Abstract
Knowledge on the spatial ecology of invasive predators positively contributes to optimizing their management, especially when involving cryptic and secretive species, such as snakes. However, this information is lacking for most invasive snakes, particularly on islands, where they are known to cause severe ecological and socio-economic impacts. This research is focused on assessing the spatial ecology of the California kingsnake (Lampropeltis californiae) on Gran Canaria to strengthen management actions. We monitored 15 radio-tagged individuals once per day on 9–11 days per month from July 2020 to June 2021 to calculate the species' home range and describe annual activity patterns in the invaded range. To account for the species' diel activity during the emergence period, we additionally monitored snakes from January to May 2021 during three consecutive days per month in four different time intervals each day. We detected movement (consecutive detections at least 6 m apart) in 31.68% of the 1146 detections during the whole monitoring period. Movements most frequently detected were shorter than 100 m (82.24%), and among them the range 0–20 m was the most recurrent (27.03%). The mean distance of movement was 62.57 ± 62.62 m in 1–2 days. Average home range was 4.27 ± 5.35 ha—calculated with the Autocorrelated Kernel Density Estimator (AKDE) at 95%—and did not significantly vary with SVL nor sex. We detected an extremely low value of motion variance (0.76 ± 2.62 σ2m) compared to other studies, with a general inactivity period from November to February, January being the less active month of the year. Diel activity was higher during central and evening hours than during early morning and night. Our results should be useful to improve control programs for this invasive snake (e.g., trap placement and visual survey guidance) on Gran Canaria. Our research highlights the importance of gathering spatial information on invasive snakes to enhance control actions, which can contribute to the management of secretive invasive snakes worldwide.
Similar content being viewed by others
Introduction
Biological invasions have a broad range of impacts1 that are particularly harsh on islands2,3. Oceanic islands, priority areas for global biodiversity conservation4, host a great proportion of endemism5,6, while being extremely vulnerable to invasive species, especially predators2,5. Therefore, predator control or eradication on islands can provide numerous ecological benefits to insular ecosystems (e.g., Jones et al.7). However, invasive species management remains a complex task8, as its success and optimization depends on the available data about target species.
Information on the biology and ecology of invasive predators can be crucial to develop control strategies and action plans8,9, but particularly home range, activity patterns, or habitat use10,11 can facilitate more effective trapping12,13. This strategic information becomes even more relevant to organisms difficult to manage, like snakes14,15, which show extremely low detectability, secretive behavior, cryptic coloration, sporadic activity patterns, or use inaccessible habitats16,17,18. As invasive snakes are becoming increasingly recognized as a major threat to biodiversity on numerous islands worldwide19,20, understanding their spatial ecology is key to optimizing control actions10,21,22. However, such important details remain unknown or poorly studied for most of the world’s invasive snakes.
The present research focuses on the California kingsnake (Lampropeltis californiae), a medium-sized colubrid native to western United States and northern Mexico23, which was detected in 1998 on Gran Canaria (Canary Islands, Spain)24. Since then, the Canary Islands' and Gran Canaria governments have led yearly control actions, based on visual surveys and trapping from March to August each year24,25, yet the species is still expanding its invaded area25. Biological information on this species is scarce and mostly comes from its native range, where it is a diurnal, generalist and wide-foraging predator that feeds on terrestrial vertebrates—mainly snakes, lizards, mammals and birds26. In California, L. californiae has a relatively small home range (5.45 ± 5.97 ha) and a sporadic and marked seasonal activity, with a peak in spring27,28. This snake generally emerge from brumation in mid-February to early March27,28. Individuals remain underground most of the time27 in rodent burrows, under rocks, human structures or logs28. Activity generally occurs during the day, although it depends on daytime temperatures28. During warm days, they may only be active at night, while milder days allow morning or late afternoon activity28. On Gran Canaria, L. californiae usually preys on the three endemic reptiles of the island, along with rodents and followed by birds29,30, causing a severe impact on the herpetofauna and ecosystems31,32. A similar phenology to that of the native range can be inferred from the number of captures in the invaded range, which increases from March to May24,25. However, a scientific understanding of the spatial ecology of L. californiae in Gran Canaria is lacking but needed, since invasive species are known to shift their ecology or behavior once established in new environments33,34, and this information is essential to guide control actions8.
In this context, our study aimed to provide basic spatial ecology information, estimated home range and described year-round phenology and diel activity of L. californiae on Gran Canaria. We also analyzed whether home range and year phenology depended on body size or sex, and explored differences in diel activity between sexes and months. These comparisons are not only interesting from an ecological point of view, but can also inform managers to improve the design of control actions. Notably, they could focus on increasing captures of sexes or sizes that are more infrequently collected (i.e., females and small or large-sized individuals24,25). Exploring diel activity more in depth should also reveal the most appropriate hours to conduct visual surveys. Based on reports from the native range, we hypothesized that L. californiae should show overall small home ranges varying between sexes, males showing larger areas. We also anticipated a marked seasonal and diurnal activity—spring and the warmest hours of the day being the periods with higher activity—but with a shorter torpor period than in the native range, due to the mild weather of Gran Canaria all year round. Our research should be useful to guide managers in designing more efficient control actions to fight against this pernicious invader. From a global perspective, we expect our research to highlight the value of movement ecology to improve the management of secretive invasive predators.
Materials and methods
Experimental design: study area and sample size
Aiming to conduct a year-round monitoring of L. californiae spatial behavior, we located our study area in Amagro Mountain Natural Monument (NW Gran Canaria), invaded since 201024, and where there were no anthropic infrastructures that would limit our monitoring or affect snake spatial behavior35.
In order to set our sample size, we had to adjust it to logistic and economic constraints, as well as to the number of snakes captured in Amagro and their weights. We only selected snakes captured in this area by the control staff between February and June 2020 to prevent homing behavioral effects36, and weighing over c. 180 g—transmitters (SI-2 9g, Holohil Systems, Ltd., Ontario, Canada) could only make up < 5% of animal weight37. We finally tagged 15 adult L. californiae individuals, measured their snout-to-vent length (SVL) with a measuring tape, and sexed them using a probe38. They comprised 6 males and 9 non-gravid females (mean ± SD SVL: 96.62 ± 11.05 cm; min–max SVL: 80–121 cm), all showing normal coloration28,39. All individuals were kept in captivity in appropriate conditions until surgical insertion of transmitters (all cases between July 4th–6th 2020), with a food intake the previous week. Surgery was carried out by an experienced veterinarian, following a protocol adapted from Melián40 (see Supplementary Material 1). Veterinarian post-surgery care lasted for 48–72 h. All individuals survived surgery, so we released them back into the wild in their capture location or its vicinity.
Field tracking
To estimate home range and describe the species phenology, we tracked individuals from July 2020 to June 2021. This one-year survey involved one field tracking session per month, each of 9–11 consecutive days, with c. 15 days between sessions, detecting all individuals once per day. Complementarily, to confirm activity during night-time, early morning or dusk, as suggested by Hubbs28, we analyzed diel activity following an experimental design adjusted from Abom et al.41. Thus, we detected individuals four times a day during three consecutive days (starting on the third day of tracking described above), only from January 2021 to May 2021—i.e., emergence period until the number of captures starts to decrease25. Each day we detected individuals at the beginning of the following periods: (1) early morning (7:30–10:30), (2) central hours (10:30–17:30), (3) evening (17:30–20:30), and (4) night (20:30–7:30) (see Abom et al.41). Period duration is approximate, as we based session times on sunrise and sunset time each month.
To locate each individual exactly, a team of 2–3 people, each provided with a Biotrack three-element Yagi antenna coupled to a Biotrack Sika handheld receiver (Biotrack, UK), followed the signal until the 2–3 antennas coincided at the same spot. We recorded detections with a 5G GPS cell phone at c. 2 m precision, using the area aerial photograph in the QField App (The QField Project/OPENGIS.ch 2019). For each detection, we recorded whether each individual was on the surface (basking or moving) or sheltered.
Data analyses
Data preparation
To estimate home range and describe the species phenology, we discarded the July 2020 data to avoid the collateral effects of surgery on movement behavior42. We defined movement as consecutive detections at least 6 m apart. An individual was assumed dead when it was detected in the same place for a long time (c. 1 month, excluding the brumation period, c. from November to February) until the end of the monitoring, assigning the date of the first detection at that place as the final detection date for subsequent analyses. Particularly for the diel activity analysis, we considered that an animal was active not only when it was located at least 6 m apart from the previous detection, but also when it was on the surface (informative of the opportunity to capture snakes for control purposes).
Tracking summary and basic spatial information
We extracted information on days that we tracked individuals, battery life, number of detections (basking/moving vs. sheltered), the percentage of detections that reflected movement, types of movements (classified by distance categories following Anguiano and Diffendorfer27), mean and maximum distance covered in 1–2 days, maximum time without performing movements, and frequency of movements. We calculated all distances as Euclidean.
Home range calculation
We calculated home range values with Autocorrelated Kernel Density Estimators (AKDE)43, a computation aid that takes into account tracking data autocorrelation while robustly dealing with data gaps and irregular sampling schedules44. To fit movement models, we used the ctmm v.0.6.1 package45, with the perturbative hybrid REML method (pHREML), and AICc to select the best fitting model46. Using the function ‘akde’ in the ctmm package45, we estimated weighted AKDE home range areas at the 95% contour, due to heterogeneous periods between samplings44. To check for AKDE's assumptions on range residency47, we first built up individual variograms using the ggplot2 v.3.35 package48 and visually decided which ones we considered were stable. Secondly, we explored the effective sample size of all individuals (DOF area resulting from the ‘akde’ function) and retained for home range calculations only those with a value greater than 10 (following Montano et al.49). We also calculated home range using the Minimum Convex Polygon (MCP)50 (100%, 95%, and 50%) and Kernel Density Estimators (KDE)51 (95% and 50%) to allow comparisons with previous studies. However, we did not perform further analyses with these estimates as both are currently considered inappropriate44 (see Supplementary Material 2 for MCP and KDE results).
Species phenology
We analyzed activity patterns throughout the year via the motion variance parameter (a measurement of animal movement intensity) calculated with dynamic Brownian Bridge Movement Models (dBBMMs)52 using the move package v.4.0.653. We set window size and margin parameters to 7 and 3 telemetry detections (7 and 3 days in our case), respectively, based on the typical movements of individuals (snakes moved every c. 3 days) and our sampling regime, as suggested by Kranstauber et al.52. Analysis of motion variance informs about behavioral changes across a tracking period, detailing how straight a movement path is, as well as how much a path varies in speed, and the scale of movements in a time window52. Motion variance is high when animals are active and their path is irregular, but low when animals are inactive and/or follow a regular path52.
Effect of SVL, sex and month in the species spatial ecology
To analyze the influence of SVL and sex on home range and motion variance, we performed Spearman’s correlation tests and Kruskal–Wallis tests54, respectively. We first checked home range and motion variance normality following the methods of Zuur et al.55, and the homogeneity of the variance for males and females with Levene's tests56. For motion variance, we additionally quantified individual variation with a repeatability estimation test57 using the rptR v. 0.9.22 package58, and seasonal patterns using month as the explanatory variable in a Welch's heteroscedastic F Test with trimmed means (rate set at 0.1) and Winsorized variances59 performed on the onewaytest package60.
We assessed snakes’ diel activity with a Generalized Linear Mixed-Effects Model (GLMM) using a binomial error distribution for activity occurrence, including sex, month, and period of day as fixed factors, and the individual as a random factor. We also tested the interaction between month and period of day as it was our primary interest, but discarded it due to convergence issues. After verifying model assumptions—i.e., homogeneity of variance, normality and dispersion of residuals—with DHARMa v.0.4.3 package61, we retrieved the models' main effects using type-II Wald Chi square tests performed with ‘Anova’ function62, conducting post hoc analyses with the emmeans v.1.7.0 package63 to obtain differences between each factor level.
We performed all analyses using R v.4.1.164, presenting all results as mean ± SD.
Ethical statement
Each procedure complied with relevant laws and institutional guidelines. Experimental protocols were all approved by the University of La Laguna Ethical Committee (Authorization no. 2978440) and later authorized by the competent institution, the Government of the Canary Islands (Authorization no. 133/2020 and 159/2021). All experiments complied with the ARRIVE guidelines (https://arriveguidelines.org). Thirty minutes before surgery, each individual received morphine as anesthetic agent, and IM medetomidine and IV alphaxalone as an anesthesia inducing agent (see Supplementary Material 1). No animal was sacrificed during this study.
Results
Tracking summary of Lampropeltis californiae on Gran Canaria
We tracked individuals for a mean of 220.20 ± 96.56 days (Table 1). We lost 13% of transmitter signals in March 2021 and 33.33% in both April and May 2021, with only three transmitters remaining active in June 2021 (one of them inserted in an individual that was dead by that time). Average transmitter battery life was 302 ± 23.36 days (12 months, ranging 6–18 months, according to the manufacturer; Holohil Systems, Ltd., Ontario, Canada). Four individuals died during our monitoring (310, 550, 670 and 930), two of which (310 and 550) were excluded from all analyses as they stopped moving in August and September 2020, respectively (Table 1). We added individual 670 to all analyses as it died in March 2021 and accumulated enough data, while individual 930 was only included in motion variance analysis as it did not show enough effective sample size.
Monitoring resulted in a total of 1146 detections for all 15 individuals (76.40 ± 33.69 per individual; Table 1), 31.68% of which occurred after a movement (> 6 m) (Table 1). Movements most frequently detected were shorter than 100 m (82.24%), and among them the range 0–20 m was the most recurrent (27.03%) (Supplementary Material 3, Table S3.1). Mean distance covered in 1–2 days by individuals during the entire period was 62.57 ± 62.62 m, with the maximum distance covered by an individual in one day being 438.09 m (Table 1). Maximum time without moving (> 6 m) averaged 61.38 ± 41.90 consecutive days (min–max: 3–122 days), occurring between November and February for all individuals, although we detected short movements (2–6 m) all through the tracking period (Table 1). After brumation, movement usually occurred in blocks of consecutive days, averaging 2.57 ± 1.70 days (min–max: 1–6 days). Animals were sheltered in 95.99% of the detections.
Home range
We considered that all individuals’ variograms were stable (Fig. 1). However, the effective sample size of four individuals was exceptionally low (< 10; 010, 590, 930, 950) (Table 2), so we excluded them from home range analyses. Average L. californiae AKDE 95% contour home range was 4.27 ± 5.35 ha (males: 2.86 ± 2.52 ha, females: 5.40 ± 6.99 ha; Table 2, Fig. 2). There was no correlation between home range and SVL (rS = -0.37, P = 0.323), nor significant difference between sexes (\(\upchi_{1}^{2}\)= 0.24, P = 0.624).
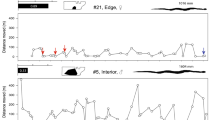
Variograms of the semi-variance at 50% (dark) and 95% (light) confidence intervals of each individual of Lampropeltis californiae in Gran Canaria. We show individuals remaining in the analyses in blue and those discarded in coral. We also indicate each individual's sex. We do not present information on dead 310 and 550, as we discarded them from all analyses.
Estimates of the 50% (dark) and 95% (light) AKDE home range contours of each individual of Lampropeltis californiae in Gran Canaria. We show individuals remaining in the analyses in blue and those discarded in coral. Dots represent all detections of each animal. The whole scale bar represents 200 m in each representation. Last section shows 95% AKDE home range contours of all individuals, with discarded individuals in coral. We do not show information on dead 310 and 550, as we discarded them from all analyses.
Activity patterns
Average motion variance was 0.76 ± 2.62 σ2m during the whole period, although individuals exhibited consistent and significant differences (R = 0.09, CI = 0.03–0.17, P < 0.005). No significant correlation existed between motion variance and SVL (rS = − 0.016, P = 0.608), nor differences between sexes (males: 0.62 ± 2.29 m, females: 0.85 ± 2.81 m; \(\upchi_{1}^{2}\) = 1.25, P = 0.263). Motion variance significantly differed among months (F9, 210.78 = 8.45, P < 0.005; Fig. 3).
Motion variance values (σ2m) along the whole year tracking period colored by individuals. Black line represents the mean value of motion variance for all individuals. Individuals with a motion variance close to the mean are shown in A, whereas B shows those with higher values. Individuals discarded for this analysis (310 and 550) are not shown.
Results from the GLMM revealed that diel activity was significantly affected by sex (\(\upchi_{1}^{2}\) = 4.26, P = 0.039), month (\(\upchi_{1}^{2}\) = 20.03, P < 0.005), and also period of day (\(\upchi_{1}^{2}\) = 29.51, P < 0.005) during the intense monitoring (January-May 2021). Females were more frequently active (basking/moving) than males (15.68% female detections and 8.55% male detections) (Fig. 4A). Post-hoc comparisons between months showed that January was the month with a significantly lower activity (P < 0.05 for all comparisons: Fig. 4B). Snakes were significantly more active during the central hours of the day and the evening than in the early morning and night (P < 0.05 for all comparisons; Fig. 4C).
Discussion
Our research provides essential information on the spatial ecology of the invasive L. californiae on Gran Canaria, which contributes to guide control actions for this pernicious invader. Home range data can be incorporated into designing control actions to manage invasive terrestrial vertebrates10,12,65, and are key to defining the density and distribution of traps13. On Gran Canaria, trap placement did not follow a specific density and distribution. Extracted from our home range calculation, to maximize the probability of a snake encountering a trap, these should not be separated by more than the minimum range detected, 104 m (diameter of a 0.85 ha circle, assuming a circle to be the most parsimonious design for a home range area; see Roy et al.66 for this calculation). Since this action is resource-consuming, trap separation could alternatively be no more than 233 m, based on the average home range detected (4.27 ± 5.35 ha). In relation to trap layout and based on previous studies67,68, we recommend using grids or linear designs, particularly in biodiversity-sensitive areas of Gran Canaria or along the expansion front. The use of drift-fences can also increase the probability of capture69. Distance traveled in a day can be used to infer the width of control buffers to prevent invasion of adjacent areas. Given that most movements detected were smaller than 100 m, we recommend a containment buffer width of 100 m. These recommendations can be improved in the future by calculating trap distance using simulations65 or the most cost-effective trap arrangement70,71. Control staff can also incorporate snake density information, as it could influence individual home range12,35.
Lampropeltis californiae phenology on Gran Canaria is highly seasonal, with a brumation period from late November to early February, which coincides with information reported for the island/coastal regions of its native range28. The species is active the rest of the year, with an activity peak between March and May, presumably linked to the breeding season30. Each year, environmental managers have reinforced control actions by hiring extra personnel between March and August. Following our results, this reinforcement should be extended from early February to mid-November, increasing efforts before the breeding season and until the end of the activity period. From an ecological perspective, the average motion variance of L. californiae, a wide-foraging predator26 (0.76 ± 2.62 σ2m, mean ± SD), is lower than in other active-searching or even ambush predators (mean ± SE for active predators: Ophiophagus hannah: 27.9 ± 0.6 m72, Boiga cyanea: 2.8 ± 0.8 σ2m73; mean ± SE for an ambush predator: Python bivittatus: 2.66 ± 0.14 m74). This seems to indicate that the species continuously forages until finding prey and then shelters and remains stationary for several days, lying dormant until it surfaces again to prey—which coincides with our findings that movements usually occurred in blocks of consecutive days. This result can be applied to management when a snake is detected but not captured, because the animal will probably remain in the same shelter/area for 2–3 days. Consequently, prospection of the surrounding area during that period could increase the probability of capture. In addition, as a notable amount of movements were short, intense visual surveys in the proximity of fresh snake tracks (e.g., scats or shed skins) for a similar amount of days can be also appropriate. Finally, it is worth noting that, since individuals were sheltered in most of the detections, the development of novel techniques to detect animals while immobile or sheltered is crucial to improve control success67,75,76,77. Due to visual surveys being extremely time- and resource-consuming15 for such a secretive snake, increasing detection on surface still requires further technological advances—e.g., remote sensing techniques78.
Diel activity analysis showed that females were more frequently active than males between January and May 2021, potentially due to their different behaviors during the breeding season (i.e., feeding, basking)79 or the associated reproductive costs of breeding for females80. This strengthens our previous recommendation to reinforce control staff from mid-February onwards to increase the probability of capturing females before reproduction begins. We also determined that L. californiae is mainly active during central hours and the evening, although a certain proportion of activity occurred at night during normal weather (15.40% of all activity detected), and even in the rain. To link visual surveys to species activity pattern81, the former should be made during central hours and evenings.
The overall spatial parameters studied for L. californiae on Gran Canaria showed a wide variation. This disparity may be explained mainly by the effect of individual heterogeneity on spatial behavior, already demonstrated for other invasive snake species82. This spatial heterogeneity can be due to individual body condition affecting movement ecology83, individual personality influencing exploratory and defensive behaviors84 or boldness and sociability85, as well as dispersive movements (such as may have happened with our individual 010). In addition, although some deaths are expected in this type of studies (e.g., due to tagging surgery, predators, health conditionsee 27,73,86), we noted a higher death rate than expected. This reduced our final sample size, and possibly skewed the results obtained in some comparisons, mainly regarding sexes. Finally, several ecological parameters can also influence animal spatial behavior (for instance density, prey and shelter availability, degree of anthropization, habitat type12,35,87). We are however highly confident that our results provide robust knowledge on home range, phenology and activity patterns of L. californiae, which can be used to improve control action designs for the whole island of Gran Canaria and possibly elsewhere.
Technological progress is still needed to facilitate the acquisition of reliable spatial ecology data for small, low-mobile and secretive snakes. GPS-based techniques for the study of animal movement have greatly advanced in recent years88, including for invasive snakes89. Nevertheless, this technology is still difficult to apply to cases like ours that involve a fossorial, less mobile, and small-bodied species89,90. To accumulate enough effective sample size for this animal, small but long-duration batteries are needed, a trade-off that still remains defying (see Mitchell et al.91). Against this backdrop, the combined use of radiotelemetry and the novel AKDE home range estimation method allowed us to mitigate common telemetry data issues (e.g., irregular sampling regimes, data autocorrelation) and those deriving from snake behavior (e.g., spatial autocorrelation, small effective sample size), without compromising data accuracy. Therefore, this combination is a promising tool to unveil the spatial ecology of other secretive species whose studies may encounter similar methodological difficulties arising from their particular behavior92,93.
From a broader perspective, our study contributes to highlight the advantages of gaining information on spatial behavior in the management of invasive species. Spatial ecology studies have enabled managers to understand invasive species' home range, activity patterns and habitat use11,65, can be also useful to plan when and where to place traps94 and conduct visual surveys10, determine sources of detection bias15, identify areas at risk95 or manage local habitats to prevent spread of invasive species96. Therefore, we argue that efforts should be made to turn spatial behavior information into an essential tool for optimizing invasive species management.
Conclusions
The high ecological adaptability of L. californiae, its secretive behavior and unique propensity to survive28, its reproductive plasticity97, devastating ecological impacts32,98, and the climatic suitability of the archipelago99 makes the strengthening of actions to control this invasion an urgent matter for Gran Canaria. Our research provides basic and applicable insights into the spatial ecology of L. californiae that can be directly incorporated into trapping and control action designs. In particular, traps should not lie more than 233 m apart and containment buffers should be less than 100 m. Moreover, control action reinforcement should be extended from early February to mid-November to increase captures (particularly of females), that is, before the breeding season starts and until the end of the activity period. Since L. californiae individuals move every 2–3 days and most movements are smaller than 100 m, intense prospection in the surroundings of a detected but uncaptured individual or of fresh tracks could increase the probability of capture. To link visual surveys to the species activity patterns, the former should be made during central hours and evenings. In addition, this study underlines the value of spatial ecology in the context of biological invasions. Such an approach can be an essential step in designing more effective control strategies, especially on other islands worldwide (e.g., Cozumel, Christmas or the Balearic Islands19,100,101,102). Moreover, the combined use of AKDE method and radio tracking for less mobile small species like L. californiae is very appropriate. For a broader perspective, we appeal to the need to innovate and develop new technologies to improve management of invasive snakes, given their devastating impacts on many islands of the world.
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
References
IPBES. Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Debating Nature’s Value (2019).
Doherty, T. S., Glen, A. S., Nimmo, D. G., Ritchie, E. G. & Dickman, C. R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA. 113, 11261–11265 (2016).
Mace, G. M. et al. Biodiversity targets after 2010. Curr. Opin. Environ. Sustain. 2, 3–8 (2010).
CBD. Decisions adopted by the conference of the parties to the convention on biological diversity at its eighth meeting (Decision VIII/15, Annex IV). Conv. Biol. Divers. Curitiba, Brazil 1–27 (2006).
Blackburn, T. M., Cassey, P., Duncan, R. P., Evans, K. L. & Gaston, K. J. Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958 (2004).
Kier, G. et al. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. 106, 9322–9327 (2009).
Jones, H. P. et al. Invasive mammal eradication on islands results in substantial conservation gains. Proc. Natl. Acad. Sci. USA. 113, 4033–4038 (2016).
IUCN. Guidelines for invasive species planning and management on islands. (IUCN, International Union for Conservation of Nature, 2018). https://doi.org/10.2305/IUCN.CH.2018.15.en.
Courchamp, F., Chapuis, J. L. & Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 78, 347–383 (2003).
Bartoszek, I. A., Smith, B. J., Reed, R. N. & Hart, K. M. Spatial ecology of invasive Burmese pythons in southwestern Florida. Ecosphere 12, e03564 (2021).
Rouco, C., Norbury, G. L. & Anderson, D. P. Movements and habitat preferences of pests help to improve population control: The case of common brushtail possums in a New Zealand dryland ecosystem. Pest Manag. Sci. 73, 287–294 (2017).
Bengsen, A. J. et al. Feral cat home-range size varies predictably with landscape productivity and population density. J. Zool. 298, 112–120 (2016).
Genovesi, P. Guidelines for eradication of terrestrial vertebrates: a European contribution to the invasive species issue (Council of Europe T-PVS, 2000).
Avery, M. L., Humphrey, J. S., Keacher, K. L. & Bruce, W. E. Detection and removal of invasive Burmese pythons: Methods development update. Proc. Vertebr. Pest Conf. 26, 145–148 (2014).
Boback, S. M., Nafus, M. G., Yackel Adams, A. A. & Reed, R. N. Use of visual surveys and radiotelemetry reveals sources of detection bias for a cryptic snake at low densities. Ecosphere 11, e03000 (2020).
Guzy, J. C. et al. Burmese pythons in Florida: A synthesis of biology, impacts, and management tools. NeoBiota 80, 1–119 (2023).
Parker, W. S. & Plummer, M. V. Population ecology. In Snakes: Ecology and Evolutionary Biology (eds Seigel, R. A. et al.) 253–301 (1987).
Turner, F. B. The dynamics of populations of squamates, crocodilians and rhynchocephalians. Biol. Reptil. 7, 157–264 (1977).
Kraus, F. Impacts from invasive reptiles and amphibians. Annu. Rev. Ecol. Evol. Syst. 46, 75–97 (2015).
Wiles, G. J., Bart, J., Beck, R. E. & Aguon, C. F. Impacts of the brown tree snake: Patterns of decline and species persistence in Guam’s avifauna. Conserv. Biol. 17, 1350–1360 (2003).
Mullin, S. J. & Seigel, R. A. Snakes: Ecology and Conservation (Cornell University Press, 2009).
Tobin, M. E., Sugihara, R. T., Pochop, P. A. & Linnell, M. A. Nightly and seasonal movements of Boiga irregularis on Guam. J. Herpetol. 33, 281–291 (1999).
Pyron, R. A. & Burbrink, F. T. Systematics of the Common Kingsnake (Lampropeltis getula; Serpentes: Colubridae) and the burden of heritage in taxonomy. Zootaxa 32, 22–32 (2009).
Cabrera-Pérez, M. Á., Gallo-Barneto, R., Esteve, I., Patiño-Martínez, C. & López-Jurado, L. F. The management and control of the California kingsnake in Gran Canaria (Canary Islands): Project LIFE+ Lampropeltis. Aliens Invasive Species Bull. 32, 20–28 (2012).
GESPLAN. #Stopculebrareal. https://www.stopculebrareal.com/ (2023).
Wiseman, K. D., Greene, H. W., Koo, M. S. & Long, D. J. Feeding ecology of a generalist predator, the california kingsnake (Lampropeltis californiae): Why rare prey matter. Herpetol. Conserv. Biol. 14, 1–30 (2019).
Anguiano, M. P. & Diffendorfer, J. E. Effects of fragmentation on the spatial ecology of the California Kingsnake (Lampropeltis californiae). J. Herpetol. 49, 420–427 (2015).
Hubbs, B. Common Kingsnakes: A Natural History of Lampropeltis Getula (Tricolor Books, 2009).
Monzón-Argüello, C. et al. Snakes on an island: Independent introductions have different potentials for invasion. Conserv. Genet. 16, 1225–1241 (2015).
Patiño Martínez, C. Acción C.4: obtención de parámetros biológicos de ejemplares de Lampropeltis californiae capturados. Informe final (2012–2015). Official report. (2015).
Piquet, J. C. The Perils of an Invasive Snake: The California Kingsnake in the Canary Islands (Universidad de La Laguna, 2022).
Piquet, J. C. & López-Darias, M. Invasive snake causes massive reduction of all endemic herpetofauna on Gran Canaria. Proc. R. Soc. B Biol. Sci. 288, 20211939 (2021).
Holway, D. A. & Suarez, A. V. Animal behavior: An essential component of invasion biology. Trends Ecol. Evol. 14, 328–330 (1999).
Parker, J. D. et al. Do invasive species perform better in their new ranges?. Ecology 94, 985–994 (2013).
Šálek, M., Drahníková, L. & Tkadlec, E. Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mamm. Rev. 45, 1–14 (2015).
Pittman, S. E. et al. Homing of invasive Burmese pythons in South Florida: Evidence for map and compass senses in snakes. Biol. Lett. 10, 20140040 (2014).
Dodd, C. K. Reptile Ecology and Conservation: A Handbook of Techniques (Oxford University Press, 2016). https://doi.org/10.1093/acprof:oso/9780198726135.001.0001.
Fitch, H. S. Criteria for determining sex and breeding maturity in snakes. Herpetologica 16, 49–51 (1960).
GESPLAN. Informe Layman. Control de la especie invasora Lampropeltis getula californiae en la isla de Gran Canaria http://www.lifelampropeltis.com (2015).
Melián, A. Técnica de inserción quirúrgica de radiotransmisores en L. C.. LIFE+ LAMPROPELTIS. In Seminario Internacional sobre gestión de reptiles exóticos invasores (ed. GESPLAN) http://www.lifelampropeltis.com (2014).
Abom, R., Bell, K., Hodgson, L. & Schwarzkopf, L. Moving day and night: highly labile diel activity patterns in a tropical snake. Biotropica 44, 554–559 (2012).
Withey, J. C., Bloxton, T. D. & Marzluff, J. M. Effects of tagging and location error in wildlife radiotelemetry studies. In Radio Tracking and Animal Populations 43–75 (Academic Press, 2001). https://doi.org/10.1016/B978-012497781-5/50004-9.
Fleming, C. H. & Calabrese, J. M. A new kernel density estimator for accurate home-range and species-range area estimation. Methods Ecol. Evol. 8, 571–579 (2017).
Silva, I. et al. Autocorrelation-informed home range estimation: A review and practical guide. Methods Ecol. Evol. 13, 534–544 (2022).
Fleming, C. H. & Calabrese, J. M. ctmm: Continuous-Time Movement Modeling (2021).
Burnham, K. P. & Anderson, D. R. Information and likelihood theory: A basis for model selection and inference. In Model Selection and Multimodel Inference 49–97 (Springer, 2002). https://doi.org/10.1007/978-0-387-22456-5_2.
Calabrese, J. M., Fleming, C. H., Noonan, M. J. & Dong, X. ctmmweb: A graphical user interface for autocorrelation-informed home range estimation. Wildl. Soc. Bull. 45, 162–169 (2021).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016).
Montano, Y. et al. A stable home: Autocorrelated kernel density estimated home ranges of the critically endangered Elongated tortoise. Herpetol. J. 32, 120–129 (2022).
Mohr, C. O. Table of equivalent populations of North American small mammals. Am. Midl. Nat. 37, 223–249 (1947).
Worton, B. J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168 (1989).
Kranstauber, B., Kays, R., Lapoint, S. D., Wikelski, M. & Safi, K. A dynamic Brownian bridge movement model to estimate utilization distributions for heterogeneous animal movement. J. Anim. Ecol. 81, 738–746 (2012).
Kranstauber, B., Smolla, M. & Scharf, A. move: Visualizing and Analyzing Animal Track Data. (2015).
Kruskal, W. H. & Wallis, W. A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621 (1952).
Zuur, A. F., Ieno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14 (2010).
Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics; Essays in Honor of Harold Hotelling (ed. Olkin, I.) 279–292 (Stanford University Press, 1960).
Nakagawa, S. & Schielzeth, H. Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biol. Rev. 85, 935–956 (2010).
Stoffel, M. A., Nakagawa, S. & Schielzeth, H. rptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644 (2017).
Welch, B. L. On the comparison of several mean values: An alternative approach. Biometrika 38, 330 (1951).
Dag, O., Dolgun, A. & Konar, N. M. Onewaytests: An R package for one-way tests in independent groups designs. R J. 10, 175–199 (2018).
Hartig, F. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. (2022).
Langsrud, Ø. ANOVA for unbalanced data: Use type II instead of type III sums of squares. Stat. Comput. 13, 163–167 (2003).
Lenth, R. V. emmeans: estimated marginal means, aka least-squares means. (2021).
R Core Team. R: A Language and Environment for Statistical Computing (2022).
Smith, D. H. V., Clayton, R., Anderson, D. & Warburton, B. Using home-range data to optimise the control of invasive animals. N. Z. J. Ecol. 39, 286–290 (2015).
Roy, S., Jones, C. & Harris, S. An ecological basis for control of the mongoose Herpestes javanicus in Mauritius: is eradication possilbe? In Turning the Tide: The Eradication of Invasive Species (eds Veitch, C. & Clout, M.) 266–273 (IUCN SSC Invasive Species Specialist Group, 2016).
Clark, L., Clark, C. & Siers, S. Brown tree snakes methods and approaches for control. In Ecology and Management of Terrestrial Vertebrate Invasive Species in the United States (eds Pitt, W. C. et al.) 107–134 (CRC Press, 2018).
Engeman, R. M. & Linnell, M. A. Trapping strategies for deterring the spread of Brown Tree Snakes from Guam. Pacific Conserv. Biol. 4, 348–353 (1998).
Fisher, R. et al. Herpetological monitoring using a pitfall trapping design in southern California. US Geol. Surv. Tech. Methods 2, 44 (2008).
Engeman, R. M. & Linnell, M. A. The effect of trap spacing on the capture of brown tree snakes on Guam. Int. Biodeterior. Biodegrad. 54, 265–267 (2004).
Gormley, A. M. & Warburton, B. Refining kill-trap networks for the control of small mammalian predators in invaded ecosystems. PLoS ONE 15, e0238732 (2020).
Marshall, B. M. et al. No room to roam: King Cobras reduce movement in agriculture. Mov. Ecol. 8, 1–14 (2020).
D’souza, A. et al. Space use and activity of Boiga cyanea—A major songbird nest predator in a seasonal tropical forest in Thailand. Glob. Ecol. Conserv. 32, e01875 (2021).
Smith, S. N. et al. Native Burmese pythons exhibit site fidelity and preference for aquatic habitats in an agricultural mosaic. Sci. Rep. 11, 7014 (2021).
Savidge, J. A., Stanford, J. W., Reed, R. N., Haddock, G. R. & Adams, A. A. Y. Canine detection of free-ranging brown treesnakes on Guam. N. Z. J. Ecol. 35, 174–181 (2011).
Liu, J., Tong, Y. & Liu, J. Review of snake robots in constrained environments. Rob. Auton. Syst. 141, 103785 (2021).
Hirata, Y., Oda, H., Osaki, T. & Takeuchi, S. Biohybrid sensor for odor detection. Lab Chip 21, 2643–2657 (2021).
Aota, T., Ashizawa, K., Mori, H., Toda, M. & Chiba, S. Detection of Anolis carolinensis using drone images and a deep neural network: An effective tool for controlling invasive species. Biol. Invasions 23, 1321–1327 (2021).
Raiti, P. Captive Care of the Common Kingsnake, Lampropeltis getula. Bull. Assoc. Reptil. Amphib. Vet. 5, 9–10 (1995).
Shine, R. Reproductive strategies in snakes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 270, 995–1004 (2003).
Christy, M. T., Yackel Adams, A. A., Rodda, G. H., Savidge, J. A. & Tyrrell, C. L. Modelling detection probabilities to evaluate management and control tools for an invasive species. J. Appl. Ecol. 47, 106–113 (2010).
Feuka, A. B., Nafus, M. G., Yackel Adams, A. A., Bailey, L. L. & Hooten, M. B. Individual heterogeneity influences the effects of translocation on urban dispersal of an invasive reptile. Mov. Ecol. 10, 1–18 (2022).
Siers, S. R., Yackel Adams, A. A. & Reed, R. N. Behavioral differences following ingestion of large meals and consequences for management of a harmful invasive snake: A field experiment. Ecol. Evol. 8, 10075–10093 (2018).
Maillet, Z., Halliday, W. D. & Blouin-Demers, G. Exploratory and defensive behaviours change with sex and body size in eastern garter snakes (Thamnophis sirtalis). J. Ethol. 33, 47–54 (2015).
Skinner, M., Brown, S., Kumpan, L. T. & Miller, N. Snake personality: Differential effects of development and social experience. Behav. Ecol. Sociobiol. 76, 135 (2022).
Shonfield, J., King, W. & Koski, W. R. Habitat use and movement patterns of butler’s gartersnake (Thamnophis butleri) in southwestern Ontario, Canada. Herpetol. Conserv. Biol. 14, 680–690 (2019).
Boback, S. M., Nafus, M. G., Yackel Adams, A. A. & Reed, R. N. Invasive brown treesnakes (Boiga irregularis) move short distances and have small activity areas in a high prey environment. Sci. Rep. 12, 12705 (2022).
Cagnacci, F., Boitani, L., Powell, R. A. & Boyce, M. S. Animal ecology meets GPS-based radiotelemetry: A perfect storm of opportunities and challenges. Philos. Trans. R. Soc. B Biol. Sci. 365, 2157–2162 (2010).
Smith, B. J., Hart, K. M., Mazzotti, F. J., Basille, M. & Romagosa, C. M. Evaluating GPS biologging technology for studying spatial ecology of large constricting snakes. Anim. Biotelemetry 6, 1–13 (2018).
Frair, J. L. et al. Resolving issues of imprecise and habitat-biased locations in ecological analyses using GPS telemetry data. Philos. Trans. R. Soc. B Biol. Sci. 365, 2187–2200 (2010).
Mitchell, L. J., White, P. C. L. & Arnold, K. E. The trade-off between fix rate and tracking duration on estimates of home range size and habitat selection for small vertebrates. PLoS ONE 14, e0219357 (2019).
Durso, A. M., Willson, J. D. & Winne, C. T. Needles in haystacks: Estimating detection probability and occupancy of rare and cryptic snakes. Biol. Conserv. 144, 1508–1515 (2011).
Seigel, R. A., Collins, J. T. & Novak, S. S. Snakes: Ecology and Evolutionary Biology (Macmillan Publishing Company, 1987).
Nafus, M. G., Yackel Adams, A. A., Klug, P. E. & Rodda, G. H. Habitat type and structure affect trap capture success of an invasive snake across variable densities. Ecosphere 9, e02339 (2018).
Lapointe, N. W. R., Thorson, J. T. & Angermeier, P. L. Seasonal meso- and microhabitat selection by the northern snakehead (Channa argus) in the Potomac river system. Ecol. Freshw. Fish 19, 566–577 (2010).
Minowa, S., Miyashita, T. & Senga, Y. Microhabitat selection of the introduced bullfrogs (Rana catesbeiana) in paddy fields in eastern Japan. Curr. Herpetol. 27, 55–59 (2008).
Fisher, S. et al. Reproductive plasticity as an advantage of snakes during island invasion. Conserv. Sci. Pract. 3, e554 (2021).
Piquet, J. C., Maestresalas, B. & López-Darias, M. Coupling phenotypic changes to extinction and survival in an endemic prey community threatened by an invasive snake. Sci. Rep. 12, 18249 (2022).
Piquet, J. C. et al. Could climate change benefit invasive snakes? Modelling the potential distribution of the California Kingsnake in the Canary Islands. J. Environ. Manag. 294, 112917 (2021).
Emery, J. et al. The lost lizards of Christmas Island : A retrospective assessment of factors driving the collapse of a native reptile community. Conserv. Sci. Pract. 3, e358 (2021).
Martínez-Morales, M. A. & Cuarón, A. D. Boa constrictor, an introduced predator threatening the endemic fauna on Cozumel Island, Mexico. Biodivers. Conserv. 8, 957–963 (1999).
Silva-Rocha, I., Salvi, D., Sillero, N., Mateo, J. A. & Carretero, M. A. Snakes on the balearic islands: An invasion tale with implications for native biodiversity conservation. PLoS ONE 10, 1–18 (2015).
Fleming, C. H. et al. From fine-scale foraging to home ranges: A semivariance approach to identifying movement modes across spatiotemporal scales. Am. Nat. 183, E154–E167 (2014).
Calabrese, J. M., Fleming, C. H. & Gurarie, E. ctmm: An R package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol. Evol. 7, 1124–1132 (2016).
Acknowledgements
We received inestimable support from M. Á. Cabrera (Canary Islands Government), R. Gallo, J. Saavedra, J. Sánchez (GESPLAN S.A), M. Amador and F. Navas (Cabildo de Gran Canaria). Special thanks to A. Melián, who performed surgical procedures and post-surgery care. Our thanks for antennas and receptors on loan from the Department of Zoology (University of La Laguna), Cabildo de Gran Canaria and Asociación de Amigos de las Pardelas. Benjamin M. Marshall, Christen Fleming, J.M. Pérez-García and E. Arrondo provided us with key help and suggestions for ADKE estimates. Guido Vaughan edited English in the final version. Funding was received by the BBVA Foundation (2018 call “Ayudas a Equipos de Investigación Científica en Ecología y Biología de la Conservación”) and the Canary Islands Government (LAMPROIMPACT agreement). JCP was beneficiary of a PhD contract from the Agencia Canaria de la Investigación, Innovación y Sociedad de la Información and the European Social Fund (ESF) (Operational Programme of the Canary Islands 2014–2020—Priority Axis 3: Priority Theme 74, 85 %). MLD was financed by the Cabildo de Tenerife under the TF INNOVA Program 2016-2021 (with funds from MEDI & FDCAN).
Author information
Authors and Affiliations
Contributions
B.M.: conceptualization, methodology, investigation, formal analysis, writing—original draft, writing—review and editing, visualization. J.C.P.: conceptualization, methodology, investigation, formal analysis, writing–original draft, writing—review and editing, visualization. M.L.D: conceptualization, methodology, investigation, formal analysis, writing—original draft, writing—review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maestresalas, B., Piquet, J.C. & López-Darias, M. Spatial ecology to strengthen invasive snake management on islands. Sci Rep 13, 6731 (2023). https://doi.org/10.1038/s41598-023-32483-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32483-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.