergone surgery. (B) Funnel plot of comparison: Exercise vs Control in HNC survivors undergone surgery, outcome: Shoulder Pain.
Functionality related to muscle strength
There was a limited evidence of good methodological quality, moderate risk of bias, and high heterogeneity showing exercise based on progressive RET was not superior over controls [SMD = 0.84 [0.27, 1.41] CI 95%, Z = 2.90, p = 0.004] in patients with HNC treated by radical neck dissection59.
Functionality related to fatigue
Evidence of good methodological quality, moderate risk of bias, and high heterogeneity [Tau2 = 1.28; I2 = 96%] showed there were not differences of exercise based on progressive RET59 or muscle relaxation65 versus controls [SMD = 0.83 [− 0.49, 2.14] CI 95%, Z = 1.23, p = 0.22] in patients with HNC treated by radical neck dissection. In contrast, there was only a limited and not lasting of short-term effect on cancer-related fatigue in a group who performed inspiratory muscle training60. [SMD = − 0.05 [− 0.30, 0.21] CI 95%, Z = 0.37, p = 0.71] (Fig. 8).
Forest plot and Funnel Plot of the effect of the exercise functionality related to fatigue in HNC undergone surgery. (A) Forest plot of the effect of the exercise on functionality related to fatigue in HNC undergone surgery. (B) Funnel plot of comparison: Exercise vs Control in HNC survivors undergone surgery, outcome: Fatigue.
Functionality related to range of motion
No differences have been found between exercise and controls in HNC survivors treated by surgery. Evidence of good methodological quality, moderate risk of bias, and high heterogeneity [Tau2 = 92; I2 = 90%] showed practicing exercise after MRND was not superior to promote active shoulder abduction in any of following-up terms [SMD = 0.89 [− 0.26, 2.04] CI 95%, Z = 1.52, p = 0.13]52,57,63.
Quality of life
There was evidence of good methodological quality, moderate risk of bias, and probably not important heterogeneity [Tau2 = 0.00; I2 = 0%] that stablishes there were not better results in relation to quality of life in comparison with controls of HNC surgically treated [SMD = − 0.41 [0.10, 0.73] CI 95%, Z = 2.56, p = 0.01]52,60.
Discussion
Effectiveness of exercise-based rehabilitation in HNC survivors undergone chemo-radiotherapy
The aim of this meta-analysis was to quantify the effect of exercise-based rehabilitation on functionality and quality of life in HNC survivors who underwent surgery and/or chemoradiotherapy. This modality has a positive effect on overall pain in HNC survivors undergoing chemoradiation. Bragante et al. found that RET combined with joint mobility training and stretching reduce overall pain on long-term in patients undergoing CHT or RDT compared to controls62. Cancer pain use to be neuropathic and can be caused both in the Central Nervous System (CNS) and the Peripheral Nervous System (PNS). During tumor growths, compression, and even invasion, can occur triggering painful sensation. CHT and RDT contributes to this neuropathic cancer pain (NCP)68. RDT induced pain could be attributed to fibrosis or sensitization of peripheral nerves69. Although the underlying effects of the exercise on NCP are not fully understood different hypotheses are postulated. One of the most plausible hypotheses is that dynamic RET produces hypoalgesia by the activation of baroreceptors induced blood pressure changes which are linked to central pain inhibition pathway70. Other potential mechanism is that RET increases not only delayed-onset muscle soreness (DOMS), responsible of the increase muscle pain thresholds, but also a stimulation of low-threshold motor units. Both joint mobility and stretching exercises activate centers of descending inhibitory opioid dependent axes and others non-opioids pathways71.
Although no positive results were found in favor of exercise in patients with HNC undergoing radio-chemotherapy, Pauli et al.64 found that RET combined with joint mobility and stretching exercises reduce OP in the medium term64. OP is a localized pain disorder in face and jaw that causes moderate to severe deterioration of chewing72. OP uses to be the result of a combination of several joint, myofascial and/or neuropathic mechanisms73. In the study of Pauli et al.64 pain can be attributed to radio-chemotherapy as well. In this sense RDT, when it acts in the environment of the temporomandibular joint (TMJ), provokes radiation induced fibrosis that arise NCP69,74. RET progressively stimulates a local anti-inflammatory response and activation of central microglial activity75 that explains the amelioration in neuropathic OP as it is observed in Pauli et al.64.
Despite not positive effects of exercise on upper limb muscle strength were observed in patients who received radio-chemotherapy, Capozzi et al. found that the combination of RET with health education and behavioral therapy has a positive effect on the long-term enhancement of upper limb muscles in patients receiving CHT or RDT30,55. All these patients with HNC suffer from sarcopenia, a condition characterized by the skeletal muscle and weight loss resulting in a decrease of physical performance.
According to some authors the prevalence of sarcopenia in patient treated by HNC is 35.5–54.5%, which is mainly attributed to weight loss and malnutrition76,77. Among the main mechanisms of sarcopenia include an intensification of protein catabolism probably related to tumor growths78.
In addition to this, CHT and RDT exacerbate this malnutrition and weight loss79. In some cases, CHT has a direct influence on muscle catabolism leading to a loss of muscle strength78, in others, the loss of muscle mass can be explained by CHT adverse effects such as fatigue, loss of appetite, nausea, vomiting or diarrhea, as a result of several reduction of food intake and physical activity78. When toxicities such as xerostomia, dysphagia or oral mucositis occur, a reduction of oral intake may imply malnutrition, weight loss and subsequent sarcopenia79. Unlike CHT, the mechanism of RDT in the onset of sarcopenia remain poorly understood. Sarcopenia is thought to decrease survival and increase disease relapses or the likelihood of death of these patients80.
Exercise prevents sarcopenia because it increases muscle mass and function77,81. Strength exercises are one of the most effective modalities to promote the upper limb functionality. However, long time exposure to exercise is required to draw muscular and neural adaptations82.
Given that CHT produces malnutrition due to a decreased intake and physical performance an improvement of nutritional status is mandatory83. Healthy lifestyle education in HNC patients encourages the acquisition of healthy feeding behavior in the long term. It is previously shown that doing exercise reduces carbohydrates and lipids intake leading to an indirect incorporation through daily meals of proteins, oligoelements and other minerals improving muscle health84.
Exercise-based rehabilitation increases lower limb strength in patients with HNC who had received CHT or RDT. In this sense, Lønbro et al.58 found that RET have a positive effect on the potentiation of lower limb muscle strength in the medium term in patients receiving CHT or RDT. As describe above a reduction of muscle strength as a result of cancer-related sarcopenia or the administration of CHT and RDT agents affect muscles equally. Thus, a RET program in the lower limb promotes the knee extension peak of force through an increase of hamstring complex muscle mass in the medium term. In this case, muscle mass changes could be associated to the fact, on the one hand, that lower limb musculature allows higher force loads compared to upper limb, and on the other hand, the higher frequency and longer duration program carried out as it has been formerly published in other populations85,86.
Rogers et al.53 also found that RET has a positive effect on the enhancement of lower limb muscle strength in the medium term in patients receiving CHT or RDT. For his part, Zhao et al.56 found that in HNC patients receiving CHT or RDT, the combination of RET with AET has a positive effect on the enhancement of lower limb muscle strength in the medium term. Authors differ on the effects of RET combined with AET. For some of them, hypertrophy produced by AET depends on modality, frequency, and intensity so that the higher they are the more hypertrophy there is. For many others, when they are combined, the effects can be inhibited by each other87,88. Nevertheless, more clinical trials are needed to delve deeper into this question.
Flexibility exercises within a multimodal program also impact on the increase of muscle maximum strength. In this sense, Lin et al.50 found when combining RET with AET flexibility exercises has a positive effect on the enhancement of lower limb muscle strength in the medium term in patients receiving CHT and RDT. Despite the discrepancies between some studies, recent works have shown that chronic exposure to elasticity exercises can contribute to an increase in maximum contraction force due to changes in both musculotendinous stiffness and neural impulse. It is probably due to changes in the muscle tension-length relationship and the rate of sarcomere shortening. In the case of Lin et al.50 active stretching exercises in combination with maximal strength exercises seem to be more effective than when they are performed with explosive force exercise, currently, not supported by evidence89,90.
Exercise-based rehabilitation was successful in reducing disease-related fatigue in patients with HNC. Samuel et al. found that the combination of RET with AET has a positive effect on short-term fatigue in patients receiving CHT and RDT51. Fatigue occurs both in the active phase of the disease and during the treatment phases producing a significant impairment of the functionality of the HNC survivor91. This disorder is defined as a distressing, persistent, and subjective feeling of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment non-proportional to activity that interferes with functioning92. The prevalence of fatigue in oncologic population ranges from 26.2 to 56.3% depending on the study93, but, in the HNC is moderate to severe only in 18% of patients94.
Fatigue can be caused by a set of mechanism whose etiologies are not clearly elucidated. In general, it can be attributed to both factors related to the CNS (neuroinflammation, hypothalamic pituitary adrenal (HPA) axis, etc.) and the PNS (cachexia, alteration of energy metabolism, etc.). It is central when the patient is not able to perfom physical or mental task without major cognitive or motor impairment92. Fatigue is peripheral if the patient’s muscles are not able to perform a task after being stimulated or there is a reduction in endurance92.
Both cancer and its treatment activate the inflammation cascade increasing cytokines releasing that act on the CNS. Other potential mechanism is the alteration of the HPA axis that is induced by cytokines as part of this neuroinflammation. Activation of the HPA axis increases cortisol releasing to limits systemic damages due to this inflammatory state. This will lead to physical fatigue, circadian cycle disturbances and lack of sleep92.
Fatigue in these patients is a result of loss of physical condition, combined with radiation and chemotherapy treatments, resulting in changes in anaerobic metabolism that lower lactate thresholds leading to fatigue and weakness. In addition, loss of appetite and the presence of nausea and vomiting can lead to caloric and nutrient deficiencies.
Fatigue-related functions may also occur at the peripheral level due to changes in energy metabolism. Not only from cachexia, but also from sarcoplasmic reticulum and/or mitochondrial damage caused by CHT and RDT, skeletal muscle may be compromised, increasing fatigue more than random or milky, depending on the primary fuel consumed92. The lactic acid system converts glucose into cellular energy nucleotides (AMP, ADP, ATP), which generate lactic acid as a by-product, and when lactic acid accumulates in the muscles, it produces fatigue and the patient manifests weakness, which is an illness with symptoms caused by CHT or RDT can exacerbate anorexia, nausea, or vomiting, ultimately reducing caloric intake and thus the availability of energy substrates. The lactate pathway was the predominant pathway activated when the various RETs of the program of Samuel et al. performed on HNC patients who had undergone CHT or RDT. This improvement could be explained by exercise shifting the lactate threshold to the right, leading to increased lactate tolerance in cancer patients undergoing CHT or RDT (whose threshold is generally lower) by preventing the onset of delayed fatigue91.
Fatigue-related functions may also occur at the peripheral level due to changes in energy metabolism. Not only from cachexia, but also from sarcoplasmic reticulum and/or mitochondrial damage caused by CHT and RDT, skeletal muscle may be compromised, increasing fatigue more than random or milky, depending on the primary fuel consumed92. The lactic system generates by-product lactic acid that when accumulates in the muscle generates fatigue that the patient manifests as weakness, a symptom that can worsen by CHT or RDT which lack of appetite, nausea or vomiting that end up reducing caloric intake and consequently decreasing the availability energy substrates. The lactate pathway was the predominant pathway activated when the various RETs of the program of Samuel et al.51 performed on HNC patients who had undergone CHT or RDT. This improvement could be explained by exercise shifting the lactate threshold to the right, leading to increased lactate tolerance in cancer patients undergoing CHT or RDT (whose threshold is generally lower) by preventing the onset of delayed fatigue91.
Effectiveness of exercise-based rehabilitation in HNC survivors undergone surgery
Exercise-based rehabilitation has a positive effect on overall pain in HNC survivors undergoing surgery. In this sense, Loh et al.65 found that relaxation exercises have a positive short-term effect on reducing overall pain in patients undergoing head and neck surgery. As noted, apart from CHT and RDT, HNC treatment also includes surgery which appear to be effective as in early as advanced stages of the disease18.
Among surgical techniques, modified radical neck dissection (MRND), selective dissection of the lymph nodes of the neck, with preservation of the accessory nerve or resection of cervical nerve root branches and subsequent flap reconstruction are the most commonly used procedures95,96,97.
Neuromuscular complications due to surgical treatment are more frequent in HNC survivors compared to those who only received CHT or RDT. When cancer invades laryngopharyngeal region, musculoskeletal structures related to speech and swallowing (i.e. dysphagia, hoarseness, etc.) need to be removed97,98. Although the surgical procedure is safe, some neuromuscular complications due to sacrifice of the accessory nerve have been reported in cases related to cancers or inadvertent injury to the nerve during surgery. As a consequence, an alteration of the strength and motor coordination of the cervico-scapular musculature could develop, which could alter the functionality of the upper limb99,100,101. Another serious post-operative complication is shoulder pain which depends on the type of surgical technique performed102,103.
In order to relieve pain, Loh et al. found that progressive relaxation exercises have a positive effect on the reduction of overall pain in the short-term65. Muscle relaxation techniques improve pain in HNC undergoing surgery by decreasing anxiety and stress104. The mechanism that could best explain these positive effects is decreasing serum cortisol levels that has been previously associated in with the onset of myofascial pain in these subgroup cancer patients105. However, there is not consensus among authors about this hypothesis regarding the role of relaxation exercise on pain releasing as Kim et al. do not find blood cortisol reductions in patients with colorectal cancer106. Further studies are required to identify the mechanisms of the effects of exercise on postoperative pain in HNC cancer.
Overall pain in patients undergoing surgery can be reduced by joint mobility exercise in the short-term. This kind of exercise consist of the displacement of joint segments in different dimensions of space with the aim of reaching the maximum range of joint play107. Behind these beneficial effects on overall pain are probably the improvement on somatosensory cortical representation of movement108, the activation of the endogenous inhibition analgesic system109, and the decreasing of psychological factors related to pain (fear-avoidance behavior, etc.)110,111.
The amelioration of functionality associated with overall pain produced by the program was probably due the fact that joint mobility exercises, combined with maximum active stretching to the limit of pain tolerance, relying on through the counterirritation mechanism a phenomenon that, according to Wall and Melzack (1965), occurs as a result of introducing a noxious stimulus more intense than the base pain achieving a downward modulation of it112.
Also exercise-based rehabilitation has a positive effect on shoulder pain in HNC survivors who undergo surgery. McGarvey et al.57 found that RET, cervical mobilization exercises have a positive long-term effect on reducing shoulder pain in patients undergoing surgery. Shoulder pain is present in at least 70% of patients after non-selective dissection of the lymph nodes of the neck. Unlike radical neck resection, MRND removes all lymph nodes by radical dissection, but respects one or more of the non-lymphatic structures (spinal accessory nerve, jugular vein, or sternocleidomastoid muscle). In nodal selective dissection, non-lymphatic structures are preserved, and nodule removal is done according to the location of the metastases113.
The positive effects of the exercise program of McGarvey et al.57 were probably attributed to that the included participants were undergone a surgical technique with nerve root, nerve, or its spinal branches preservation, and thus, reducing the possibility of neuropathic pain linked, not only to the mechanical damage of the nerve but also with the entrapment produced by post-surgical fibrosis at the interfaces of the nerve path57. The positive effects of RET program on shoulder pain depend, as discussed above for CHT patients, on the increase in muscle pain threshold. Its combination with cervical mobility exercises, which the subsequent activation of endogenous pain inhibition systems, and in the study of McNeely59, with muscle stretching, that decreases the activation of muscle nociceptors, result in a significant improvement of shoulder pain59,71.
Despite not having found effects in favor of exercise on fatigue in patients with post-surgical HNC, Valkenet et al. found that inspiratory RET has a positive short-term effect on reducing fatigue in those who were operated60.
As commented, fatigue-related functionality is the result of a peripheral mechanism accompanied by a reduction in energy metabolism achieved initiation of the anaerobic metabolism pathway leading to fatigue of the muscles involved in ventilation. This fatigue can also be explained by the increased ventilatory rate as part of the onset of systemic inflammatory response syndrome (SIRS) due to surgery in which it is aggravated by the previous state of cancer cachexia that occurs with loss of muscle mass and loss of function114.
Limitations
The methodology carried out to conduct this study implies several limitations. First, most of the RCTs include in this meta-analysis have significant heterogeneity and low sample sizes could be an important limitation for external validity of our results. Secondly, the good quality and the high risk of bias detected on the allocation concealment and the blinding of participants or therapist can produce an overestimation of the impact of exercise in the studied outcomes resulting in the possibility of misinformed conclusions. These limitations imply the need for further research into the effectiveness of standardized clinical trial protocols to refute the results and obtain more evidence to reach the level of clinical recommendation.
Conclusions
There is evidence of fair to good methodological quality, low to moderate risk of bias, and weak recommendation supporting the use of exercise-based rehabilitation to increase functionality. However, no evidence was found in favor of the use of this modality for improving the quality of life of HNC survivors who underwent chemoradiotherapy or surgery. The lack of standardization in the development of exercise programs, the diversity of randomized trials, and the heterogeneity of interventions and evaluations warrant further study.
Change history
22 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-37292-w
References
Sherrod, A. M. et al. Caregiving burden in head and neck cancer. J. Clin. Oncol. 32, suppl 15 (2014).
Mishra, A. & Meherotra, R. Head and neck cancer: Global burden and regional trends in India. Asian Pacific J. Cancer Prevent. https://doi.org/10.7314/APJCP.2014.15.2.537 (2014).
Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. https://doi.org/10.1097/CCO.0000000000000629 (2020).
Oliveira, C. C., Marques, M. E. A. & Nóbrega, V. C. Lymphoepithelioma-like carcinoma of the skin. Bras. Dermatol. 93, 256–258 (2018).
Dubey, P. et al. Nonnasopharyngeal lymphoepithelioma of the head and neck. Cancer 82, 1556–1562 (1998).
Nazir, S. et al. Spindle Cell Carcinoma in Head and Neck Region. Pakistan J. Med. Health Sci. 15, 594 (2021).
Wang, N., Huang, M. & Lv, H. Head and neck verrucous carcinoma. Medicine. 99, e18660 (2020).
Franchi, A. & Skalova, A. Undifferentiated and dedifferentiated head and neck carcinomas. Semin. Diagnostic Pathol. https://doi.org/10.1053/j.semdp.2021.09.001 (2021).
Villagómez-Ortíz, V. J. et al. Prevalencia de infección por virus del papiloma humano en carcinoma espinocelular de cavidad oral, orofaringe y laringe. Cir. Cir. 84, 363–368 (2016).
Lop, J. et al. Causes of long-term mortality in patients with head and neck squamous cell carcinomas. Eur. Archives Oto-Rhino-Laryngol. 279, 3657–3664 (2022).
Mody, M. D., Rocco, J. W., Yom, S. S., Haddad, R. I. & Saba, N. F. Head and neck cancer. Lancet 398, 2289–2299. https://doi.org/10.1016/S0140-6736(21)01550-6 (2021).
Cohen, N., Fedewa, S. & Chen, A. Y. Epidemiology and demographics of the head and neck cancer population. Oral Maxillofacial Surg. Clin. N. Am. 30, 381–395. https://doi.org/10.1016/j.coms.2018.06.001 (2018).
Beynon, R. A. et al. Tobacco smoking and alcohol drinking at diagnosis of head and neck cancer and all-cause mortality: Results from head and neck 5000, a prospective observational cohort of people with head and neck cancer. Int. J. Cancer 143, 1114–1127 (2018).
Sato, T. High-risk factors in the development of head and neck cancers. Japan. J. Cancer Chemother. 14, 2626–2631 (1987).
Chang, C. C. et al. Oral hygiene and the overall survival of head and neck cancer patients. Cancer Med. 8, 1854–1864 (2019).
Kawakita, D. et al. Impact of oral hygiene on head and neck cancer risk in a Chinese population. Head Neck. 39, 2549–2557 (2017).
Buchakjian, M. R., Davis, A. B., Sciegienka, S. J., Pagedar, N. A. & Sperry, S. M. Longitudinal perioperative pain assessment in head and neck cancer surgery. Ann. Otol. Rhinol. Laryngol. 126, 646–653 (2017).
Losi, E. et al. Undergoing head and neck cancer surgery: A grounded theory. Eur. J. Cancer Care (Engl.) 28, e13062 (2019).
Kiong, K. L. et al. Impact of neoadjuvant chemotherapy on perioperative morbidity after major surgery for head and neck cancer. Cancer 126, 4304–4314 (2020).
Strnad, V., Geiger, M., Lotter, M. & Sauer, R. The role of pulsed-dose-rate brachytherapy in previously irradiated head-and-neck cancer. Brachytherapy 2, 158–163 (2003).
A.S. Kirthi Koushik & R.C. Alva. Brachytherapy in head and neck cancer: A forgotten art or a skill to be remembered!! J. Anal. Oncol. 6, 14–22 (2017).
Peiffert, D., Coche-Dequéant, B., Lapeyre, M. & Renard, S. Brachytherapy for head and neck cancers. Cancer/Radiotherapie. https://doi.org/10.1016/j.canrad.2017.12.005 (2018).
Dubey, P., Sertorio, M. & Takiar, V. Therapeutic advancements in metal and metal oxide nanoparticle-based radiosensitization for head and neck cancer therapy. Cancers https://doi.org/10.3390/cancers14030514 (2022).
Kanotra, S. P., Kanotra, S., Gupta, A. & Paul, J. Chemoradiation in advanced head and neck cancers: A comparison of two radiosensitizers, paclitaxel and cisplatin. Indian J. Otolaryngol. Head Neck Surg. 63, (2011).
Cnossen, I. C. et al. Multimodal guided self-help exercise program to prevent speech, swallowing, and shoulder problems among head and neck cancer patients: A feasibility study. J. Med. Internet Res. 16, e74 (2014).
Yen, C. J. et al. Effect of exercise training on exercise tolerance and level of oxidative stress for head and neck cancer patients following chemotherapy. Front. Oncol. 10, 1536 (2020).
Dsouza, M., Samuel, S. & Saxena, P. Effects of exercise training during concomitant chemoradiation therapy in head-and-neck cancer patients: A systematic review. Indian J. Palliative Care. https://doi.org/10.4103/IJPC.IJPC_14_20 (2020).
Midgley, A. W., Lowe, D., Levy, A. R., Mepani, V. & Rogers, S. N. Exercise program design considerations for head and neck cancer survivors. Eur. Arch. Oto-Rhino-Laryngol. 275, 169–179 (2018).
Scherpenhuizen, A., Van Waes, A. M. A., Janssen, L. M., Van Cann, E. M. & Stegeman, I. The effect of exercise therapy in head and neck cancer patients in the treatment of radiotherapy-induced trismus: A systematic review. Oral Oncol. https://doi.org/10.1016/j.oraloncology.2015.05.001 (2015).
Capozzi, L. C., Nishimura, K. C., McNeely, M. L., Lau, H. & Nicole Culos-Reed, S. The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: A systematic review. Br. J. Sports Med. https://doi.org/10.1136/bjsports-2015-094684 (2016).
Chee, S., Byrnes, Y. M., Chorath, K. T., Rajasekaran, K. & Deng, J. Interventions for trismus in head and neck cancer patients: A systematic review of randomized controlled trials. Integr. Cancer Therapies. https://doi.org/10.1177/15347354211006474 (2021).
Fang, Y. Y. et al. Physical activity and fitness in survivors of head and neck cancer. Support. Care Cancer. 29, 6807–6817 (2021).
Dhodapkar, M. V. & Dhodapkar, K. M. Immune modulation in hematologic malignancies. Semin. Oncol. https://doi.org/10.1053/j.seminoncol.2015.05.009 (2015).
Koelwyn, G. J., Wennerberg, E., Demaria, S. & Jones, L. W. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology (United States) 29, 214800 (2015).
Meng, H. & Rogers, C. J. Exercise impact on immune regulation of cancer. Exercise Energy Balance Cancer. https://doi.org/10.1007/978-1-4614-4493-0_4 (2013).
Chen, Y. et al. Body mass index and the risk of head and neck cancer in the Chinese population. Cancer Epidemiol. 60, 208–215 (2019).
Gama, R. R. et al. Body mass index and prognosis in patients with head and neck cancer. Head Neck. 39, 1226–1233 (2017).
Hashibe, M. et al. Tobacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head Neck 35, 914–922 (2013).
Duyur Caklt, B., Pervane Vural, S. & Ayhan, F. F. Complex decongestive therapy in breast cancer-related lymphedema: Does obesity affect the outcome negatively? Lymphat. Res. Biol. 17, 45–50 (2019).
J Tsai, R. et al. Lymphedema following breast cancer: The importance of surgical methods and obesity. Front. Womens Health 3, 1–17 (2018).
Lahart, I. M., Metsios, G. S., Nevill, A. M. & Carmichael, A. R. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Datab. Systematic Rev. https://doi.org/10.1002/14651858.CD011292.pub2 (2018).
Trommer, M. et al. Exercise interventions for adults with cancer receiving radiation therapy alone. Cochrane Datab. Systematic Rev. 2023, CD013448 (2023).
Shao, C. H., Chiang, C. C. & Huang, T. W. Exercise therapy for cancer treatment-induced trismus in patients with head and neck cancer: A systematic review and meta-analysis of randomized controlled trials. Radiother. Oncol. 151, 249–255. https://doi.org/10.1016/j.radonc.2020.08.024 (2020).
Bye, A. et al. Exercise and nutrition interventions in patients with head and neck cancer during curative treatment: A systematic review and meta-analysis. Nutrients 12, 1–26. https://doi.org/10.3390/nu12113233 (2020).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372, n71 (2021).
Maher, C. G., Sherrington, C., Herbert, R. D., Moseley, A. M. & Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 83, 713–721 (2003).
Higgins J.P. et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials BMJ 343, d5928 (2011)
Guyatt, G. H. et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008).
Ashton, R. E. et al. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: Systematic review with meta-analysis. Br. J. Sports Med. https://doi.org/10.1136/bjsports-2017-098970 (2020).
Lin, K. Y. et al. Effects of exercise in patients undergoing chemotherapy for head and neck cancer: A pilot randomized controlled trial. Int. J. Environ. Res. Public Health 18, 1–14 (2021).
Samuel, S. R. et al. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support. Care Cancer 27, 3913–3920 (2019).
Su, T. L. et al. The effect of home-based program and outpatient physical therapy in patients with head and neck cancer: A randomized, controlled trial. Oral Oncol. 74, 130–134 (2017).
Rogers, L. Q. et al. Pilot, randomized trial of resistance exercise during radiation therapy for head and neck cancer. Head Neck 35, 1178–1188 (2013).
Hajdú, S. F., Wessel, I., Dalton, S. O., Eskildsen, S. J. & Johansen, C. Swallowing exercise during head and neck cancer treatment: Results of a randomized trial. Dysphagia 37, 749–762 (2022).
Capozzi, L. C. et al. Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: Results from an exploratory randomized controlled exercise trial. Cancer 122, 1185–1200 (2016).
Zhao, S. G. et al. Maintaining physical activity during head and neck cancer treatment: Results of a pilot controlled trial. Head Neck 38, E1086–E1096 (2016).
McGarvey, A. C., Hoffman, G. R., Osmotherly, P. G. & Chiarelli, P. E. Maximizing shoulder function after accessory nerve injury and neck dissection surgery: A multicenter randomized controlled trial. Head Neck 37, 1022–1031 (2015).
Lønbro, S. et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy—Results from the randomized DAHANCA 25B trial. Radiother. Oncol. 108, 314–319 (2013).
McNeely, M. L. et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: A randomized controlled trial. Cancer 113, 214–222 (2008).
Valkenet, K. et al. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br. J. Surg. 105, 502–511 (2018).
Eades, M. et al. Effect of an interdisciplinary rehabilitation program on quality of life in patients with head and neck cancer: Review of clinical experience. Head Neck 35, 343–349 (2013).
Bragante, K. C. et al. Efficacy of exercise therapy during radiotherapy to prevent reduction in mouth opening in patients with head and neck cancer: A randomized controlled trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 129, 27–38 (2020).
Thomas, A., D’Silva, C., Mohandas, L., Pais, S. M. J. & Samuel, S. R. Effect of muscle energy techniques V/S active range of motion exercises on shoulder function post modified radical neck dissection in patients with head and neck cancer-A randomized clinical trial. Asian Pac. J. Cancer Prev. 21, 2389–2393 (2020).
Pauli, N., Andréll, P., Johansson, M., Fagerberg-Mohlin, B. & Finizia, C. Treating trismus: A prospective study on effect and compliance to jaw exercise therapy in head and neck cancer. Head Neck 37, 1738–1744 (2015).
Loh, E. W., Shih, H. F., Lin, C. K. & Huang, T. W. Effect of progressive muscle relaxation on postoperative pain, fatigue, and vital signs in patients with head and neck cancers: A randomized controlled trial. Patient Educ. Couns. 105, 2151–2157 (2022).
Cnossen, I. C. et al. Prophylactic exercises among head and neck cancer patients during and after swallowing sparing intensity modulated radiation: Adherence and exercise performance levels of a 12-week guided home-based program. Eur. Arch. Otorhinolaryngol. 274, 1129–1138 (2017).
Dotevall, H. et al. Treatment with head-lift exercise in head and neck cancer patients with dysphagia: results from a randomized, controlled trial with flexible endoscopic evaluation of swallowing (FEES). Support. Care Cancer. 31, 56 (2023).
Yoon, S. Y. & Oh, J. Neuropathic cancer pain: Prevalence, pathophysiology, and management. Korean J. Internal Med. https://doi.org/10.3904/kjim.2018.162 (2018).
Delanian, S., Lefaix, J. L. & Pradat, P. F. Radiation-induced neuropathy in cancer survivors. Radiother. Oncol. https://doi.org/10.1016/j.radonc.2012.10.012 (2012).
Labianca, R. et al. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin. Drug Investigat. https://doi.org/10.2165/11630080-000000000-00000 (2012).
Idorn, M. & Thor Straten, P. Exercise and cancer: From “healthy” to “therapeutic”?. Cancer Immunol. Immunother. https://doi.org/10.1007/s00262-017-1985-z (2017).
Velly, A. M. et al. Management of painful temporomandibular disorders. J. Am. Dental Assoc. 153, 144–157 (2022).
Oral, K., Küçük, B. B., Ebeoǧlu, B. & Dinçer, S. Etiology of temporomandibular disorder pain. Agri. 21, 89–94 (2009).
Azzam, P., Mroueh, M., Francis, M., Daher, A. A. & Zeidan, Y. H. Radiation-induced neuropathies in head and neck cancer: Prevention and treatment modalities. ecancermedicalscience. 14. https://doi.org/10.3332/ECANCER.2020.1133 (2020).
Leitzelar, B. N. & Koltyn, K. F. Exercise and neuropathic pain: A general overview of preclinical and clinical research. Sports Med. Open. 7. https://doi.org/10.1186/s40798-021-00307-9 (2021).
Silva, P. B., Ramos, G. H. A., Petterle, R. R. & Borba, V. Z. C. Sarcopenia as an early complication of patients with head and neck cancer with dysphagia. Eur. J. Cancer Care (Engl.) 30, e13343 (2021).
Cao, A., Ferrucci, L. M., Caan, B. J. & Irwin, M. L. Effect of exercise on sarcopenia among cancer survivors: A systematic review. Cancers https://doi.org/10.3390/cancers14030786 (2022).
Bozzetti, F. Chemotherapy-induced sarcopenia. Curr. Treat Options Oncol. 21, 7 (2020).
De Bree, R., Van Beers, M. A. & Schaeffers, A. W. M. A. Sarcopenia and its impact in head and neck cancer treatment. Curr. Opin. Otolaryngol. Head Neck Surg. https://doi.org/10.1097/MOO.0000000000000792 (2022).
Karavolia, E. et al. Impact of sarcopenia on acute radiation-induced toxicity in head and neck cancer patients. Radiother. Oncol. 170, 122–128 (2022).
Supriya, R., Singh, K. P., Gao, Y., Gu, Y. & Baker, J. S. Effect of exercise on secondary sarcopenia: A comprehensive literature review. Biology. https://doi.org/10.3390/biology11010051 (2022).
Newton, R. U. & Galvão, D. A. Exercise in prevention and management of cancer. Curr. Treat Options Oncol. 9, 135–46 (2008).
Ravasco, P. Nutritional approaches in cancer: Relevance of individualized counseling and supplementation. Nutrition 31, 603–604 (2015).
Oikawa, S. Y., Holloway, T. M. & Phillips, S. M. The impact of step reduction on muscle health in aging: Protein and exercise as countermeasures. Front. Nutr. https://doi.org/10.3389/fnut.2019.00075 (2019).
Jenkins, N. D. M. et al. Greater neural adaptations following high- vs. low-load resistance training. Front. Physiol. 8, 331 (2017).
Aagaard, P., Simonsen, E. B., Andersen, J. L., Magnusson, P. & Dyhre-Poulsen, P. Neural adaptation to resistance training: Changes in evoked V-wave and H-reflex responses. J. Appl. Physiol. 92, 2309–2318 (2002).
Wilson, J. M. et al. Concurrent training: A meta-analysis examining interference of aerobic and resistance exercises. J. Strength Cond. Res. 26, 2293–2307 (2012).
Fyfe, J. J., Bishop, D. J. & Stepto, N. K. Interference between concurrent resistance and endurance exercise: Molecular bases and the role of individual training variables. Sports Med. https://doi.org/10.1007/s40279-014-0162-1 (2014).
Gurjão, A. L. D., Gonçalves, R., De Moura, R. F. & Gobbi, S. Acute effect of static stretching on rate of force development and maximal voluntary contraction in older women. J. Strength Cond. Res. 23, 2149–2154 (2009).
Smajla, D., García-Ramos, A., Tomažin, K. & Strojnik, V. Selective effect of static stretching, concentric contractions, and a balance task on ankle force sense. PLoS One 14, e0210881 (2019).
Morrow, G. R., Andrews, P. L. R., Hickok, J. T., Roscoe, J. A. & Matteson, S. Fatigue associated with cancer and its treatment. Support. Care Cancer. https://doi.org/10.1007/s005200100293 (2002).
Thong, M. S. Y., van Noorden, C. J. F., Steindorf, K. & Arndt, V. Cancer-related fatigue: Causes and current treatment options. Curr. Treat Options Oncol. 21, 17 (2020).
Al Maqbali, M., Al Sinani, M., Al Naamani, Z., Al Badi, K. & Tanash, M. I. Prevalence of fatigue in patients with cancer: A systematic review and meta-analysis. J. Pain Sympt. Manag. https://doi.org/10.1016/j.jpainsymman.2020.07.037 (2021).
Bossi, P. et al. Prevalence of fatigue in head and neck cancer survivors. Ann. Otol. Rhinol. Laryngol. 128, 413–419 (2019).
Mesia, R. et al. SEOM clinical guidelines for the treatment of head and neck cancer (2020). Clin. Transl. Oncol. 23, 1001 (2021).
Pfister, D. G. et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Comprehensive Cancer Netw. 18, 873–898 (2020).
Pauloski, B. R. Rehabilitation of dysphagia following head and neck cancer. Phys. Med. Rehabilit. Clin. N. Am. https://doi.org/10.1016/j.pmr.2008.05.010 (2008).
Mura, F., Bertino, G., Occhini, A. & Benazzo, M. Surgical treatment of hypopharyngeal cancer: A review of the literature and proposal for a decisional flow-chart. Acta Otorhinolaryngol. Ital. 33, 299–306 (2013).
Erisen, L. et al. Shoulder function after accessory nerve-sparing neck dissections. Head Neck 26, 967–971 (2004).
Kallappa, S. & Dange, P. An analysis of complications of neck dissection in head and neck cancers. Int. J. Clin. Oncol. Cancer Res. 5, 24 (2020).
Chiesa-Estomba, C. M. et al. Complications after functional neck dissection in head and neck cancer patients: An observational, retrospective, single-centre study. ORL 83, (2021).
Wang, H.-L. Shoulder pain after neck dissection among head and neck cancer patients. Shoulder Pain After Neck Dissection Among Head Neck Cancer Patients 148, 478–482 (2009).
Sheikh, A., Shallwani, H. & Ghaffar, S. Postoperative shoulder function after different types of neck dissection in head and neck cancer. Ear Nose Throat J. 93, E21–E26 (2014).
Garssen, B. et al. Stress management training for breast cancer surgery patients. Psychooncology 22, 572–580 (2013).
Nadendla, L. K., Meduri, V., Paramkusam, G. & Pachava, K. R. Evaluation of salivary cortisol and anxiety levels in myofascial pain dysfunction syndrome. Korean J. Pain. 27, 30–34 (2014).
Kim, K. J., Na, Y. K. & Hong, H. S. Effects of progressive muscle relaxation therapy in colorectal cancer patients. West J. Nurs. Res. 38, 959–973 (2016).
Thomas, A., D’Silva, C., Mohandas, L., Pais, S. M. J. & Samuel, S. R. Effect of muscle energy techniques V/S active range of motion exercises on shoulder function post modified radical neck dissection in patients with head and neck cancer—A randomized clinical trial. Asian Pac. J. Cancer Prev. 21, 2389–2393 (2020).
Bunno, Y. Effectiveness of motor imagery on physical therapy: Neurophysiological aspects of motor imagery. Phys. Therapy Effectiveness. https://doi.org/10.5772/intechopen.90277 (2020).
Koltyn, K. F., Brellenthin, A. G., Cook, D. B., Sehgal, N. & Hillard, C. Mechanisms of exercise-induced hypoalgesia. J. Pain. 15, 1294–1304 (2014).
Carayol, M. et al. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: What is the optimal dose needed?. Ann. Oncol. https://doi.org/10.1093/annonc/mds342 (2013).
Mustian, K. M. et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. 3, 961–968 (2017).
Melzack, R. & Wall, P. D. Pain mechanisms: A new theory. Science (1979) 150, 971–979 (1965).
DeVita, V. T., Lawrence, T. S. & Rosenberg, S. A. DeVita, Hellman, and Rosenberg’s cancer: Principles & practice of oncology. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology (2018).
Roberts, B. M. et al. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J. 27, 2600–2610 (2013).
Author information
Authors and Affiliations
Contributions
Theoretical conceptualization, R.P.G., D.Z.L., and G.B.M.; literature searching, D.Z.L., G.B.M., C.R.G.; statistical analysis, I.M.P. and S.M.P.; elaboration of draft, I.M.P. and S.M.P.; review, N.K.Y. and F.D.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Nart Keituqwa Yáñez, which was incorrectly given as Nart Keituqwa Yán.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez, I.M.M., Pérez, S.E.M., García, R.P. et al. Exercise-based rehabilitation on functionality and quality of life in head and neck cancer survivors. A systematic review and meta-analysis. Sci Rep 13, 8523 (2023). https://doi.org/10.1038/s41598-023-35503-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35503-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.