Abstract
Evidence has also shown that oxidative stress and systemic inflammation, or in other words, disruption of the oxidant and antioxidant balance, can play an important role in the initiation or progression of NAFLD. The purpose of this study was to investigate the associations between the oxidative balance scores (OBS) and the risk of NAFLD. 552 healthy and 340 patients adult over the age of 18 with NAFLD participated in this case–control research. A validated 168-item quantitative food frequency questionnaire (FFQ) and indicators of physical activity, obesity, and smoking status were used to assess OBS score. The connection between OBS and NAFLD was discovered using binary logistic regression. The mean (± SD) age and (body mass index) BMI of the study population was 40.22 ± 9.79 years and 29.06 ± 3.92 kg/m2, respectively. The mean ± SD of OBS was 41.48 ± 5.23. After adjustment for potential confounders, higher scores of adherence to the OBS conferred a protection for the presence of NAFLD (odds ratio [OR]: 0.29; 95% confidence interval [CI]: 0.15–0.49; P for trend < 0.001). The findings of the present study indicate an approximately 80% reduction in the odds of developing NAFLD with higher OBS adherence in the overall population. However, prospective studies are needed to further investigate this association.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is becoming the most common form of chronic liver disease; it is characterized by a wide range of fat liver illnesses that can result in severe liver disease and cirrhosis1. The worldwide prevalence of NAFLD in adults is estimated to be at 20–25, 5–18, and 25–31% in populations in Asian countries and Iran, respectively2,3,4. NAFLD creates a significant economic burden on the health care system and affects the quality of life as the illness advances; thus, it is crucial to find effective solutions to prevent and treat this condition5. This high frequency of NAFLD is assumed to be mostly related to poor dietary habits, notably the consumption of a high-calorie diet that is rich in saturated fatty acids (SFA) or simple carbohydrates6. Regarding the pharmacological options of NAFLD, there is currently no consensus. Nonetheless, lifestyle therapies focusing on physical activity and well-balanced diet in terms of both quality and quantity are regarded as the cornerstone of NAFLD management1. Thus, a viable strategy for the prevention and treatment of NAFLD may involve alterations to eating habits and diet composition.
On the other hand, scientists have been interested in a number of diet-related risk factors that appear to contribute to NAFLD, including obesity, insulin resistance, inflammation, and oxidative and antioxidant systems7,8,9. Despite earlier research indicating a relationship between particular nutrients/food with oxidative stress and inflammation, dietary interactions may affect the effects of combined nutrients10,11. As a result, a novel method based on dietary patterns connected to oxidative and antioxidant systems is required.
Evidence has also shown that oxidative stress and systemic inflammation, or in other words, disruption of the oxidant and antioxidant balance, can play an important role in the initiation or progression of NAFLD through increasing lipid peroxidation in the cell membrane12,13.
Oxidative balance score (OBS) was first introduced by van Hooydonk et al. in 200214 as a measure of antioxidant-prooxidant status, which included vitamin C and beta-carotene as antioxidant components and iron as a prooxidant factor. However, in later research and evidence, these dietary index components increased to 2015,16,17. In general, the components of OBS include two components of diet and lifestyle, which are categorized as antioxidant and prooxidant factors18.
Recent studies also show the significant and beneficial effect of antioxidant agents such as vitamin E and C on the development and even the onset of NAFLD disease19,20,21. In addition, there are various evidences for the role of various types of prooxidants and oxidative stress, such as increased intake of saturated fatty acids, obesity and smoking, on increasing the risk of this disease19,22,23,24,25,26,27.
Although some research has been done to correlate this index with the risk of chronic illnesses such as diabetes, cancer, and metabolic syndrome, no research has been done in NAFLD patients. On the other hand, due to the rising prevalence of NAFLD and the fact that chronic diseases, particularly NAFLD, have imposed significant costs on the health-care system, we conducted this study to assess OBS in NAFLD patients in order to identify a critical approach to improving or controlling NAFLD.
Methods
Study design and population
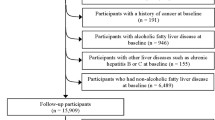
Between 2020 and 2022, this case–control study included adults over the age of 18 who had recently been diagnosed with NAFLD and healthy controls who had been admitted to Taleghani Hospital in Tehran, Iran, and the academic liver disease clinics of Shahid Sadoughi University of Medical Sciences in Yazd, Iran. The control group included 552 people without a history of NAFLD who were recruited from the same hospital, whereas the case group included 340 consecutive patients with NAFLD who had been diagnosed by a gastroenterologist. The procedure for patient sample was assessed by two dietitians. The following criteria were used to diagnose NAFLD28,29,30: chronic elevation in liver enzymes (liver enzymes > 19 U/L for women and > 30 U/L for men), liver ultrasound compatible with NAFLD, Having a grade II, III NAFLD based on liver biopsy, abstinence from alcohol usage, and the elimination of other possible causes of liver disease. A gastroenterologist confirmed the diagnosis of NAFLD when the case group was submitted to our facilities for assessment by Fibroscan28, which indicated a controlled attenuation parameter score of more than 237 and a fibrosis score of more than 7. Also, patients from other outpatient clinics at the same hospital, including dermatology, ophthalmology, and otorhinolaryngology, were recruited for the control group, which had no history of NAFLD. The healthy controls had no history of chronic or inflammatory illnesses, had been eating consistently for the past six months, and had been physically active (such as diabetes, gastrointestinal or cardiovascular disorders, cancer, etc.). Laboratory tests and liver ultrasonography, which confirmed they were free of any hepatic steatosis in any stage, served as the foundation for the inclusion criteria for the control group. Also, matching of people in the case and control group (1:1) was done based on age variables (± 3 years) and body mass index (BMI) (± 1 kg/m2). The following conditions precluded patients from participating: long-term dietary changes, weight loss, a specific illness, a history of hepatic or renal disease (such as non-alcoholic steatohepatitis (NASH), alcoholic fatty liver disease, Wilson's disease, cirrhosis, autoimmune liver disease, hemochromatosis, viral infections), diabetes, cancer, thyroid disorder, and autoimmune disease. By completing demographic, economic, and social questionnaires, information regarding age, education level, work status, medical history, smoking status, usage of certain pharmaceuticals (other than typical NAFLD medications), and dieting history during the past six months was gathered. The levels of physical activity of the participants were assessed using general practice physical activity questionnaires (GPPAQs). The GPPAQ is a short survey that gauges one's current level of physical activity30 and occupation and is scored into active, moderately active, moderately inactive or inactive categories (see Fig. 1). In this study, nutritionists served as the interviewers. As a consequence, every patient answered every item in the survey honestly. Also, informed consent was obtained from all subjects and all methods were performed in accordance with the relevant guidelines and regulations. This study was approved by Shahid Beheshti University of Medical Sciences, Tehran, Iran, and Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Calculation of sample size
Required minimal sample size for the current work was calculated based on the hypothesis of 1.5 times decreased odds of NAFLD by OBS. Terefore, considering type I error of 5%, the study power of 90% and the ratio of controls to cases as approximately 1.5, the minimum required sample size was calculated.
Anthropometric measurement
The researchers carried out an anthropometric analysis. The weight was recorded to the closest 100 g using a standard SECA 700 Digital Scale (SECA, Hamburg, Germany) with little clothing and no shoes. The patient's height requirements were evaluated using a Seca portable height gauge with 0.1 cm of precision. In addition, a Seca waist measuring device was used to establish the waist circumference (WC) in the central area between the iliac crown and the last gear. In addition, the hip circumference was calculated in centimeters by placing the same measuring tape parallel to the floor at the fullest part of the buttocks. Weight (kg)/Height2(m) was used to determine body mass index (BMI) after weight and height were measured in the aforementioned method. All anthropometric measures were conducted by the researcher in order to reduce observational variance.
Dietary assessment
A semi-quantitative validated food-frequency questionnaire (FFQ) with 168 food items was used to collect data on dietary consumption during the preceding year31. The FFQ consisted of a list of typical Iranian foods and their serving sizes. Self-reports on the FFQ determined the average portion size and frequency of consumption for each food item. The frequency of consumption of each food item was as follows: never, 2–3 times per month, once per week, 2–4 times per week, 5–6 times per week, and daily. Using standard Iranian household measurements, the serving quantities were provided in grams32. Utilizing the United States Department of Agriculture's (USDA) national nutritional databank, daily nutrient consumptions for each individual were calculated33. The nutritional and calorie content of the foods were analyzed using a customized version of Nutritionist 4 (First DatabankInc., Hearst Corp., San Bruno, CA, USA) for Iranian meals.
Calculation of oxidative balance scores (OBS)
In the present study, we used the method described by Goodman et al.34 to calculate the OBS of each participant. According to this method, a total of 13 dietary and nondietary pro- and antioxidant components, based on a priori knowledge about their association to oxidative stress, were selected. The components were divided into four groups: (1) dietary antioxidants (selenium, fiber, β-carotene, vitamin D, vitamin C, vitamin E, and folate); (2) dietary prooxidants (iron, saturated (SFA), and polyunsaturated (PUFA) fatty acids); (3) nondietary antioxidant (physical activity); and (4) nondietary prooxidants (smoking and obesity). Dietary factors were ranked into quintiles. For dietary antioxidants and physical activity, the first to fifth quintiles were assigned scores of 1–5. An inverse scoring was used for dietary prooxidants. For obesity, we assigned 1: BMI ≥ 30 kg/m2 and WC ≥ 102 cm in males and ≥ 88 cm in females, 3: either BMI ≥ 30 kg/m2 or WC ≥ 102 cm in males or ≥ 88 cm in females, and 5: BMI < 30 kg/m2 and WC < 102 in males or < 88 cm in females. For smoking, it was assigned 1: current smoking, 3: former smoking, and 5: never smoking. The score of four components was then summed to calculate the OBS for each participant. A higher score of OBS indicates more adherence to this score derived from diet and a lower score indicates less adherence to this score. The minimum and maximum scores possible are, respectively, 5 and 65.
Biochemical measurement:
The laboratory technician took 10 ml of venous blood from the individuals at the beginning and end of the trial, following 10–12 h of fasting. After clotting in the environment, the serum was quickly separated by centrifugation and stored at −70 °C until it was transported to the laboratory for testing. The concentrations of triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and fasting blood sugare (FBS) were determined using a kit from Pars Azmon Company (Tehran, Iran) using an enzymatic colorimetric technique. Enzyme photometry was used with the Pars test kit (Parsazmun, Tehran, Iran) to determine the total cholesterol content. Using the Friedewald formula35, the concentration of low-density lipoprotein cholesterol (LDL-C) was also determined. LDL-C = TC (mg/dL) − HDL-C (mg/dL) − TG (mg/dL)/5. On the basis of an auto analysis (BT-3000)., the enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using commercially available enzymatic reagents (Pars Azmoon, Tehran, Iran).
Statistical analysis
The Statistical Package Software for Social Science v.21 (SPSS Inc., Chicago, IL, USA) was used to conduct the statistical analysis. The data's normality was examined using the Kolmogorov–Smirnov’s test and histogram charts. The baseline characteristics and dietary intakes were recorded as mean standard deviation (SD) for quantitative variables, and for qualitative variables, as number and percentages. We used independent sample t-tests (or one-way ANOVA) and chi-squared tests to compare data between two groups (or across quartiles of OBS) for continuous and categorical variables, respectively. Nutrients were adjusted for total energy intake (kcal) using the residual method. Logistic regression was used to examine the relationship between OBS and the risk of NAFLD. The analyses were adjusted for potential confounders such as age, sex, hip circumference, education, drug use, disease history, FBS, ALT, AST, Lipid profiles, and energy intake. The odds ratio (OR) of NAFLD across quartiles of scores was estimated with a 95% confidence interval (CI). P-values < 0.05 were considered statistically significant.
Ethics approval and consent to participate
This study was approved by the research council and ethics committee Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Results
The mean (± SD) age of the study population was 40.22 ± 9.79 years. The mean (± SD) BMI was 29.06 ± 3.92 kg/m2. The mean ± SD of OBS was 41.48 ± 5.23.
Table 1 illustrates the general characteristics and biochemical parameters of participants between NAFLD patients and control groups as well as across the quartile of OBS. Compared with controls, NAFLD subjects had significantly higher hip circumference, ALT, AST, FBS, TC, TG, and LDL-C concentration, but had lower physical activity and mean of OBS. There was also a significant difference between the level of education, diseases history, drug use, and smoking between the case and control groups. The mean age of subjects in the highest quartile of OBS vs the lowest quartile, significantly decreased. Also, the mean BMI, weight, WC, Hip- circumference, as well as use of smoking, drug, and disease history decreased across the quartile of OBS, but physical activity increased. In addition, a significant difference was observed between the levels of education among the quartile of OBS. There were no significant differences between the quartile of OBS and rest of variables.
Dietary intake of subjects between groups and across the quartile of OBS are presented in Table 2. NAFLD subjects had higher intakes of energy, protein, saturated fatty acid (SFA), and red and processed meats, but lower intakes of calcium, magnesium, folate, vitamin D, legume, whole grain, fruits, and vegetables as compared to controls. Compared with those in the lowest quartile of OBS, subjects in the highest quartile had higher intake of energy, carbohydrate, fiber, calcium, magnesium, zinc, vitamin C, E, D, B9, total dairy, whole grains, fruits, and vegetables as well as lower intake of protein, fat, monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), cholesterol, sodium, iron, nuts, and red and processed meat.
The ORs and 95% CIs for NAFLD subjects based on quartile of OBS of are reported in Table 3.
In crude and first adjusted model (based on age and sex), there was a significant association for OBS in the highest quartile compared with the lowest quartile (odds ratio [OR] = 0.35, 95% confidence interval [CI] 0.21–0.56; P for trend < 0.001; OR = 0.38, 95% CI 0.26–0.63; P for trend < 0.001, respectively). Furthermore, after adjusting for confounders according to the final model (based on model 1 + hip circumference, education, drug use, disease history, FBS, ALT, AST, Lipid profiles, and energy intake), higher scores of adherence to the OBS conferred a protection for the presence of NAFLD (OR: 0.29; 95% CI 0.15–0.49; P for trend < 0.001).
Discussion
This study examined the link between an OBS and NAFLD risk in 340 NAFLD patients and 552 controls, all of whom were men and women over the age of 18 who visited a hospital in Tehran or Yazd, Iran. We used a previously used score in other studies that is based on a remarkable compilation of 13 components that extensively assess exposure through dietary and lifestyle-related antioxidant and pro-oxidant components, allowing us to approach the complexity of individual oxidative balance assessment comprehensively. After controlling for relevant confounders, our data revealed a statistically significant strong inverse relationship between OBS score and decreased NAFLD risk. So that the findings show a protective effect on NAFLD following OBS adherence.
To our knowledge, this is the first study to investigate the OBS as a protective factor against NAFLD. However, several studies have investigated the potential beneficial effects of this index on other chronic diseases such as metabolic syndrome36, diabetes37, cancer38, kidney39,40, and cardiovascular diseases40 which seemed to have a similar mechanism to the risk of NAFLD. In addition, the relationship between this index with the reduction of oxidative stress and systemic inflammation has been investigated as a major risk factor in NAFLD41. So that a study in 2019 by Golmohammadi et al37 showed that higher compliance with OBS, which indicates increased exposure to antioxidants and decreased exposure to prooxidants, improved insulin resistance as well as better glycemic control in adults with type 2 diabetes. A cross-sectional study conducted among 6,400 Korean adults over 40 years of age showed that compared to the lowest tertile, those in the highest tertile of OBS had a 35% lower risk of developing metabolic syndrome after adjustment for potential confounders36. In addition, in a study, increased adherence to OBS was associated with a decreased incidence of end-stage renal disease (ESRD)39. However, in another study, no significant relationship between this index and the incidence of ESRD and cardiovascular disease was observed after adjustment of confounders40, which according to the author's statement could be due to the specific dietary restrictions of these patients at the time of study entry.
In a study by Kong et al.41, the relationship between OBS and markers of oxidative stress and inflammation was investigated. The findings of this study indicated that people with higher OBS adherence had a decrease in the levels of F2-isoprostanes (FIP) and C-reactive protein (CRP) by 80% and 40%, respectivcely, compared to people with lower adherence to this index.
Furthermore, evidence shows that micronutrients with antioxidant, antifibrotic, immunomodulatory, and lipoprotective capabilities, such as vitamins A, C, D, and E, carotenoids, zinc, selenium, and magnesium, may have favorable effects on NAFLD42. On the other hand, the findings of the present case–control study are consistent with recent studies and evidence on the protective role of healthy dietary patterns. These dietary patterns, characterized by high intake of fruits, vegetables, legumes, and low-fat dairy products, which are rich in antioxidant nutrients and with minimal prooxidants, can reduce the incidence of NAFLD43. The formation and progression of NAFLD are both influenced by poor eating, which is defined as a diet high in calories, carbohydrates, and saturated fats and lacking in fiber, MUFA, and antioxidant micronutrients44. Additionally, a systematic review and meta-analysis of the available data showed that western dietary patterns with high intakes of processed foods, red meat, high-fat dairy products, and refined grains with minimal intake of antioxidant nutrients and rich in prooxidants might considerably increase the likelihood of developing NAFLD (OR 1.56; 95% CI 1.27–1.92). Furthermore, research revealed that cooking beef at high temperatures for an extended period of time produces heterocyclic amines (HCAs), which are linked to oxidative stress and NAFLD45.
It is also important to note that lifestyle as one of the components of OBS can also play an important role in the incidence of NAFLD in our study. This suggests that each of the healthy lifestyle variables, such as physical exercise, an appropriate BMI, and quitting smoking, played a significant effect in avoiding NAFLD in addition to nutrition. Obesity, a sedentary lifestyle, smoking, as well as other individual variables and environmental factors, have been demonstrated to impact NAFLD46.
The study offers both advantages and disadvantages. One of the study's shortcomings is that it is unable to evaluate the causal connection. Another drawback is that, despite accounting for a number of potential confounding factors, it is impossible to rule out the chance that any more potential confounding factors may exist that were not taken into account in the studies. Self-reported data on food consumption might also lead to memory bias. Another weakness of this study may be that because the subjects were Muslims, alcohol intake was not examined. A review of this OBS component, though, would have no impact on the outcomes because neither the control group nor the study case group drank alcohol.
The recent research has a number of advantages. This is the first study that we are aware of that evaluated the relationship between OBS and the risk of NAFLD in Iranian adults. Trained workers were used to conduct interviews and gather food frequency questionnaires. The sample size we used was adequate, and we made an effort to account for a variety of confounding factors by utilizing a validated questionnaire and large-scale variable adjustments.
In conclusion, by calculating the study power of approximately 90%, the findings of the present study indicate an approximately 80% reduction in the odds of developing NAFLD with higher OBS adherence. Our findings confirm earlier research on the prevention of NAFLD by good food and lifestyle choices. Additionally, they can be used as tactics to stop or even slow the progression of NAFLD.
Data availability
Data is available upon request from the corresponding author for the article due to privacy/ethical restrictions.
References
Anania, C., Perla, F. M., Olivero, F., Pacifico, L. & Chiesa, C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 24, 2083–2094. https://doi.org/10.3748/wjg.v24.i19.2083 (2018).
Williams, C. D. et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140, 124–131. https://doi.org/10.1053/j.gastro.2010.09.038 (2011).
Lankarani, K. B. et al. Non alcoholic fatty liver disease in southern Iran: A population based study. Hepat. Mon. 13, e9248. https://doi.org/10.5812/hepatmon.9248 (2013).
Browning, J. D. et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 40, 1387–1395. https://doi.org/10.1002/hep.20466 (2004).
Younossi, Z. M. et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 64, 1577–1586. https://doi.org/10.1002/hep.28785 (2016).
Giraldi, L. et al. Mediterranean diet and the prevention of non-alcoholic fatty liver disease: Results from a case-control study. Eur. Rev. Med. Pharmacol. Sci. 24, 7391–7398. https://doi.org/10.26355/eurrev_202007_21907 (2020).
Younossi, Z. et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018).
Yilmaz, Y. In Seminars in Liver Disease 14–21.
Paik, J. M. et al. Dietary risks for liver mortality in NAFLD: global burden of disease data. Hepatol. Commun. 6, 90–100 (2022).
Serafini, M. & Del Rio, D. Understanding the association between dietary antioxidants, redox status and disease: Is the total antioxidant capacity the right tool?. Redox Rep. 9, 145–152 (2004).
Meydani, M. Dietary antioxidants modulation of aging and immune-endothelial cell interaction. Mech. Ageing Dev. 111, 123–132 (1999).
Ashraf, N. & Sheikh, T. Endoplasmic reticulum stress and oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Free Radic. Res. 49, 1405–1418 (2015).
Cichoż-Lach, H. & Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. WJG 20, 8082 (2014).
Van Hoydonck, P. G., Temme, E. H. & Schouten, E. G. A dietary oxidative balance score of vitamin C, beta-carotene and iron intakes and mortality risk in male smoking Belgians. J. Nutr. 132, 756–761. https://doi.org/10.1093/jn/132.4.756 (2002).
Goodman, M., Bostick, R. M., Dash, C., Flanders, W. D. & Mandel, J. S. Hypothesis: Oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann. Epidemiol. 17, 394–399. https://doi.org/10.1016/j.annepidem.2007.01.034 (2007).
Goodman, M. et al. Combined measure of pro- and anti-oxidant exposures in relation to prostate cancer and colorectal adenoma risk: An update. Ann. Epidemiol. 20, 955–957. https://doi.org/10.1016/j.annepidem.2010.08.011 (2010).
Lakkur, S. et al. Oxidative balance score and risk for incident prostate cancer in a prospective U.S. cohort study. Ann. Epidemiol. 24, 475-478.e474. https://doi.org/10.1016/j.annepidem.2014.02.015 (2014).
Hernández-Ruiz, Á. et al. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients 11, 774. https://doi.org/10.3390/nu11040774 (2019).
Oliveira, C. P. et al. Vitamin C and vitamin E in prevention of nonalcoholic fatty liver disease (NAFLD) in choline deficient diet fed rats. Nutr. J. 2, 1–5 (2003).
Ji, H.-F., Sun, Y. & Shen, L. Effect of vitamin E supplementation on aminotransferase levels in patients with NAFLD, NASH, and CHC: Results from a meta-analysis. Nutrition 30, 986–991 (2014).
Ipsen, D. H., Tveden-Nyborg, P. & Lykkesfeldt, J. Does vitamin C deficiency promote fatty liver disease development?. Nutrients 6, 5473–5499 (2014).
Mosca, A. et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig. Liver Dis. 53, 1154–1158 (2021).
Burke, A. & FitzGerald, G. A. Oxidative stress and smoking-induced vascular injury. Prog. Cardiovasc. Dis. 46, 79–90 (2003).
Liu, Y. et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): A population-based study in China. J. Epidemiol. 23, 115–121 (2013).
Hopps, E., Noto, D., Caimi, G. & Averna, M. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 20, 72–77 (2010).
Cheng, Y. et al. Associations between dietary nutrient intakes and hepatic lipid contents in NAFLD patients quantified by 1H-MRS and dual-Echo MRI. Nutrients 8, 527 (2016).
Targher, G. & Byrne, C. D. Metabolically healthy obesity and NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 442–444 (2016).
Yamamura, S. et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 40, 3018–3030. https://doi.org/10.1111/liv.14675 (2020).
Piazzolla, V. A. & Mangia, A. Noninvasive diagnosis of NAFLD and NASH. Cells 9, 1005. https://doi.org/10.3390/cells9041005 (2020).
Semmler, G. et al. Novel reliability criteria for controlled attenuation parameter assessments for non-invasive evaluation of hepatic steatosis. United Eur. Gastroenterol. J. 8, 321–331. https://doi.org/10.1177/2050640619900820 (2020).
Mirmiran, P., Esfahani, F. H., Mehrabi, Y., Hedayati, M. & Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 13, 654–662. https://doi.org/10.1017/s1368980009991698 (2010).
Ghafarpour, M., Houshiar-Rad, A., Kianfar, H. & Ghaffarpour, M. (Tehran: Keshavarzi Press, 1999).
Bowman, S. A., Friday, J. E. & Moshfegh, A. J. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004: Documentation and User Guide. (US Department of Agriculture, 2008).
Goodman, M. et al. Combined measure of pro-and anti-oxidant exposures in relation to prostate cancer and colorectal adenoma risk: An update. Ann. Epidemiol. 20, 955–957 (2010).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 (1972).
Lee, H.-S. & Park, T. Pathway-driven approaches of interaction between oxidative balance and genetic polymorphism on metabolic syndrome. Oxid. Med. Cell. Longev. 2017, 1–9 (2017).
Golmohammadi, M., Ayremlou, P. & Zarrin, R. Higher oxidative balance score is associated with better glycemic control among Iranian adults with type-2 diabetes. Int. J. Vitam. Nutr. Res. 91, 31–39 (2019).
Sohouli, M. H. et al. Adherence to oxidative balance scores is associated with a reduced risk of breast cancer. A case-control study. Nutr. Cancer 75, 164–173 (2022).
Ilori, T. O. et al. Oxidative balance score and chronic kidney disease. Am. J. Nephrol. 42, 320–327 (2015).
Ilori, T. O. et al. Oxidative balance score and the risk of end-stage renal disease and cardiovascular disease. Am. J. Nephrol. 45, 338–345 (2017).
Kong, S. Y. J. et al. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammationoxidative balance score and markers of oxidative stress. Cancer Epidemiol. Biomark. Prev. 23, 545–554 (2014).
Berná, G. & Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 40(Suppl 1), 102–108. https://doi.org/10.1111/liv.14360 (2020).
Tutunchi, H., Saghafi-Asl, M., Asghari-Jafarabadi, M. & Ostadrahimi, A. Association between dietary patterns and non-alcoholic fatty liver disease: Results from a case-control study. Arch. Iran. Med. 24, 35–42. https://doi.org/10.34172/aim.2021.06 (2021).
Vancells Lujan, P., ViñasEsmel, E. & SacanellaMeseguer, E. Overview of non-alcoholic fatty liver disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients 13, 1442. https://doi.org/10.3390/nu13051442 (2021).
Zelber-Sagi, S. et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 68, 1239–1246 (2018).
Juanola, O., Martínez-López, S., Francés, R. & Gómez-Hurtado, I. Non-Alcoholic fatty liver disease: Metabolic, genetic, epigenetic and environmental risk factors. Int. J. Environ. Res. Public Health 18, 5227. https://doi.org/10.3390/ijerph18105227 (2021).
Acknowledgements
We thank the Student Research Committee and the Research & Technology Chancellor of Shahid Beheshti University of Medical Sciences for their financial support of this study.
Author information
Authors and Affiliations
Contributions
A.H, and Mh.S contributed in conception, design, and statistical analysis. Mh.S, P.R., M.H, and A.H contributed in data collection and manuscript drafting. Mh.S and A.H supervised the study. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sohouli, M.H., Rohani, P., Hosseinzadeh, M. et al. Adherence to oxidative balance scores and lower odds of non-alcoholic fatty liver disease: a case–control study. Sci Rep 13, 6140 (2023). https://doi.org/10.1038/s41598-023-33407-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33407-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.