Abstract
To successfully establish itself in a novel environment, an animal must make an inherent trade-off between knowledge accumulation and exploitation of knowledge gained (i.e., the exploration–exploitation dilemma). To evaluate how habitat quality affects the spatio-temporal scale of switching between exploration and exploitation during home range establishment, we conducted experimental trials comparing resource selection and space-use of translocated animals to those of reference individuals using reciprocal translocations between habitat types of differing quality. We selected wild pigs (Sus scrofa) as a model species to investigate hypotheses related to the movement behavior of translocated individuals because they are globally distributed large mammals that are often translocated within their introduced range to facilitate recreational hunting. Individuals translocated to higher quality habitat (i.e. higher proportions of bottomland hardwood habitats) exhibited smaller exploratory movements and began exploiting resources more quickly than those introduced to lower quality areas, although those in lower-quality areas demonstrated an increased rate of selection for preferred habitat as they gained knowledge of the landscape. Our data demonstrate that habitat quality mediates the spatial and temporal scale at which animals respond behaviorally to novel environments, and how these processes may determine the success of population establishment.
Similar content being viewed by others
Introduction
Animals are exposed to novel environments through natural and anthropogenic processes: natal dispersal, dispersal beyond their native range (e.g. Eurasian waterfowl in North America)1, passive transport by environmental conditions (e.g. terrestrial animals carried by ocean currents)2, accidental introduction (e.g., marine organisms transported in ship ballast)3 or deliberate translocation for conservation (e.g., reintroductions or population augmentation of endangered and game species)4 or recreational purposes (e.g., desired angling species)5. To successfully establish in a novel environment, appropriate resources and niche space must be available, and an animal must gain sufficient knowledge of the distribution and availability of these resources to allow its survival and reproductive success. However, there is an inherent and well-recognized trade-off between knowledge accumulation and exploitation of the knowledge gained, referred to as the exploration–exploitation dilemma6,7.
The cost of gaining knowledge through exploration can outweigh the benefit of exploiting short-term opportunities, or vice-versa, and thus optimal strategies should balance present and future benefits while accounting for resource heterogeneity in time or space8. It has been proposed the optimal trade-off between exploration and exploitation of habitats will vary depending upon the life-stage of an animal (e.g., it is sub-optimal to invest in gaining new information near the end of life); this pattern may also be evident in animals introduced to novel environments, as dispersal events essentially represent a reversion in knowledge levels9. Examination of fine-scale spatial–temporal patterns, such as speed and distance travelled, may allow scientists to identify phases of an animal’s establishment in a novel landscape to determine the period over which the animal is primarily exploring versus exploiting their environment10. Research suggests memory-based foraging is linked to the establishment and use of animal home ranges, which generally constitute a restricted portion of the total available landscape11. Although other factors can affect home range size (e.g., mating system, sex, type of forager)12, greater evaluation of spatial patterns observed as an animal establishes a home range in a novel environment is necessary to facilitate conservation and management programs.
Based on the exploration–exploitation dilemma, knowledge accumulation or exploration by an animal should be highest immediately following its introduction to a novel environment9. At this stage, the animal may live off energy reserves and explore the landscape to identify resources required for survival, and this exploration requires reduced exploitation of identified resources. Corroborating this hypothesis, multiple studies provide evidence of increased movements by animals immediately following translocation, e.g.,13,14,15,16. Once an animal attains familiarity with locations of sufficient resources in the novel environment, it is likely to begin exploiting these resources, and exhibiting reduced exploratory behavior. Eventually, movement patterns should resemble those of resident animals of the same species under similar conditions. In addition, research on translocations suggests habitat quality and competition may influence spatial patterns exhibited by animals in novel environments. For example, Frair et al.17 found site fidelity of translocated elk (Cervus elaphus) was related to patch forage quality surrounding the release site. Therefore, it might be expected that in areas where high quality habitat is present and readily accessible, animals will locate and map necessary resources in their novel environment more quickly and occupy a smaller home range due to the proximal availability of resources. Additionally, density of conspecifics or other species with overlapping niche space are likely to influence post-release behavior18,19. Nonetheless, as an animal accumulates spatial and temporal knowledge of the distribution of resources within the novel landscape following introduction, aspects of animal movement such as home range size, average daily distance moved, and resource selection should also change.
Previous studies have presented broad comparisons of home range size between translocated and resident animals20,21,22,23, but rarely are changes in home range size and movement patterns described at a fine temporal scale (e.g., daily or weekly movements post translocation), allowing elucidation of processes by which animals colonize new areas (but see10,24). Knowledge of fine-scale patterns in movement by animals in novel environments is necessary as a foundation for greater understanding of the mechanisms of species establishment25, and therefore has implications for improving existing exploration–exploitation theory and the conservation and management of wildlife populations.
In this study, we used a 7-day moving window to assess a suite of movement metrics (i.e., daily distance traveled, use area, and net squared displacement) to evaluate the processes by which animals establish a home range in novel areas to which they are translocated. We selected wild pigs (Sus scrofa) as a model species to investigate hypotheses related to the movement behavior of translocated individuals because they are a globally distributed large mammal that is considered an invasive species in many areas and expanding in range due to frequent translocations26. In regions where they are non-native, wild pigs are often translocated to facilitate recreational hunting opportunities, which is a primary mechanism contributing to the extensive range expansion of this species in their introduced range27,28. Previous research suggests pigs have well-developed spatial memories29, which likely facilitates their ability to successfully establish populations when introduced to a novel environment. Once introduced, wild pigs cause extensive ecological and economic impacts, and thus information is critically needed on post-release movements to facilitate management programs30. Although generalist foragers, wild pigs are heavily dependent on wetland and riparian habitats for foraging, resting, and thermoregulation31,32,33. Thus, riparian areas and bottomland hardwood swamps are considered high quality habitats for wild pigs, particularly relative to pine-dominated forests, and the distribution of these preferred habitats has a strong influence on wild pig movements34. Thus, the composition and configuration of bottomland hardwood forests, wetlands, and riparian areas within the landscape likely plays an important role in the movement behavior of translocated wild pigs or those dispersing into novel areas.
We hypothesized, based upon current exploration–exploitation theory, that translocated wild pigs would exhibit extensive exploratory movements (relative to typical movements for the species) following release into a novel environment (exploration phase). Subsequently, during the exploitation phase, we predicted these patterns would resemble those of established resident animals as evidenced by various attributes of movement such as home range size, daily movement distances, and resource selection. To test these predictions, we conducted experimental translocation trials complete with reverse translocations and control individuals, where we translocated female wild pigs from high quality habitat to low quality habitat and vice versa and compared movement patterns and resource selection of each experimental group to those of reference females to evaluate whether patterns of movement are mediated by habitat quality. We predicted translocated individuals would show reduced movements when introduced into higher quality habitat (bottomland hardwoods) compared to relatively low-quality habitat (upland pines), as exploitation of resources should require less travel and exploration in areas with abundant high quality habitat35. Similarly, we predicted animals translocated to higher quality habitat would take less time to exhibit movement patterns similar to resident animals than those translocated to lower quality habitat.
Methods
Data collection
We conducted this research on the Savannah River Site (SRS), a 780 km National Environmental Research Park on the border of South Carolina and Georgia, USA. Approximately 68% of habitat on the SRS consists of upland pine forest containing interspersed riparian areas, with another 22% comprised of bottomland hardwood forest, and the remaining areas consisting of open water, shrublands, industrial areas, and grasslands36. Although wild pigs occur throughout both upland pine and bottomland hardwood habitats on the SRS37, movements are often concentrated within bottomland hardwood and other riparian habitats33,38. Upland pine forests on the SRS are dominated by loblolly pine (Pinus taeda), long-leaf pine (P. palustris), and slash pine (P. elliotii) and are managed by the USDA Forest Service for timber and wildlife habitat36. Bottomland hardwood forests incorporate swamp and wetlands and are dominated by oaks (Quercus spp.), tulip-poplars (Liriodendron tulipifera), and other hardwood species36.
We trapped breeding-age female wild pigs in baited corral and box traps in upland pine and bottomland hardwood habitat from June 2014 to July 2016 (Table S1). We used a dart rifle (X-Caliber, Pneu-Dart Inc., Pennsylvania, USA) to anesthetize pigs using a combination of Telazol® (4.4 mg/kg; MWI Veterinary Supply, Idaho, USA) and Xylazine (2.2 mg/kg; Wildlife Pharmaceuticals Inc., Colorado, USA). We recorded sex, assessed age through examination of tooth eruption, and collected morphological measurements from each animal. We collared pigs with a 3000S Global Positioning System (GPS) collar (Lotek Wireless Inc., Ontario, Canada) or a GPS Plus X Collar (Vectronic Aerospace GmbH, Berlin, Germany). Lotek 3000S GPS collars were programmed to take locations every 2 h, while GPS Plus X collars were programmed to collect locations at 15-min intervals for the first 6 weeks post capture, at which point we reprogrammed them to take locations at 1-h intervals. Anesthetized pigs were either reversed with Yohimbine (0.15 mg/kg) at their capture site and released to serve as reference animals or were transported while anesthetized to a novel release site to serve as translocated animals. South Carolina laws regarding the transportation of captured wild pigs limited translocations to within the boundary of the SRS property. Nonetheless, given the large area encompassed by the SRS we were able to translocate pigs at least 8 km from their point of capture, which is greater than the 95% credible interval for wild pig home range size in North America39 and greater than most reported dispersal events40. Additionally, although capture and translocation efforts were restricted to inside the SRS, wild pigs could move outside of the SRS boundaries. Among translocated animals, individuals captured in upland pine (low quality) habitat were translocated to bottomland hardwood (high quality) habitat and vice-versa. To assess overall habitat quality at each release site, we estimated the utilization distribution (UD) for each resident wild pig using kernel density estimation from the R package adehabitatHR41. The 95% isopleth of UD’s was used to define the home range size for each animal. We then buffered release sites by the average home range size (8.3 km2) and calculated percent upland pine and bottomland hardwood for each release habitat type. For wild pigs released in our classified bottomland hardwood habitat, 26% of the area contained bottomland hardwood while upland pine constituted 44%. Percentages for animals released into upland pine was 21% and 71% for bottomland hardwood and upland pine, respectively. Additional information on study species, study site, and data collection can be found in supplemental material. Additionally, to assess social integration of translocated wild pigs with local groups of wild pigs, in 2016 we placed baited white-flash trail cameras (Reconyx PC900 Hyperfire, Reconyx, Holmen, Wisconsin, USA) in locations that would maximize probability of detection based on local habitat conditions and known locations from collar data. We attempted to determine whether translocated individuals were travelling with other uncollared animals every 30 days by setting cameras for approximately 1 week, or until we were able to confirm target animals were travelling with other individuals or had left the area. All experimental methods were carried out with the approval of the University of Georgia’s Animal Care and Use Committee under protocol A2015 05004Y2A3 and were carried out in accordance with the ARRIVE guidelines for the immobilization of animals for studies conducted in the field.
Data analysis
Space use metrics
Prior to analyses, we subsampled data from collars to 2-h intervals, the minimum common temporal resolution among animals used in this research. We also removed all locations with Position Dilution of Precision values > 942. To evaluate effects of translocation, we calculated a suite of movement and space use statistics for resident and translocated pigs in both upland pine and bottomland hardwood habitats using a 7-day moving window analysis24. We first built the UD across the entire monitoring period for each animal, then used a moving window to incorporate each individual day as well as the 3 days prior and after. For example, day 4 would be included in calculations for days 1 through 7. Within the moving window framework, we calculated: (1) a 50% (hereafter, core area) and 95% (hereafter, range area) dynamic Brownian bridge movement model (dBBMM)43 utilization distribution, (2) Euclidian distance (m) to release site, and (3) daily distance (m) travelled for each pig. For all animals, we evaluated fidelity to the release site by calculating the daily mean distance from release site using the 12 GPS locations taken each day. For resident animals, we calculated distance to release site based on capture/release location. We calculated mean distance travelled for each individual by summing distance between each GPS location (i.e., step lengths) taken each day (hereafter, distance travelled) to evaluate daily movement rates24. For each metric, group (translocated or resident), and habitat type (bottomland hardwood or upland pine), we computed a mean and standard error across the 7-day periods for 90 days post release, or until the collar was retrieved, whichever was shorter. Three translocated wild pigs returned to their original home range within 10 days of translocation (Table S1). We considered animals returning to their original home range once they were within 1.6 km (radius of average resident home range size) of initial capture location. When this occurred, we removed data from the time of release until the animal had returned to their original home range and considered the individual as a resident at that time. We also censored three pigs after they each began travelling with another collared animal (Table S1). All analyses were conducted using program R (version 4.2.1) and dBBMM utilization distributions were calculated using the R package move44.
The dBBMM method requires a time-stamped series of locations and an estimate of telemetry error. We used an error estimate of 15 m for all locations based on vendor estimates. The dBBMM varies the Brownian motion variance (\({\upsigma }_{m}^{2}\)) for different subsections of the trajectory by moving a sliding window encompassing n number of locations along a path (see Kranstauber et al.43 for details). To fit the dBBMM, we specified a window size of 11 fix locations (equivalent to 22 h) and a margin of 5 fix locations based on the temporal resolution of each track and our a priori assumptions of the time-scale of behavioral breaks43.
Resource selection
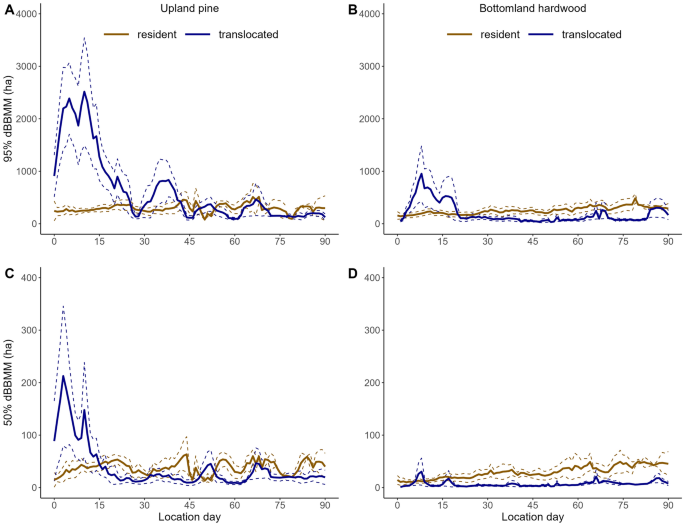
To quantify resource selection, we used data from the National Land Cover Database (https://www.mrlc.gov/data) with a resolution of 30 × 30 m. We condensed the 15 habitat classifications to three coarse land cover types: bottomland (e.g., marsh, wetlands, bottomland hardwood, deciduous forest), upland pine (e.g. pine woodlands), and shrub/grassland (e.g. herbaceous and shrub/scrub) based on results of prior studies within our landscape33,34, which we assumed differed in provision of food and cover for wild pigs31,33,45. We excluded four other categories; developed (consisting of 4 levels), cultivated (consisting of 2 categories), open water, and barren land due to their limited distribution in and around the SRS site. To assess selection of these habitat types we used step selection functions (SSFs)46. Each step is compared to n number of random points drawn from a distribution of step lengths and turning angles. Thus, SSF’s constrain selected and available habitat types in both space and time. Animals introduced into novel locations are likely to exhibit different movement parameters when exploring versus exploiting new environments24,47, therefore, we used results from our moving window analysis to assign a distribution of step lengths and turning angles for generating random points based upon establishment phase (i.e., exploration vs. exploitation) and release habitat type (i.e., bottomland hardwood vs. upland pine). In addition, we created a single distribution of step lengths and turning angles for resident animals regardless of habitat type. To distinguish between exploration and exploitation for translocated individuals, we used results from our moving window analysis assessing 95% dBBMM use areas. We considered animals switching from exploration to exploitation once the 95% dBBMM for each translocated group reached the average for their respective resident group (see results; Fig. 1). On average, animals translocated to upland pine exhibited similar use areas (95% dBBMM; Fig. 1A) to residents on day 29, while those translocated to bottomland hardwood achieved similar patterns on day 22 (Fig. 1A). Consequently, we generated separate distributions for step length and turning angles for days 1–29 (exploratory) and days 30–90 (exploitation) for upland pine wild pigs, and for days 1–22 (exploration) and days 23–90 (exploitation) for bottomland hardwood wild pigs. As used area for resident wild pigs was similar between habitat types based on dBBMM (Fig. 1), we used one turning angle and step length distribution for both. We generated 20 random steps for each wild pig step using step lengths and turning angle distributions observed in this study while excluding the individual for which the distributions are being applied to47. Habitat type was recorded at the endpoint of each observed step. To reduce bias from animals being collared for long periods of time, we truncated all locations at 90 days or whenever the collar failed, whichever was shorter. GPS tracks were processed using the AdehabitatLT package in the R statistical Framework48.
Dynamic Brownian bridge movement models at the 95% utilization distribution contour (A upland; B bottomland) and 50% contour (C upland; D bottomland) for resident and translocated wild pigs within a 7-day moving window on the Savannah River Site, SC, USA. Solid lines indicate means and dashed lines are ± 1 standard error. Note the Y axis not on same scale for both graphs.
To compare habitat types of used and available steps, we applied a conditional logistic regression with amt’s fit_clogit function49. Our first objective was to test whether resource selection differed between resident and translocated individuals, release habitat type, or the interaction of these factors. To examine this, we fit four models (Table 1A): (1) habitat only (3 habitat covariates and step length), (2) habitat*status (resident or translocated), (3) habitat*release habitat (dummy variable indicating whether the animal was released in primarily upland pine or bottomland hardwood habitat), and (4) habitat*status + release habitat. We also included a random effect term to account for individual variation and strata (strata term accounts for autocorrelation by grouping gps locations that are sequential and thus more likely to be correlated). We ranked all models using Akaike Information Criterion (AIC)50. Additionally, we performed a post-hoc test to assess whether resource selection differed between diel periods as previous research has indicated wild pigs are likely to exhibit different behaviors across the diel cycle33. We added an interaction term indicating whether the location was taken during diurnal or nocturnal time periods to our top-ranked model above. We considered models ≤ 2 AIC as equivalent51. Our second objective was to determine how selection for our hypothesized preferred habitat type (bottomland) differed through time based on release habitat type, and between resident and translocated individuals (Table 1B). For this analysis we estimated 7-day selection coefficients for bottomland habitat for each group based only on nocturnal locations as previous research has indicated female wild pigs tend to spend a higher proportion of time resting during diurnal hours33. We added a fixed effect for each 7-day period that an animal was on the air post release and calculated the average selection for bottomland hardwood for that group. We used a generalized linear mixed-effects model (GLMM) from the lme4 package52 in R with a term to account for temporal autocorrelation.
Results
We captured 31 female wild pigs and removed two animals from analysis due to capture-related mortality < 1 day post capture. We tracked the remaining 29 individuals from 14 to 209 days (mean = 103.6 day, SE = 10.7 day; Table S1), although as mentioned previously we truncated the data to 90 days post release for all analyses. Three females were initially released at the capture location then subsequently re-trapped and translocated, thus they were included in both resident and translocated datasets; the remaining individuals were included as either reference or translocated animals. On average, we released translocated animals 17.1 km (SD = 3.8 km) from their original capture location and monitored them for 113.6 days (range 14–209 days; Table S1). One translocated wild pig was harvested by a hunter outside of SRS 53 days after release and was censored at that time. In 2016, we obtained post-translocation sounder formation data from cameras for four females at 30 days and seven females at 60 days. Three of four (75%) translocated wild pigs were documented travelling with other conspecifics at 30 days, and six of seven (86%) at 60 days.
Of the 26 radio-collared wild pigs that were included in our analyses, we classified 14 as residents (upland pine = 4; bottomland hardwood = 10) and 14 as translocated (upland pine = 8; bottomland hardwood = 6; Table S1); two individuals were captured initially as residents then subsequently recaptured and translocated. Overall, during the exploration phase, translocated individuals exhibited larger use areas, travelled further from their release site, and had larger daily movements than resident animals (Figs. 1, 2, Figure S1). Resident animals had relatively consistent range and core areas across the 90-day window (Fig. 1), and tended to remain within ~ 1 km of their release location (Fig. 2).
Space use metrics
Wild pigs translocated from bottomland hardwood to upland pine habitat moved furthest from the release location (Figs. 1A, 2) and had the largest range and core areas, exhibiting two peaks in home range area on day five (2230.1 ha; SE = 604.5 ha) and day 10 (2,202.9 ha; SE = 934.8 ha; Fig. 1A). In contrast, ranges of individuals translocated from upland pine to bottomland hardwood habitat peaked on day seven (947 ha; SE = 524.02) at less than half the area of animals translocated from bottomland hardwood to upland pine habitat (Fig. 1B). Core areas followed a similar trajectory, although the magnitude was reduced for animals translocated to bottomland hardwood habitat (Fig. 1C,D). Range area for individuals translocated to upland pine was > 2 times that of those translocated to bottomland hardwood for both the exploration (upland pine = 1217.3 ha, SE = 121.7 ha; bottomland hardwood = 421.7 ha, SE = 50.6 ha) and exploitation phases (upland pine = 290.4 ha, SE = 20.3 ha; bottomland hardwood = 110.1 ha, SE = 8.0 ha); however, during the exploitation phase, both translocated groups had smaller use areas on average than resident animals from those habitat types (Fig. 1).
Distance to release site was consistent across resident animals. Among translocated wild pigs, individuals showed the greatest change in distance moved from the release site during the exploration phase before stabilizing during the exploitation phase (Fig. 2). Wild pigs translocated to upland pine dispersed furthest from release location, with the greatest rate of increase occurring from day one (2.4 km; SE = 0.5 km) to day 15 (6.5 km; SE = 1.7 km; Fig. 2A). Wild pigs translocated to bottomland hardwood followed a similar trajectory, although the magnitude was less; dispersal distance on day one was 0.7 km (SE = 0.2 km) and increased to 2.7 km (SE = 1.1 km) on day 13 (Fig. 2B). During the exploration phase (first 29 days for upland pine; 22 days for bottomland hardwood), range area for individuals translocated to upland pine (1,217.3 ha; SE = 121.7 ha) was > 4 times that of individuals translocated to bottomland hardwood habitat (290.4 ha; SE = 20.3 ha). All animals translocated to high quality bottomland hardwood habitat were residing in similar areas at the end of their respective monitoring periods. In contrast, 50% (n = 4) of animals translocated to upland pine dispersed to areas that were predominately bottomland habitat at the end of their monitoring periods.
Resource selection
Our analysis strongly supported the model incorporating both status (i.e., translocated or resident) and release habitat (bottomland hardwood or upland pine; Tables S2–S4). Despite the addition of 16 parameters, our post-hoc test accounting for diel period ranked higher than our previous top-ranked model, indicating wild pigs were exhibiting different nocturnal and diurnal selection patterns. The most significant changes in resource selection we observed were for wild pigs in low quality upland pine habitat. Residents of upland pine demonstrated avoidance of both grassland and bottomland habitat during nocturnal hours and selection for both habitat types during diurnal hours (Fig. 3). In contrast, animals translocated to upland pine showed significant avoidance of grassland habitat types during diurnal hours, and, though not significant, tended to exhibit greater selection for both grassland and bottomland habitat types during nocturnal hours (Fig. 3). Wild pigs translocated from upland pine to bottomland habitat exhibited no significant changes from their resident counterparts, although they did tend to select for all three habitat types more during diurnal hours than residents (Fig. 3).
Standardized coefficients and 95% confidence interval from our top-ranked model for translocated and resident wild pigs on the Savannah River Site, SC, USA: model habitat × treatment × diel period. Intercept line (0) indicates neither selection nor avoidance. Estimates shown in relation to resident bottomland hardwood wild pigs.
Nocturnal weekly resource selection
Overall, each of our treatment groups exhibited varied selection for bottomland hardwood habitat over the 13-week period (Fig. 4). Both resident bottomland hardwood and resident upland pigs showed relatively little temporal change in selection, with resident upland animals exhibiting slightly lower selection overall. Wild pigs translocated to primarily bottomland habitat exhibited a decrease in selection of bottomland areas through time, while those translocated to upland habitat increased (Fig. 4).
Discussion
Our results revealed differences in space use patterns of translocated wild pigs through time that were consistent with the exploration–exploitation hypothesis. Further, our data demonstrate habitat quality can mediate both the spatial and temporal scale at which animals respond behaviorally to novel environments as wild pigs introduced into high quality bottomland hardwood habitats displayed reduced exploratory periods and made less extensive movements than those introduced into lower quality upland pine habitats. Movement patterns of translocated animals, whether conservation-related, accidental, or intentional release of invasive or game species, may influence the success of population establishment as individuals that move greater distances or for longer periods of time may incur increased mortality risk and have a higher probability of emigrating from the release site17,22. Although, as resources become more abundant the need for an individual to explore their surrounding environment is reduced9.
Animals translocated to new landscapes will have incomplete knowledge of the environment; thus, developing a spatial representation of this new system through exploration requires a substantial expenditure of time and energy53. This increased energy output could result in reduced foraging time or have other fitness-related consequences. Indeed, longer acclimatization times have been associated with increased mortality. For instance, Frair et al.17 found site fidelity for translocated elk was directly related to forage resources encountered, and higher movement rates in low fidelity areas reduced elk survival. Similarly, Moehrenschlager and Macdonald13 found survival of swift foxes (Vulpes velox) was negatively correlated with distance translocated individuals moved from the release site. Longer exploratory bouts, both spatially and temporally, could also decrease reproductive success13, further reducing the founder population’s probability of establishment. For example, Poirier and Festa-Bianchet54 found translocated bighorn sheep required 1 year to acclimate and integrate into the local population. This delayed integration resulted in lower mass gain that ultimately led to a 1–2 year delay in reproduction, and those that did integrate more rapidly received more aggressive behavior (e.g. kicked, displaced).
In our study, we translocated only individual adult females that were not relocated with their social group, yet we suspect many illegal translocations could also involve groups of individuals to promote establishment of reproductive populations. Interestingly, we observed several instances of translocated wild pigs locating other translocated individuals or joining resident groups, reflecting the strong social dynamics of this species. Thus, it is possible group dynamics could affect the results we observed under scenarios involving group translocations. Group foragers such as wild pigs may be able to acquire social information from other conspecifics rather than solely relying on personal sampling55,56,57 to reduce some fitness-related costs associated with exploration of novel environments. For example, Clapp et al.58 documented a 19.5 day reduction in acclimation period from second and third releases of bighorn sheep compared with initial translocation efforts. However, although we did not test this directly, we observed three instances where two translocated wild pigs came into contact and began travelling together shortly after translocation and demonstrated similar movement patterns, both spatially and temporally, to those travelling alone. In fact, two of these individuals (135 and 136) were initially part of the same social group and were translocated separately, yet after rejoining had the largest movement rates of any translocated animals.
Results of SSF models using both resident and translocated animals indicate both extrinsic (e.g., habitat type) and intrinsic (e.g., translocated or resident) variables can influence wild pig resource selection, and highlights the adaptability of this invasive species. Distance to wetlands and bottomland habitat has been shown to be an important driver of wild pig movements33,39 and occurrence probability59, and was likely a key factor affecting resource selection and movement metrics in our study. Water sources are more abundant in bottomland hardwood habitats on our study site. Thus, the fact that wild pigs translocated to upland pine areas exhibited much lower selection for this feature, especially during the first few weeks post translocation, likely required these individuals to make larger movements through low-quality areas to meet basic physiological needs.
Selection for bottomland hardwood habitat generally supported our main hypotheses; (1) resident animals would exhibit relatively static selection through time, and (2) animals translocated to less suitable habitat (upland pine) would show a greater increase in selection of preferred habitat (bottomland hardwood) than those translocated directly into bottomland hardwoods as both groups developed a mental map of the area. Overall, wild pigs translocated to upland pine areas had the lowest selection for bottomland habitat during the first few weeks post translocation, suggesting initial movement behavior may have been focused attempting to relocate to their former range, but showed a positive trend over the 13 week monitoring period. Indeed, during the exploration phase, wild pigs tended to make relatively rapid straight-line movements followed by longer duration periods in which animals moved as if feeding or resting. Additionally, during the exploration phase wild pigs often made quick foray loops (e.g., < 1 day) after which the animal returned to a central location. These relatively quick-duration movements were possibly diluted in our analysis by the longer periods of time in these centralized locations. More fine-scale temporal resolution from GPS collars could elucidate whether animals are selecting for habitats differently during these quick duration exploratory movements, and warrants further investigation. It is also worth highlighting that at the end of the monitoring period, except for the resident upland group, the other treatment groups tended to converge near the same probability of selection for our hypothesized preferred habitat type. This might indicate some optimal amount of bottomland hardwood habitat is required to meet basic biological needs (e.g., as thermal refugia31,33).
Recent technological advancements in GPS telemetry equipment have facilitated a greater understanding of animal movement ecology, and provided the opportunity to understand how a variety of species respond to novel environments13,22,44,58,60. However, most studies have assessed movement dynamics from a conservation-oriented perspective and less attention has focused on how large invasive species respond to similar introductions into novel environments. Expansion of wild pig populations from illegal translocations by humans for hunting purposes has been well documented27,28,61,62, and our analysis indicates there is high dispersal potential from initial release sites prior to establishment within a relatively short time frame. These large-scale movements could hamper mitigation efforts aimed at removing newly established populations and increase risk of cross-species transmission of disease from wild pigs to wildlife, livestock, and humans63. Furthermore, more erratic movements during exploratory phases may increase risk to humans and property through vehicle collisions38,64. Based on our findings, these risks are likely to decrease temporally from initial release to acclimation and associated home range establishment and to be habitat dependent.
Regardless, predictions of an individual’s response to translocation can provide important insight into risks associated with these activities and facilitate more effective translocation strategies for species of conservation concern, e.g.,10. Indeed, nearly 30% of conservation translocation efforts are unsuccessful4 and a primary reason translocation efforts fail is long-distance or frequent movements exhibited by released animals65,66, which may increase the animal’s probability of mortality or emigration from the site20. Similarly, invasive species can greatly affect ecosystems and cause extirpation or extinction of native species67,68, and knowledge of the movement ecology of animals in novel environments may allow improved mitigation of risks posed by invasive animals following their introduction. However, there is a need for additional information regarding how group dynamics may affect the process of exploratory behavior and acclimation to novel environments, as well as the long-term consequences of translocation on overall fitness in both occupied and unoccupied habitats60,69. Thus, we recommend future research address these knowledge gaps to facilitate more effective strategies for the application of species introductions and translocations as potential management and conservation tools, as well as to better mitigate threats from introductions of invasive species.
Currently our understanding of how individuals integrate multiple sources of information to make decisions regarding where to establish is underdeveloped. While there is a substantial body of literature characterizing existing home ranges, using translocations we can observe the process of home range development. This can contribute to improved understanding of higher order processes governing not only home ranges but of species and individuals assorting across the landscape. Detailed tracking of translocated individuals also can shed light on the processes of exploration and settlement, and ultimately help link population-level fitness with space use. Thus, continued expansion of our understanding of underlying processes contributing to the successful establishment of species within diverse landscapes remains critical to the refinement of policies and strategies governing the conservation and management of species.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Edgell, M. C. Trans-hemispheric movements of Holarctic Anatidae: The Eurasian wigeon (Anas penelope L.) in North America. J. Biogeogr. 20, 27–39 (1984).
Gerlach, J., Muir, C. & Richmond, M. D. The first substantiated case of trans-oceanic tortoise dispersal. J. Nat. Hist. 40, 2403–2408 (2006).
Manchester, S. J. & Bullock, J. M. The impacts of non-native species on UK biodiversity and the effectiveness of control. J. Appl. Ecol. 37, 845–864 (2000).
Fischer, J. & Lindenmayer, D. B. An assessment of the published results of animal relocations. Biol. Conserv. 96, 1–11 (2000).
Rahel, F. J. Homogenization of fish faunas across the United States. Science 288, 854–856 (2000).
Schumpeter, J. A. & Opie, R. The Theory of Economic Development: An Inquiry into Profits, Capital, Credit, Interest, and the Business Cycle (Harvard University Press Cambridge, 1934).
March, J. G. Exploration and exploitation in organizational learning. Organ. Sci. 2, 71–87 (1991).
Eliassen, S., Jørgensen, C., Mangel, M. & Giske, J. Exploration or exploitation: Life expectancy changes the value of learning in foraging strategies. Oikos 116, 513–523 (2007).
Berger-Tal, O., Nathan, J., Meron, E. & Saltz, D. The exploration–exploitation dilemma: A multidisciplinary framework. PLoS One 9, e95693 (2014).
Berger-Tal, O. & Saltz, D. Using the movement patterns of reintroduced animals to improve reintroduction success. Curr. Zool. 60, 515–526 (2014).
Merkle, J., Fortin, D. & Morales, J. M. A memory-based foraging tactic reveals an adaptive mechanism for restricted space use. Ecol. Lett. 17, 924–931 (2014).
Ofstad, E. G., Herfindal, I., Solberg, E. J. & Sæther, B. E. Home ranges, habitat and body mass: Simple correlates of home range size in ungulates. Proc. R. Soc. B 283, 20161234 (2016).
Moehrenschlager, A. & Macdonald, D. W. Movement and survival parameters of translocated and resident swift foxes Vulpes velox. Anim. Conserv. 6, 199–206 (2003).
Farnsworth, M. L. et al. Short-term space-use patterns of translocated Mojave desert tortoise in southern California. PLoS One 10, e0134250 (2015).
Flanagan, S. E., Brown, M. B., Fennessy, J. T. & Bolger, D. T. Use of home range behaviour to assess establishment in translocated giraffes. Afr. J. Ecol. 20, 1–10 (2016).
Mertes, K. et al. Management background and release conditions structure Post-release movements in reintroduced ungulates. Front. Ecol. Evol. 7, 470 (2019).
Frair, J. L., Merrill, E. H., Allen, J. R. & Boyce, M. S. Know thy enemy: Experience affects elk translocation success in risky landscapes. J. Wildl. Manage. 71, 541–554 (2007).
van Heezik, Y., Maloney, R. F. & Seddon, P. J. Movements of translocated captive-bred and released Critically Endangered kaki (black stilts) Himantopus novaezelandiae and the value of long-term post-release monitoring. Oryx 43, 639–647 (2009).
Shier, D. M. & Swaisgood, R. R. Fitness costs of neighborhood disruption in translocations of a solitary mammal. Conserv. Biol. 26, 116–123 (2012).
Benson, J. F. & Chamberlain, M. J. Space use, survival, movements, and reproduction of reintroduced Louisiana black bears. J. Wildl. Manage. 71, 2393–2403 (2007).
Rittenhouse, C. D., Millspaugh, J. J., Hubbard, M. W. & Sheriff, S. L. Movements of translocated and resident three-toed box turtles. J. Herpetol. 41, 115–121 (2007).
Bauder, J. M., Castellano, C., Jensen, J. B., Stevenson, D. J. & Jenkins, C. L. Comparison of movements, body weight, and habitat selection between translocated and resident gopher tortoises. J. Wildl. Manage 78, 1444–1455 (2014).
Attum, O. & Cutshall, C. D. Movement of translocated turtles according to translocation method and habitat structure. Restor. Ecol. 23, 588–594 (2015).
Cohen, B., Prebyl, T., Stafford, N., Collier, B. & Chamberlain, M. Space use, movements, and habitat selection of translocated wild turkeys in northwestern Louisiana. In Proceedings of the National Wild Turkey Symposium, Tuscon, Arizona, pp. 165–174 (2015).
Jesmer, B. R. et al. Is ungulate migration culturally transmitted? Evidence of social learning from translocated animals. Science 361, 1023–1025 (2018).
Lewis, J. S. et al. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep. 7, 44152 (2017).
Bevins, S. N., Pedersen, K., Lutman, M. W., Gidlewski, T. & Deliberto, T. J. Consequences associated with the recent range expansion of nonnative feral swine. Bioscience 64, 291–299 (2014).
Tabak, M. A., Piaggio, A. J., Miller, R. S., Sweitzer, R. A. & Ernest, H. B. Anthropogenic factors predict movement of an invasive species. Ecosphere 8, 25 (2017).
Mendl, M., Laughlin, K. & Hitchcock, D. Pigs in space: Spatial memory and its susceptibility to interference. Anim. Behav. 54, 1491–1508 (1997).
Beasley, J. C., Ditchkoff, S. S., Mayer, J. J., Smith, M. D. & VerCauteren, K. C. Research priorities for managing invasive wild pigs in North America. J. Wild. Manage. 82, 674–681 (2018).
Choquenot, D. & Ruscoe, W. A. Landscape complementation and food limitation of large herbivores: Habitat-related constraints on the foraging efficiency of wild pigs. J. Anim. Ecol. 72, 14–26 (2003).
Eckert, K. D., Keiter, D. A. & Beasley, J. C. Animal visitation to wallows and implications for disease transmission. J. Wild. Dis. 55, 488–493 (2019).
Clontz, L. M., Pepin, K. M., VerCauteren, K. C. & Beasley, J. C. Connecting the dots: Behavioral state resource selection in wild pigs in the southeast United States. Sci. Rep. 11, 6924 (2021).
Clontz, L. M., Pepin, K. M., VerCauteren, K. C. & Beasley, J. C. Influence of biotic and abiotic factors on home range size and shape of invasive wild pigs (Sus scrofa). Pest Manage. Sci. 78, 914–928 (2022).
Fortin, D. Optimal searching behaviour: The value of sampling information. Ecol. Modell. 153, 279–290 (2002).
Imm, D. & McLeod, K. Plant Communities. Ecology and Management of a Forested Landscape: Fifty Years of Natural Resource Stewardship on the Savannah River Site 106–160 (Island Press, 2005).
Keiter, D. A. et al. Effects of scale of movement, detection probability, and true population density on common methods of estimating population density. Sci. Rep. 7, 9446 (2017).
Beasley, J. C., Grazia, T. E., Johns, P. E. & Mayer, J. J. Habitats associated with vehicle collisions with wild pigs. Wildl. Res. 40, 654–660 (2014).
Kay, S. L. et al. Quantifying drivers of wild pig movement across multiple spatial and temporal scales. Mov. Ecol. 5, 14 (2017).
McClure, M. L., Burdett, C. L., Farnsworth, M. L., Sweeney, S. J. & Miller, R. S. A globally-distributed alien invasive species poses risks to United States imperiled species. Sci. Rep. 8, 5331 (2018).
Calenge, C. Home Range Estimation in R: The AdehabitatHR Package (Saint Benoist, France, 2019).
Lewis, J. S., Rachlow, J. L., Garton, E. O. & Vierling, L. A. Effects of habitat on GPS collar performance: Using data screening to reduce location error. J. Appl. Ecol. 44, 663–671 (2007).
Kranstauber, B., Kays, R., LaPoint, S. D., Wikelski, M. & Safi, K. A dynamic Brownian bridge movement model to estimate utilization distributions for heterogeneous animal movement. J. Anim. Ecol. 81, 738–746 (2012).
Kranstauber, B., Smolla, M. & Kranstauber, M. Move: Visualizing and analyzing animal track data. R Package Version 1, r365 (2013).
Wood, G. W. & Brenneman, R. E. Feral hog movements and habitat use in coastal South Carolina. J. Wildl. Manage. 20, 420–427 (1980).
Fortin, D. et al. Wolves influence elk movements: Behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86, 1320–1330 (2005).
Fryxell, J. M. et al. Multiple movement modes by large herbivores at multiple spatiotemporal scales. Proc. Natl. Acad. Sci. USA 105, 19114–19119 (2008).
Calenge, C. Analysis of Animal Movements in R: The adehabitatLT Package (R Foundation for Statistical Computing, 2011).
Signer, J., Fieberg, J. & Avgar, T. Animal movement tools (amt): R package for managing tracking data and conducting habitat selection analyses. Ecol. Evol. 9, 880–890 (2019).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2003).
Arnold, T. W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manage. 74, 1175–1178 (2010).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48 (2015).
Chittka, L., Skorupski, P. & Raine, N. E. Speed–accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407 (2009).
Poirier, M. A. & Festa-Bianchet, M. Social integration and acclimation of translocated bighorn sheep (Ovis canadensis). Biol. Conserv. 218, 1–9 (2018).
Galef, B. G. & Giraldeau, L. A. Social influences on foraging in vertebrates: Causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 (2001).
Fortin, D. et al. Group-size-mediated habitat selection and group fusion–fission dynamics of bison under predation risk. Ecology 90, 2480–2490 (2009).
Jones, T. B., Aplin, L. M., Devost, I. & Morand-Ferron, J. Individual and ecological determinants of social information transmission in the wild. Anim. Behav. 129, 93–101 (2017).
Clapp, J. G., Beck, J. L. & Gerow, K. G. Post-release acclimation of translocated low-elevation, non-migratory bighorn sheep. Wildl. Soc. Bull. 38, 657–663 (2014).
McClure, M. L. et al. Modeling and mapping the probability of occurrence of invasive wild pigs across the contiguous United States. PLoS One 10, e0133771 (2015).
Haydon, D. T. et al. Socially informed random walks: Incorporating group dynamics into models of population spread and growth. Proc. R. Soc. Lond. Biol. 275, 1101–1109 (2008).
Gipson, P. S., Hlavachick, B. & Berger, T. Range expansion by wild hogs across the central United States. Wildl. Soc. Bull. 26, 279–286 (1998).
Spencer, P. B. & Hampton, J. O. Illegal translocation and genetic structure of feral pigs in Western Australia. J. Wildl. Manage. 69, 377–384 (2005).
Miller, R. S. et al. Cross-species transmission potential between wild pigs, livestock, poultry, wildlife, and humans: Implications for disease risk management in North America. Sci. Rep. 7, 7821 (2017).
Sáenz-de-Santa-María, A. & Tellería, J. L. Wildlife-vehicle collisions in Spain. Eur. J. Wildl. Res. 61, 399–406 (2015).
Berger-Tal, O., Blumstein, D. T. & Swaisgood, R. R. Conservation translocations: A review of common difficulties and promising directions. Anim. Conserv. 23, 121–131 (2020).
Kemink, K. & Kesler, D. Using movement ecology to inform translocation efforts: A case study with an endangered lekking bird species. Anim. Conserv. 16, 449–457 (2013).
Davis, M. A. Biotic globalization: Does competition from introduced species threaten biodiversity?. AIBS Bull. 53, 481–489 (2003).
Pitt, W. C., Beasley, J. C. & Witmer, G. W. Ecology and Management of Terrestrial Invasive Species in the United States (CRC Press, 2018).
Sigaud, M. et al. Collective decision-making promotes fitness loss in a fusion–fission society. Ecol. Lett. 20, 33–40 (2017).
Acknowledgements
This research was supported financially by the US Department of Energy under Award No. DE-EM0005228 to the UGA Research Foundation and the US Department of Agriculture’s Animal and Plant Health Inspection Service.
Disclaimer
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness. Or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States.
Author information
Authors and Affiliations
Contributions
J.C.B., R.S.M., and S.J.S. conceived the idea; J.B.S. analyzed the data; J.B.S., D.A.K., P.E.S., and J.C.B. collected the data; J.B.S. and D.A.K. wrote an initial version of the manuscript, which was then substantially edited by J.C.B. and all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smith, J.B., Keiter, D.A., Sweeney, S.J. et al. Habitat quality influences trade-offs in animal movement along the exploration–exploitation continuum. Sci Rep 13, 4814 (2023). https://doi.org/10.1038/s41598-023-31457-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31457-3
This article is cited by
-
Spatial ecology of translocated raccoons
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.