Abstract
The pathophysiology of degenerative cervical myelopathy (DCM) is characterized by chronic compression-induced damage to the spinal cord leading to secondary harm such as disruption of the blood spinal cord barrier (BSCB). It is therefore the purpose of this study to analyze BSCB disruption in pre- and postoperative DCM patients and to correlate those with the clinical status and postoperative outcome. This prospectively controlled cohort included 50 DCM patients (21 female; 29 male; mean age: 62.9 ± 11.2 years). As neurological healthy controls, 52 (17 female; 35 male; mean age 61.8 ± 17.3 years) patients with thoracic abdominal aortic aneurysm (TAAA) and indication for open surgery were included. All patients underwent a neurological examination and DCM-associated scores (Neck Disability Index, modified Japanese Orthopaedic Association Score) were assessed. To evaluate the BSCB status, blood and cerebrospinal fluid (CSF) samples (lumbar puncture or CSF drainage) were taken preoperatively and in 15 DCM patients postoperatively (4 female; 11 male; mean age: 64.7 ± 11.1 years). Regarding BSCB disruption, CSF and blood serum were examined for albumin, immunoglobulin (Ig) G, IgA and IgM. Quotients for CSF/serum were standardized and calculated according to Reiber diagnostic criteria. Significantly increased preoperative CSF/serum quotients were found in DCM patients as compared to control patients: AlbuminQ (p < .001), IgAQ (p < .001) and IgGQ (p < .001). IgMQ showed no significant difference (T = − 1.15, p = .255). After surgical decompression, neurological symptoms improved in DCM patients, as shown by a significantly higher postoperative mJOA compared to the preoperative score (p = .001). This neurological improvement was accompanied by a significant change in postoperative CSF/serum quotients for Albumin (p = .005) and IgG (p = .004) with a trend of a weak correlation between CSF markers and neurological recovery. This study further substantiates the previous findings, that a BSCB disruption in DCM patients is evident. Interestingly, surgical decompression appears to be accompanied by neurological improvement and a reduction of CSF/serum quotients, implying a BSCB recovery. We found a weak association between BSCB recovery and neurological improvement. A BSCB disruption might be a key pathomechanism in DCM patients, which could be relevant to treatment and clinical recovery.
Similar content being viewed by others
Introduction
Degenerative cervical myelopathy (DCM) is a result of a chronic mechanical harm to the spinal cord leading to a composition of highly complex pathomechanisms on the molecular and cellular level1,2. As part of the central nervous system, the spinal cord has a special barrier along vessels, the blood-spinal cord barrier (BSCB)3. This BSCB protects the spinal cord mechanically and biochemically from substances and cells within the intravascular compartment, thereby maintaining a healthy microenvironment4. In a physiological state, proteins such as albumin and immunoglobulin (Ig) can only pass from the blood to the spinal cord tissue by passive diffusion in capillaries. Larger vessels prevent this passage through the BSCB, resulting in a characteristic CSF/serum quotient for each protein5. It is known that a structural damage of the BSCB is associated with increased permeability leading to protein efflux and edema (among other effects). The result is a self-sustaining cascade of secondary injury to the spinal cord, as described in acute spinal cord injury3,4,6. The pathomechanism of BSCB disruption is responsible for an extended damage of the spinal cord beyond the local mechanical injury, including inflammatory cascades, increased macrophage activation, Wallerian degeneration and cell death7,8,9,10,11. Already investigated extensively in traumatic spinal cord injury (SCI), BSCB disruption is still underestimated in chronic degenerative circumstances12. This key pathomechanism seems to be an important component in DCM, also providing possible future treatment options.
BSCB disruption has been in the focus of our previous publication, demonstrating its presence in DCM patients, giving first evidence of BSCB disruption in preoperative DCM patients with a distinct association with the clinical status of the patients12. To gain a deeper understanding of these promising results, the current study aims to replicate previous findings indicating a BSCB disruption in DCM patients, and additionally addresses the postoperative status.
Methods
Study procedure and sample analysis
The study was approved by the local ethics committee of the Medical Faculty of the RWTH Aachen University (EK 164/13) including the following amendment. Before the investigation, all participants gave written informed consent according to the Declaration of Helsinki (Medical Association 2008). Any participants who had a neurological condition other than DCM (e.g., neurodegenerative diseases, ischemic diseases, cerebral hemorrhage, central nervous system infections or spinal trauma) were excluded from participation in the study. All included patients underwent a neurological examination and the objective functional status was assessed by an experienced spine surgeon using the modified Japanese Orthopedic Association score (mJOA; normal function: 18 points, mild myelopathy: 15–17 points, moderate myelopathy: 12–14 points, severe myelopathy: 0–11 points) and the Neck Disability Index (NDI)13,14,15.
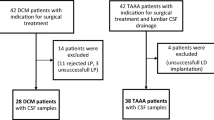
As described in a previous publication from 2020, our treatment recommendations were based on the AOSPINE guidelines for DCM12,16. We offered decompressive surgery as first-line therapy to DCM patients with moderate (mJOA 12–14) and severe (mJOA 0–11) clinical signs of myelopathy who also had correlating degenerative cervical spinal stenosis on imaging. In patients with mild signs of myelopathy (mJOA 15–17), surgery or conservative treatment with structured rehabilitation were recommended as possible option. In case of clinical deterioration, surgical intervention was strongly recommended. In DCM patients, cerebrospinal fluid (CSF) samples were obtained preoperatively by lumbar puncture (LP) or during CT myelography (if magnetic resonance imaging was contraindicated, e.g. with cardiac pacemaker). Three months after surgery (mean 121 ± 27 days), patients were electively examined in our outpatient clinic and a second LP was performed. According to our prior study, we included neurological healthy control patients12. These patients had an indication for open surgery concerning a thoracic abdominal aortic aneurysm (TAAA). Therefore, control patients routinely received a preoperative CSF drainage placement for intra- and postoperative intrathecal pressure monitoring17.
Blood serum samples of each patient were collected simultaneously with the corresponding CSF sample in all patients to perform Reiber diagnostics for detection of a BSCB disruption. A Queckenstedt maneuver was carried out in each DCM patient to rule out completely abolished CSF passage18. In addition, all CSF samples were examined for cytoalbuminous dissociation19.
The simultaneously collected CSF and blood serum samples were taken directly to the laboratory for examination. Routine laboratory values of CSF were determined: (1) CSF cell count (/µl), (2) lactate (mmol/l), and (3) protein concentration (g/l). CSF and blood serum samples were additionally analyzed for albumin, IgG, IgA and IgM (all mg/dl) by simultaneous nephelometric quantification (BN ProSpec System, Siemens Healthineers). Quotients (Q) of CSF/serum were calculated according to the standardized Reiber diagnostic criteria for AlbuminQ, immunoglobulin G (IgG)Q, IgAQ and IgMQ (all Q: n × 10–3)20. Individual age-related references of AlbuminQ were calculated using the formula: (4 + age/15) × 10–321,22. There were missing values for the following variables relevant to the preoperative/postoperative comparison: 1 for AlbuminQ, 1 for IgG, 1 for IgA and 7 for IgM.
The differentiation of a barrier disturbance from intrathecal synthesis is determined by the ratio of IgGQ and AlbuminQ, which is shown graphically in a Reiber diagram (Fig. 1). Values within the IgGQ and outside the AlbuminQ reference range indicate a barrier disorder. Conversely, intrathecal synthesis is present if the values are outside the IgGQ and inside the AlbuminQ reference range.
Reiber diagram. Please note that the black circle in the lower left area represents a normative ratio between AlbuminQ (QAlb in the figure) and IgAQ (QIgA in the figure). A barrier dysfunction and Ig synthesis are marked in the graph published by Reiber et al.5.
Data analyses
All statistical analyses were performed with SPSS 25 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.). Data measures deviating more than 1.5 standard deviations (SD) from the group-specific mean were regarded as outliers and corrected for by being replaced by the “worst” group-specific score on that respective variable. Explorative analyses revealed that this was the case for pre-operative IgMQ in two TAAA patients as well as for pre-operative IgAQ, IgGQ, IgMQ, and AlbuminQ in three DCM patients. In addition, another TAAA patient revealed extreme deviating values with regard to all parameters of interest (mJOA, IgAQ, IgGQ, IgMQ, and AlbuminQ), so this patient was excluded from further analyses. All statistical comparisons were tested two-sided with a significance level of p < 0.05 and Bonferroni-corrected for multiple testing.
Differences in mJOA as well as CSF/serum coefficients between patients and controls were explored by applying Independent Samples t-Tests, including group (DCM, TAAA) as between-subject factor, and the variable of interest (mJOA, IgAQ, IgGQ, IgMQ, and AlbuminQ) as dependent variable (adjusted p < 0.05 / 5 = 01).
Subgroup differences in CSF/serum coefficients between DCM patients with severe and non-severe mJOA scores were analyzed by means of Independent Samples t-Tests, including group (mJOA severe, mJOA non-severe) as between-subject factor, and the coefficient of interest (mJOA, IgAQ, IgGQ, IgMQ, and AlbuminQ) as dependent variable (adjusted p < 0.05 / 5 = 01).
Patients’ intra-individual changes from pre- to postoperative assessment were analyzed using Paired Samples t-Tests, including the pair of interest (pre-post for each mJOA, NDI, IgAQ, IgGQ, IgMQ, and AlbuminQ) as dependent variable (adjusted p < 0.05 / 6 = 008).
In order to assure that age as a possible confounding variable would not obscure analyses results, bivariate correlation analyses were performed, including age, mJOA, NDI, pre-/post-operative IgAQ, IgGQ, IgMQ, and AlbuminQ, tested two-sided and Bonferroni-corrected for multiple testing (adjusted p = 0.005). Results revealed no significant association between age and any variable of interest (all p > 0.05).
Results
Description of the study groups
Fifty DCM patients and 52 TAAA patients as neurologically healthy control group were included in this study. Due to rejected or unsuccessful LP, as well as unsuccessful lumbar drainage, 44 DCM and 46 TAAA patients remained for analysis. Baseline group characteristics (age, gender and comorbidities) and preoperative neurological scores (mJOA and NDI score) are shown in Table 1. As expected, there were significant group differences in neurological characteristics. There were 18 DCM patients with a mild paresis and 7 with a severe paresis (0–2/5 degree of strength) compared to the neurological healthy control group of TAAA patients. 25 DCM patients showed no paresis. A similar picture was seen with regard to ataxias: 38 patients with and 10 patients without ataxia in the DCM group compared to neurological healthy control group of TAAA patients. After approval of the amendment, a total of 15 patients could be included to receive a CSF puncture about three months after surgery.
(1) Neurological status
Comparison of preoperative clinical conditions in DCM patients and TAAA
As expected, a significant difference between the two groups was evident for the clinical NDI score (p < 0.001, data not shown) and the mJOA score (p < 0.001, Fig. 2). In total, seven patients were classified as mildly affected (mJOA 15–17), 13 as moderately (mJOA 12–14) and 30 as severe affected (mJOA 0–11).
Extent of clinical disturbance as myelopathy preoperatively. As expected, significantly lower mJOA score were detected in the DCM group (n = 49) compared to the TAAA group (n = 48). M = mean. p = p value. mJOA = modified Japanese Orthopedic Association. TAAA = thoracic abdominal aortic aneurysm. DCM = degenerative cervical myelopathy.
Comparison of clinical conditions in DCM patients with preoperative and postoperative assessment and TAAA patients
As shown in Fig. 3, we detected a significantly higher mJOA score postoperatively compared with the preoperative score (p = 0.001). However, the difference between DCM patients’ postoperative mJOA score and the mJOA score of the TAAA group remained significant (p < 0.001), albeit with a tendency to approach the values of the neurologically healthy patients. NDI score was significantly lower postoperatively as compared to preoperative assessment (Mpre = 40, Mpost = 30; p = 0.007, data not shown).
Extent of clinical myelopathy pre- and postoperatively. As expected, we detected a significant difference between pre- and postoperative DCM patients (npre/post = 16). While there are still significant differences between control patients (n = 48) and postoperative DCM patients, please note the trend of convergence. M = mean. p = p value. mJOA = modified Japanese Orthopedic Association. TAAA = thoracic abdominal aortic aneurysm. DCM = degenerative cervical myelopathy. Pre = preoperative. Post = postoperative.
(2) CSF findings
Comparison of preoperative CSF/serum quotients in DCM patients and TAAA patients
The results of Independent Samples t-Tests revealed a statistically significant difference regarding the assessment of almost all CSF/serum quotients: as compared to the control group, patients showed increases in AlbuminQ (p < 0.001, Fig. 4), IgAQ (p < 0.001, Fig. 5), and IgGQ (p < 0.001, Fig. 5). The group difference in IgMQ did not reach significance (p = 0.255, Fig. 5).
Extent of blood spinal cord barrier (BSCB) disruption preoperatively. The quotient for albumin (AlbuminQ) is shown and compared between the control (n = 43) and DCM groups (n = 43). Please note the significant difference indicating BSCB disruption. M = mean. p = p value. TAAA = thoracic abdominal aortic aneurysm. DCM = degenerative cervical myelopathy.
Extent of blood spinal cord barrier (BSCB) disruption preoperatively. The quotients for IgA (IgAQ), IgG (IgGQ) and IgM (IgMQ) are shown and compared between the control (nIgA,IgG = 42, nIgM = 28) and DCM groups (nIgA,IgG = 43, nIgM = 32). Please note the significant differences for IgAQ and IgGQ indicating BSCB disruption. M = mean. p = p value. TAAA = thoracic abdominal aortic aneurysm. DCM = degenerative cervical myelopathy.
We next investigated a possible difference in quotients between clinically severely affected (mJOA severe = 0–11) and less severely affected patients (mJOA mild = 15–17 and mJOA moderate = 12–14). However, we could not find any significant differences for AlbuminQ (p = 0.545), IgAQ (p = 0.975) and IgGQ (p = 0.688).
Comparison of CSF/serum quotients in DCM patients with preoperative and postoperative assessment and TAAA patients
After surgical decompression, AlbuminQ was significantly decreased postoperatively compared with the mean preoperative value (p < 0.01 Fig. 6).
Extent of blood spinal cord barrier (BSCB) recovery postoperatively. The AlbuminQ of the control group (n = 43) and the DCM patients with pre- and postoperative values (n = 15) are shown. Please note the significant differences pre- and postoperatively indicating BSCB recovery. M = mean. p = p value. TAAA = thoracic abdominal aortic aneurysm. DCM = degenerative cervical myelopathy. Pre = preoperative. Post = postoperative. AlbuminQ = Quotient of albumin.
The same was evident for IgGQ (p < 0.01), while IgAQ (p = 0.053) and IgMQ (p = 0.683) did not change significantly after surgery as shown in Fig. 7. Furthermore, the postoperative quotients remain significantly increased as compared to the TAAA control group regarding AlbuminQ (p = < 0.001), IgGQ (p < 0.01) and IgAQ (p < 0.01).
Extent of blood spinal cord barrier (BSCB) recovery postoperatively. The quotients of the control group (nIgA,IgG = 42, nIgM = 28) and the DCM patients with pre- and postoperative values (nIgA,IgG = 15, nIgM = 9) are shown. Please note the significant differences pre- and postoperatively for IgGQ indicating BSCB recovery. M = mean. p = p value. TAAA = thoracic abdominal aortic aneurysm. DCM = degenerative cervical myelopathy. Pre = preoperative. Post = postoperative. IgGQ = Quotient of IgG. IgAQ = Quotient of IgA. IgMQ = Quotient of IgM.
(3) Correlation between the extent of BSCB recovery and clinical improvement
Table 2 shows the postoperative cases with their respective mJOA scores and quotients pre- and postoperatively. Plotting the postoperative changes in mJOA score and AlbuminQ for each patient, a trend towards a negative linear relationship with a correlation coefficient r = − 0.20 was found, indicating that the greater the recovery of the BSCB, the better the clinical recovery (Fig. 8).
Simple linear regression model. Please note the trend towards negative linear relationship with r = − 0.2 between Δ mJOA and Δ AlbuminQ. Δ mJOA = changes in modified Japanese Orthopedic Association from postoperative to preoperative. Δ AlbQ = changes in Quotient of Albumin from postoperative to preoperative.
Discussion
While a BSCB disruption is well-known in acute SCI, its impact in chronic spinal cord disorders is rarely investigated. However, recently published studies provided first evidence that this pathomechanism may also be a key mechanism in DCM patients12,23,24,25. With an increased number of participants in this study, we confirmed our previous results of a preoperatively existing BSCB disruption according to the Reiber criteria published recently12. Using the Queckenstedt maneuver, we ruled out the possibility of a CSF stop in order to avoid false positive increased quotients. A significant correlation between the extend of preoperative symptoms (as indicated by mild, moderate and severe mJOA scores) and the extend of BSCB disruption could not be found. This is possibly due to the low number of patients in every severity classification, especially concerning patients with mild symptoms. At the molecular level, a BSCB breakdown can lead to a disruption of tight junction connectivity and basal lamina integrity, attributing to increased permeability24. The increased permeability in turn enables a secondary cascade of pathomechanisms that contributes to local spinal cord damage3,26,27,28,29. Preventing the development of this cascade or alleviating its severity could be an important therapeutic goal in DCM patients, as it is known that the BSCB remains chronically disrupted in untreated DCM30. Therefore, we also investigated the impact of surgical decompression on BSCB disruption.
The analysis of the quotient findings after surgery has not yet been described. In our study, we were able to detect a relevant BSCB recovery in 15 patients three months after surgical decompression of the spinal cord. In some cases, a BSCB disruption even restored completely according to the Reiber criteria. Our data showed a significant group difference between pre- and postoperative DCM patients for AlbuminQ and IgGQ, which tended to reach scores similar to those of the control group (Figs. 6 and 7). Although the effect can already be seen in this small sample size, it has yet to be confirmed with more patients. There is already first evidence to support BSCB recovery and axonal regeneration after surgical decompression in animal experiments31,32. To our knowledge, this is the first study to examine pre- and postoperative course of CSF findings in humans. Outcomes after surgical decompression for DCM have been prospectively investigated by AOSpine North America and AOSpine CSM International, indicating a sustained long-term improvement in neurological function33,34. The exact molecular mechanisms for this clinical recovery remain incompletely understood and require further intensive translational research. It is conceivable that not only the pathomechanisms but also the recovery processes are very similar to those in SCI. In this respect, there is already a better evidence base for SCI26,27,35,36,37,38,39,40. Astrocytes have the ability to reduce inflammation, cellular degeneration and restabilize the BSCB by forming a glial scar39,40. In addition to astrocytes, the glial scar is composed of multiple cell types such as oligodendrocyte progenitor cells, fibroblasts, macrophages and microglia interacting with each other37,38. This cell compound is capable of both, hindering and promoting vascular and axonal regeneration leading to BSCB reformation37,41. It has already been suggested in a rodent model that there is an association between restoration of BSCB and locomotor recovery after SCI due to limiting the influx of neurotoxins to the spinal cord (e.g. plasma proteins, immune cells, nitric oxide synthase)27.
Interestingly, we found such an association between recovery of the BSCB and improvement in the mJOA score in our postoperative DCM patients. Despite the low number of patients, our data indicate a decrease in AlbuminQ being accompanied with an improvement of the mJOA score. (However, it has to be reported that in one patient, the mJOA score remained the same postoperatively, but the AlbuminQ decreased. Another patient had a lower mJOA score postoperatively, but also a lower AlbuminQ.) This association needs to be verified and tested for significance in further studies with a larger patient cohort. If this relationship can be confirmed, the measurement of BSCB disruption by AlbuminQ and IgQ could be a promising biomarker for clinical recovery. Therefore, intensive research is required to understand the chronic lesion in DCM patients, which is characterized by ongoing mechanical microtrauma and persisting BSCB disruption. We believe that the regeneration process of the BSCB is one of the crucial factors for the postoperative recovery of DCM patients and thus monitoring of this BSCB recovery could become of clinical importance.
Limitations
This is a prospective single-center study with a relatively large DCM cohort, but it remains difficult to perform specific subgroup analyses. In particular, the correlation between the different clinical severity grades (as measured by mJOA subgroups) and the extent of BSCB disruption may require a multicenter study with a larger DCM patient cohort. Our definition of a BSCB disruption is based on the Reiber criteria for this study. An analysis of other indirect signs of BSCB disruption, such as spinal cord swelling on MRT, was not performed. Although the mJOA is a tool for describing neurological status in DCM patients in an objectifiable manner, it remains coarse and subjective. Postoperative clinical and laboratory data were collected from 15 patients three months after surgery. Later examination time points, e.g. after one year, would be of high interest, as would confirmation of these results with a larger sample size. However, this is the only study assessing postoperative CSF findings in association with BSCB in DCM patients.
Conclusion
Our data confirm the preoperative existence of a BSCB disruption in DCM patients. This BSCB disruption has the potential to recover after surgical decompression, which is detectable using Reiber diagnostics. The possible association between the extent of BSCB recovery and clinical improvement needs further investigations. We hypothesize that monitoring of BSCB disruption may be a promising diagnostic and potential therapeutic biomarker in DCM patients, which could lead to more individualized DCM treatments in the future.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Blume, C. et al. Decreased angiogenesis as a possible pathomechanism in cervical degenerative myelopathy. Sci. Rep. 11(1), 2497 (2021).
Akter, F. et al. The pathophysiology of degenerative cervical myelopathy and the physiology of recovery following decompression. Front. Neurosci. 14, 138 (2020).
Bartanusz, V. et al. The blood-spinal cord barrier: morphology and clinical implications. Ann. Neurol. 70(2), 194–206 (2011).
Jin, L. Y. et al. Blood-spinal cord barrier in spinal cord injury: A review. J. Neurotrauma 38(9), 1203–1224 (2021).
Reiber, H. & Peter, J. B. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J. Neurol. Sci. 184(2), 101–122 (2001).
Kumar, H. et al. Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol. Neurobiol. 54(5), 3578–3590 (2017).
Karadimas, S. K., Gatzounis, G. & Fehlings, M. G. Pathobiology of cervical spondylotic myelopathy. Eur. Spine J. 24(Suppl 2), 132–138 (2015).
Yu, W. R. et al. Human neuropathological and animal model evidence supporting a role for Fas-mediated apoptosis and inflammation in cervical spondylotic myelopathy. Brain 134(Pt 5), 1277–1292 (2011).
Karadimas, S. K. et al. A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol. Dis. 54, 43–58 (2013).
Bohlman, H.H., & Emery, S.E. The pathophysiology of cervical spondylosis and myelopathy. Spine (Phila Pa 1976), 1988. 13(7): p. 843–6.
Fehlings, M.G., & Skaf, G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976), 1998. 23(24): p. 2730–7.
Blume, C. et al. Patients with degenerative cervical myelopathy have signs of blood spinal cord barrier disruption, and its magnitude correlates with myelopathy severity: A prospective comparative cohort study. Eur Spine J 29(5), 986–993 (2020).
Yonenobu, K., et al., Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976), 2001. 26(17): p. 1890–4; discussion 1895.
Vernon, H. & Mior, S. The neck disability index: A study of reliability and validity. J. Manipulative Physiol. Ther. 14(7), 409–415 (1991).
Tetreault, L. et al. The modified Japanese Orthopaedic Association scale: Establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur. Spine J. 26(1), 78–84 (2017).
Fehlings, M. G. et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: Recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Glob. Spine J. 7(3 Suppl), 70S-83S (2017).
Gombert, A. & Simon, F. Strategies to prevent and detect intraoperative spinal cord ischemia during complex aortic surgery: From drainages and biomarkers. Neural Regen Res. 16(4), 678–679 (2021).
Queckenstedt, Zur Diagnose der Rückenmarkskompression. Deutsche Zeitschrift für Nervenheilkunde, 1916. 55(4): p. 325–333.
Froin, G., Inflammations meningées avec réactions chromatique, fibrineuse et cytologique du liquide cephalo-rachidien. Gaz d hop, 1903. 76.
Reiber, H. et al. Reporting cerebrospinal fluid data: knowledge base and interpretation software. Clin. Chem. Lab. Med. 39(4), 324–332 (2001).
Reiber, H. Flow rate of cerebrospinal fluid (CSF) — A concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 122(2), 189–203 (1994).
Reiber, H. Cerebrospinal fluid - physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult. Scler. J. 4(3), 99–107 (1998).
Tetreault, L. et al. Degenerative cervical myelopathy: A spectrum of related disorders affecting the aging spine. Neurosurgery 77(Suppl 4), S51-67 (2015).
Tu, J. et al. Degenerative cervical myelopathy: Insights into Its pathobiology and molecular mechanisms. J. Clin. Med. 10(6), 1214 (2021).
Karadimas, S.K., et al., Immunohistochemical profile of NF-κB/p50, NF-κB/p65, MMP-9, MMP-2, and u-PA in experimental cervical spondylotic myelopathy. Spine (Phila Pa 1976), 2013. 38(1): p. 4–10.
Maikos, J. T. & Shreiber, D. I. Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J. Neurotrauma 24(3), 492–507 (2007).
Cohen, D. M. et al. Blood-spinal cord barrier permeability in experimental spinal cord injury: Dynamic contrast-enhanced MRI. NMR Biomed. 22(3), 332–341 (2009).
Tachibana, N. et al. Spinal cord swelling in patients with cervical compression myelopathy. BMC Musculoskelet Disord. 20(1), 284 (2019).
Beattie, M. S. & Manley, G. T. Tight squeeze, slow burn: Inflammation and the aetiology of cervical myelopathy. Brain J. Neurol. 134(Pt 5), 1259–1261 (2011).
Kalsi-Ryan, S., Karadimas, S. K. & Fehlings, M. G. Cervical spondylotic myelopathy: The clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 19(4), 409–421 (2013).
Cheng, X. et al. Pathophysiological changes and the role of Notch-1 activation after decompression in a compressive spinal cord injury rat model. Front. Neurosci. 15, 579431–579431 (2021).
Dhillon, R. S. et al. Axonal plasticity underpins the functional recovery following surgical decompression in a rat model of cervical spondylotic myelopathy. Acta Neuropathol. Commun. 4(1), 89–89 (2016).
Fehlings, M. G. et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: Results of the AOSpine North America prospective multi-center study. J. Bone Joint Surg. Am. 95(18), 1651–1658 (2013).
Fehlings, M.G., et al., A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976), 2015. 40(17): p. 1322–8.
Noble, L. J. & Wrathall, J. R. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 482(1), 57–66 (1989).
Kim, Y. H., Ha, K. Y. & Kim, S. I. Spinal cord injury and related clinical trials. Clin. Orthop. Surg. 9(1), 1–9 (2017).
Tran, A. P., Warren, P. M. & Silver, J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 98(2), 881–917 (2018).
Venkatesh, K. et al. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 377(2), 125–151 (2019).
Gesteira, T. F., Coulson-Thomas, Y. M. & Coulson-Thomas, V. J. Anti-inflammatory properties of the glial scar. Neural Regen Res. 11(11), 1742–1743 (2016).
Faulkner, J. R. et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24(9), 2143–2155 (2004).
Mautes, A. E. et al. Vascular events after spinal cord injury: Contribution to secondary pathogenesis. Phys. Ther. 80(7), 673–687 (2000).
Acknowledgements
The authors declare that they have no conflict of financial and non-financial interests. This study was awarded und supported by: START research funding (Medical faculty of the Rheinisch-Westfälische Technische Hochschule, RWTH Aachen) and the research funding of the Deutsche Wirbelsäulenstiftung (Deutsche Wirbelsäulengesellschaft, DWG).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
C.B. and T.S. contributed to the conception and design of the study. C.B., T.S., K.J., U.B. and C.M. contributed to the acquisition, analysis and interpretation of data. C.B., T.S., K.J., L.B., T.P., D.D., A.G. and H.C. contributed to drafting the text or preparing the figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmidt, T.P., Jütten, K., Bertram, U. et al. Blood spinal cord barrier disruption recovers in patients with degenerative cervical myelopathy after surgical decompression: a prospective cohort study. Sci Rep 13, 7389 (2023). https://doi.org/10.1038/s41598-023-34004-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34004-2
This article is cited by
-
Blood-spinal cord barrier disruption in degenerative cervical myelopathy
Fluids and Barriers of the CNS (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.