Abstract
Aging process is associated with gradual change of liver function and structure. The goal of this study was to evaluate age-related hemodynamic changes in the portal vein (PV) using four-dimensional (4D) flow MRI in healthy adults. A total of 120 healthy subjects were enrolled and categorized into groups A (n = 25, 30–39 years), B (n = 31, 40–49 years), C (n = 34, 50–59 years), and D (n = 30, 60–69 years). All subjects underwent 4D flow data acquisition using a 3-T MRI system to measure the hemodynamic parameters in the main PV. The clinical characteristics and 4D flow parameters were compared among the groups using analysis of variance and analysis of covariance after controlling for significant covariates, accordingly. The outcome metric applying the age-related quadratic model to estimate the age at which 4D flow parameters are the highest (the peak age) as well as the rates of age-related 4D flow changes was estimated. The average area, average through-plane velocity, peak velocity magnitude, average net flow, peak flow, and net forward volume in group D were significantly lower than those in groups A, B and C (P < 0.05). Group C showed significantly lower values of the average through-plane velocity and peak velocity magnitude than those of group B (P < 0.05). The peak age computed was approximately 43–44 years of age for all 4D flow parameters. The rates of age-related 4D flow changes for all 4D flow parameters were negatively correlated with age (P < 0.05). The volume and velocity of the blood flow through the PV peaked at approximately 43–44 years of age and decreased significantly after 60 years of age.
Similar content being viewed by others
Introduction
The hepatic hemodynamic status is important to understand the pathophysiological mechanism and progression of the liver diseases1. In particular, the portal vein (PV) is the main vessel of the liver blood system and delivers approximately 75% of the blood supply by draining blood from the splenic and superior mesenteric veins to the liver to metabolize nutrients and remove potentially toxic substances2. The normal flow waveform in the PV shows constant, non-pulsatile, and unidirectional blood flow toward the liver2. Indeed, any changes in the blood flow pattern in the PV might be characteristic features of various pathologies including portal hypertension in liver cirrhosis3. Therefore, changes in the volume and velocity of the blood flow through the PV with age may have important implications for the health of the liver.
The process of aging is associated with gradual deterioration of hepatic function and structure accompanied by various changes in liver cells such as sinusoidal endothelial cells4. More seriously, aging can also increase mortality risk from various liver diseases as an adverse prognostic factor5. To date, although a few studies6,7,8 including a small number of subjects have used ultrasound to measure portal blood flow and shed light on the effect of the age on the hemodynamic circumstances in the PV, the hemodynamic characteristics of the PV including the volume and velocity of the blood flow through the PV with age remain poorly understood. The studies6,8 published in 1989 have reported that liver volume and portal blood flow decrease after the age of 50 years, and liver function decreases progressively with aging. Another study7 revealed that total hepatic flow measured by pulsed echo-Doppler significantly decreased with age, particularly in subjects over 75 years. A similar reduction was also observed in functional hepatic flow, and both were correlated with age. Accordingly, when reliably evaluating altered hepatic blood flow associated with liver diseases, understanding age-related hemodynamic changes in the PV as a baseline could be important. Also, ultrasonography has been mainly used to assess changes in portal and splanchnic blood flow to the liver. However, it is sometimes limited for visualizing blood flow due to overlying intestinal gas and a limited field of view. Furthermore, this modality could lead to inaccurate quantification of hemodynamics as a result of variability in the actual velocity values of the portal venous blood flow9.
Four-dimensional (4D) flow magnetic resonance imaging (MRI) has recently attracted attention as a noninvasive imaging tool to provide relevant hemodynamic and anatomic information for clinical diagnosis of various abdominal pathologies10. There was a clinical study11 using 4D flow MRI that investigated normal three-dimensional portal venous hemodynamics in healthy subjects in their 20 s and 50 s and in patients with liver cirrhosis. Nevertheless, the correlation of blood flow characteristics with age in the portal venous system has not been well characterized in healthy subjects.
Therefore, this study aimed to investigate hemodynamic changes in the PV with age using 4D flow MRI in healthy adults.
Results
Clinical characteristics
Table 1 presents the demographics and clinical characteristics of the four groups. There were significant differences in the age (P < 0.001), body weight (P = 0.007), body height (P < 0.001), and standard liver volume (P < 0.001) among the groups. Further post hoc test revealed that there was a significantly different at P < 0.05 level between the groups, as detailed in Table 1. However, there were no significant differences in other variables among the groups.
Comparison of the quantified parameters from 4D flow MRI among the four groups
As shown in Fig. 1, in group D, all hemodynamic parameters in the main PV using 4D flow MRI including the average area, average through-plane velocity, peak velocity magnitude, average net flow, peak flow, and net forward volume were significantly lower than those in groups A, B, and C (except for the average net flow in group A and net forward volume in group B) (P < 0.05). Moreover, group C showed significantly lower values of the average through-plane velocity and peak velocity magnitude than those in group B (P < 0.05). However, there were no significant differences in other 4D flow parameters among the groups.
The average intraclass correlation coefficients (ICC) for all 4D flow parameters in the PV were 0.911 ± 0.043 for group A; 0.890 ± 0.070 for group B; 0.912 ± 0.053 for group C; and 0.936 ± 0.023 for group D (Table 2). All P-values were less than 0.001 for ICC tests.
Correlation of the 4D Flow parameters with clinical characteristics in all age groups
Among various clinical characteristics, body weight (P = 0.007), body height (P < 0.001), standard liver volume (P < 0.001), and age (P < 0.001) had significant P-values among the four groups (Table 1). In Table 3, regarding Pearson’s correlation outcomes, the 4D flow MRI parameters, including the average through-plane velocity, peak velocity magnitude, average net flow, peak flow, and net forward volume in the PV, were positively correlated with body weight, body height, and standard liver volume (P < 0.05). In contrast, age was negatively correlated with these parameters (P < 0.05).
In the partial correlation outcomes, all 4D flow parameters were negatively correlated with age, even after adjusting for other factors including body weight, body height, and standard liver volume (P < 0.05).
Estimation of the peak age and age-related 4D flow change rates
Figure 2 shows the computation of the peak age for 4D flow parameters using the quadratic model. The peak age computed was 43 years of age for the average area, 43 years of age for the average through-plane velocity, 44 years of age for the peak velocity magnitude, 43 years of age for the average net flow, 43 years of age for the peak flow, and 43 years of age for the net forward volume. As shown in Fig. 3, for all parameters, the rates of age-related 4D flow change were negatively correlated with age (P < 0.05) in all groups (y-intercept: 0.041–0.045, slope: − 0.001), group A (y-intercept: 0.040, slope: − 0.001), group B (y-intercept: 0.036–0.037, slope: − 0.001), group C (y-intercept: 0.043–0.049, slope: − 0.001), and group D (y-intercept: 0.078–0.101, slope: − 0.002). The age-related 4D flow change rate observed before the peak age tended to decrease gradually, whereas this trend in the change rate gradually increased after the peak age with aging. In particular, the y-intercept and slope were higher in group D than in the other groups.
Discussion
In an effort to overcome the limitations when using ultrasonography as described in the introduction section, our study investigated the age-related hemodynamic changes in the main PV in a cohort of more than 100 healthy subjects using a 4D flow MRI, which has been shown to make reproducible flow measurements through comprehensive dataset of anatomical and functional flow information2. In fact, in our study, the range of ICC values, as an indicator of reproducibility of the measured values, was 0.89–0.93, suggesting that the reliability of 4D flow MRI is very good.
This study was consistent with the trend of a previous study7 that enrolled 40 normal subjects in four age groups (< 45, 45–60, 61–75 and > 75 years). That study, published in 1999, found that total hepatic blood flow (the sum of portal and hepatic arterial blood flow) and functional hepatic blood flow decreased significantly with age7. Our findings showed that the values of blood flow parameters in the main PV decreased significantly after 60 years of age. In our study, all portal blood flow parameters were significantly lower in normal subjects older than 60 years of age than in those younger than 40 years of age. Namely, the average area, average through plane velocity, peak velocity magnitude, average net flow, peak flow, and net forward volume decreased by 27%, 27%, 23%, 25%, 27%, and 30%, respectively. Furthermore, the temporal trajectories of 4D flow parameters of the PV were mapped in healthy subjects covering an adult lifespan of more than 40 years of age. As a result, the hemodynamic parameters of the PV peaked at approximately 43–44 years of age.
Zoli et al.7 suggested that age-related reductions in liver weight and volume might be explained by a decrease in the number and size of hepatocytes. Another ultrasound study8 showed a 20–40% decrease in liver volume with age. In fact, the volume of liver cells gradually increases as they mature but begins to decrease with aging12. An experimental study13 reported that aging-related changes in liver cells included polyploidy, accumulation of lipofuscin inside hepatocytes, decreased smooth endoplasmic reticulum area, and decreased mitochondrial number and functional decline. With aging, hepatocyte polyploidy tends to occur more frequently, accompanied by a decrease in the number of mitochondria and dysfunction, resulting in decreased adenosine triphosphate levels12. A molecular imaging study14 using 99mTc-galactosyl-human serum albumin liver scintigraphy reported a decrease in functional liver cell mass but not total liver volume associated with aging-related changes in the liver. In addition, some studies have suggested that aging causes severe morphological changes in the sinusoidal vasculature, negatively affecting liver function15. Taken together, we hypothesized that aging, defined as the gradual changes in liver structure and function that occurs over time and various changes in hepatocytes, could eventually lead to hemodynamic changes in the PV. Following this hypothesis, our data showed a positive correlation of body weight, height, and standard liver volume with 4D flow MRI parameters. Furthermore, our data demonstrated that aging itself is an important factor in reducing portal blood flow, even if the influence of variables affecting portal blood hemodynamics such as liver volume, height, and weight were excluded.
Our novel study using 4D flow MRI provided a new metric for assessing age-dependent portal blood flow characteristics in healthy subjects, implying the importance of an age-matched control cohort for assessing liver disease associated with PV blood flow. Interestingly, this study revealed for the first time that the age at which the hemodynamic parameters of PV reach the highest values (maximum age) was the mid-40 s. At the same time, the trend in the rate of change of flow hemodynamics according to age gradually increased after the peak observation; in particular, it increased more rapidly in the 60 s. This may be due to long-term risk factors such as increased oxidative stress, increased inflammatory responses, accelerated cellular aging, and progressive organ dysfunction with aging16. Similarly, only a small number of studies published 30 years ago6,8 suggested that portal blood flow decreased after age 50 and postulated that aging-related decreased blood flow may be an important component of age-related changes in the liver. These changes may negatively affect the process by which various drugs are eliminated from the body with aging. Therefore, the peak age and age-related rate of change in 4D flow values are considered important metrics for understanding the physiological and/or pathological course of liver disease with age. However, it should be noted that age is closely related to other basic metrics including weight, height, and liver volume. These key factors must be considered together when assessing the diagnosis and outcome of various liver diseases17.
There are several limitations in this study. First, because our study was conducted at a single tertiary care medical center, the results may not be representative of the entire population. Large-scale studies with populations that reflect different traits may help to better validate the results. Second, the metrics presented in this study were the results of a cross-sectional study. This potentially limits the conclusions that can be drawn about the longitudinal process of aging. Therefore, further studies with a longitudinal design are needed to confirm the cross-sectional trends observed here. Third, the age at which the 4D flow parameters reach the highest values (peak age) is considered a biologically meaningful indicator, but the clinical significance of this indicator requires further studies.
In conclusion, our study demonstrates age-related hemodynamic changes in the PV using 4D flow MRI in healthy subjects, where the volume and velocity of the blood flow through the PV peaked at approximately 43–44 years of age and decreased significantly after 60 years of age.
Methods
Study population
At least 25 subjects for each group were estimated to significantly quantify the differences in 4D flow parameters among the four groups with an α error of 0.05 and a β error of 0.2. A total of 174 participants who underwent routine laboratory tests including liver function testing and 4D flow MRI between 2018 and 2021 were included in this study as part of an IRB-approved clinical study involving healthy individuals which was approved by the institutional review board of Chonnam National University Hospital, and conformed to the ethical guidelines of the 2008 Declaration of Helsinki. Written informed consent was obtained from all subjects. And the methods in this study were carried out in accordance with the approved guideline and regulation. Of those participants, 54 subjects were excluded from our study due to the following reasons: (1) abnormal liver function testing (n = 9); (2) medical history of focal or diffuse liver disease (n = 11); (3) fatty deposition (more than 5%) in the liver (n = 13); (4) history of treatment for abdominal pathology within the last year (n = 19); and (5) cardiovascular risk factors or history of cardiac diseases (n = 2). Finally, 120 consecutive individuals were enrolled in this study. Each subject was categorized into one of four age groups: group A (n = 25, 30–39 years); group B (n = 31, 40–49 years); C (n = 34, 50–59 years); and D (n = 30, 60–69 years) (Fig. 4).
4D flow MRI acquisition
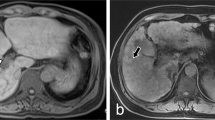
All subjects underwent 4D flow MRI using a clinical 3-T scanner (Skyra; Siemens Healthcare, Erlangen, Germany) with a 32-channel phased array coil. The 4D flow sequence covered the main PV (Fig. 5) and utilized both ECG-gated and navigator-triggering techniques. The imaging parameters for 4D flow MRI were as follows: repetition time/echo time = 6.72/2.84 ms, field-of-view = 380 × 308 mm2, slice thickness = 2.0 mm, matrix = 192 × 129, GRAPPA acceleration factor = 3, Cartesian sampling of k-space, number of excitations = 1, and scan time = 8–15 min. Four-dimensional flow MRI scans were performed after a period of fasting for at least 8 h, and the status of the stomach was reviewed on anatomic MR images (Fig. 5a). To avoid velocity aliasing, the velocity encoding was performed prior to the 4D flow MRI. Through the velocity-encoding sensitivity [VENC] scout images (Fig. 5b), the VENC was set to 30 cm/s18.
Anatomic coronal MR image (a) in the abdomen showing the main portal vein (PV) and the status of the stomach. Velocity-encoding (VENC) image (b) focusing on the PV (VENC = 30 cm/s). The red dotted circle indicates the cross-sectional area of the PV. Four-dimensional flow MRI data were post-processed; then, segmented anatomic images (left) and color-coded streamline images (right) were generated from a 38-year-old male (c), a 45-year-old female (d), a 53-year-old male (e), and a 68-year-old female (f). The red solid lines in (c) indicate planes for measuring the hemodynamic parameters, and the white arrows in (c) show the direction of blood flow.
4D flow MRI data analysis
Post-processing of 4D flow images was performed using Siemens 4D flow software (V2.4, Siemens Healthcare, Erlangen, Germany) and quantified by two radiologists with eight and 20 years of experience in interpreting abdominal MRIs, respectively; the 4D flow parameters measured by two observers were averaged to set as the 4D flow parameter values. Time-resolved images of the 3D velocity vector fields were generated to display the blood flow in the PV. Thereafter, 3D streamlines and time-density curves of the blood flow parameters were obtained from the 4D datasets. The quantitative parameters included the average area (mm2), average through-plane velocity (cm/sec), peak velocity magnitude (cm/sec), average net flow (mL/sec), peak flow (mL/sec), and net forward volume (mL) in two planes of the PV. The 4D flow parameters obtained from two cut planes were averaged to represent the values of main PV.
Clinical data acquisition
Various clinical information, including age, sex, body weight, body height, body mass index, standard liver volume, splenic diameter, platelet count, and levels of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase, were obtained through electronic chart analysis. Blood samples were analyzed using a routine clinical chemistry analyzer to measure blood cell counts and liver function. The splenic diameter was measured on MRI using electronic calipers and was defined as the greatest longitudinal dimension at the level of the splenic hilum on the PACS monitor19. Furthermore, the formulas for standard liver volume identified in the literature were used to calculate standard liver volume in each subject, and simple subject-specific parameters such as weight and height were also used20.
Statistical analysis
Data were analyzed using SPSS 24.0 software (SPSS Inc., Chicago, IL). Clinical characteristics were compared using analysis of variance (ANOVA), and 4D flow measurements were compared using analysis of covariance (ANCOVA) with body weight, body height, and standard liver volume as confounding factors in the test as covariates among the four groups. The ICC was used to assess the interobserver agreement in quantitative measurements of 4D flow parameters determined by two observers. Pearson’s correlation analysis was applied to analyze the relationship between the 4D flow parameters and clinical characteristics in all subjects, and partial correlation was performed to evaluate the relationship between the 4D flow parameters and age after controlling for body weight, body height, and standard liver volume. In addition, the outcome metric applying age-related quadratic model to estimate the age at which 4D flow parameters are the highest (the peak age) was evaluated. The rates of age-related 4D flow changes before and after the peak age were estimated by the following formula: (y2 − y1)/y1, where y2 and y1 were obtained from the age-related quadratic model for the peak age, representing the change extent in 4D flow values with age. Then, Pearson’s correlation analysis was performed using these values from all groups as well as from each group to compare the slope and intercept between those from all groups and each group.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Carneiro, C. et al. All about portal vein: A pictorial display to anatomy, variants and physiopathology. Insights Imaging 10, 38. https://doi.org/10.1186/s13244-019-0716-8 (2019).
Oechtering, T. H. et al. Clinical applications of 4D flow MRI in the portal venous system. Magn. Reson. Med. Sci. 21, 340–353. https://doi.org/10.2463/mrms.rev.2021-0105 (2022).
Maruyama, H. & Shiina, S. Collaterals in portal hypertension: Anatomy and clinical relevance. Quant. Imaging Med. Surg. 11, 3867–3881. https://doi.org/10.21037/qims-20-1328 (2021).
Maeso-Diaz, R. & Gracia-Sancho, J. Aging and chronic liver disease. Semin. Liver Dis. 40, 373–384. https://doi.org/10.1055/s-0040-1715446 (2020).
Radonjic, T. et al. Aging of liver in its different diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms232113085 (2022).
Zoli, M. et al. Portal blood velocity and flow in aging man. Gerontology 35, 61–65. https://doi.org/10.1159/000213000 (1989).
Zoli, M. et al. Total and functional hepatic blood flow decrease in parallel with ageing. Age Ageing 28, 29–33. https://doi.org/10.1093/ageing/28.1.29 (1999).
Wynne, H. A. et al. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 9, 297–301. https://doi.org/10.1002/hep.1840090222 (1989).
Bane, O. et al. Hemodynamic measurements with an abdominal 4D flow MRI sequence with spiral sampling and compressed sensing in patients with chronic liver disease. J. Magn. Reson. Imaging 49, 994–1005. https://doi.org/10.1002/jmri.26305 (2019).
Bane, O. et al. 4D flow MRI in abdominal vessels: Prospective comparison of k-t accelerated free breathing acquisition to standard respiratory navigator gated acquisition. Sci. Rep. 12, 19886. https://doi.org/10.1038/s41598-022-23864-9 (2022).
Stankovic, Z. et al. Normal and altered three-dimensional portal venous hemodynamics in patients with liver cirrhosis. Radiology 262, 862–873. https://doi.org/10.1148/radiol.11110127 (2012).
Le Couteur, D. G. et al. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology 33, 537–543. https://doi.org/10.1053/jhep.2001.22754 (2001).
Schmucker, D. L. Age-related changes in liver structure and function: Implications for disease ?. Exp. Gerontol. 40, 650–659. https://doi.org/10.1016/j.exger.2005.06.009 (2005).
Wakabayashi, H., Nishiyama, Y., Ushiyama, T., Maeba, T. & Maeta, H. Evaluation of the effect of age on functioning hepatocyte mass and liver blood flow using liver scintigraphy in preoperative estimations for surgical patients: Comparison with CT volumetry. J. Surg. Res. 106, 246–253. https://doi.org/10.1006/jsre.2002.6462 (2002).
Le Couteur, D. G. et al. Old age and the hepatic sinusoid. Anat. Rec. (Hoboken) 291, 672–683. https://doi.org/10.1002/ar.20661 (2008).
Poulose, N. & Raju, R. Aging and injury: Alterations in cellular energetics and organ function. Aging Dis 5, 101–108. https://doi.org/10.14336/AD.2014.0500101 (2014).
Hagen, F., Mair, A., Bosmuller, H. & Horger, M. Correlation between liver volume and liver weight in a cohort with chronic liver disease: A semiautomated CT-volumetry study. Quant. Imaging Med. Surg. 12, 376–383. https://doi.org/10.21037/qims-21-299 (2022).
Liu, D. et al. Quantitative study of abdominal blood flow patterns in patients with aortic dissection by 4-dimensional flow MRI. Sci. Rep. 8, 9111. https://doi.org/10.1038/s41598-018-27249-9 (2018).
Moon, C. M., Shin, S. S., Heo, S. H. & Jeong, Y. Y. Metabolic alterations associated with early-stage hepatocellular carcinoma and their correlation with aging and enzymatic activity in patients with viral hepatitis-induced liver cirrhosis: A preliminary study. J. Clin. Med. https://doi.org/10.3390/jcm9030765 (2020).
Pomposelli, J. J., Tongyoo, A., Wald, C. & Pomfret, E. A. Variability of standard liver volume estimation versus software-assisted total liver volume measurement. Liver Transpl. 18, 1083–1092. https://doi.org/10.1002/lt.23461 (2012).
Acknowledgements
Mun Young Paek (Siemens Healthineers Limited, Seoul, Korea), and Ning Jin (Siemens Medical Solutions, Chicago, USA) contributed to design of MRI sequence and protocols.
Funding
This study was financially supported by Chonnam National University (No. 2021-2159), and the Ministry of Science and ICT through the National Research Foundation of Korea (No. 2021R1A2C1005765; 2021H1D3A2A02037997; 2022R1A2C1003266).
Author information
Authors and Affiliations
Contributions
C.M.M. and S.S.S. designed the study; C.M.M. and S.K.K. performed the majority of experiments; C.M.M. and S.H.H. contributed to the analysis and interpretation of results; C.M.M. wrote the first draft of the manuscript; S.S.S. and S.H.H. has approved the final manuscript and completed manuscript; also, all authors agree with the content of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moon, CM., Kim, SK., Heo, S. et al. Hemodynamic changes in the portal vein with age: evaluation using four-dimensional flow MRI. Sci Rep 13, 7397 (2023). https://doi.org/10.1038/s41598-023-34522-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34522-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.