Abstract
Many people eat polished rice, while rice bran, a by-product known to be rich in protein and expected to have potential functions for health benefits, has not been effectively utilized. In this study, we determined that orally administered Val-Tyr-Thr-Pro-Gly (VYTPG) derived from rice bran protein improved cognitive decline in mice fed a high-fat diet (HFD). It was demonstrated that VYTPG was released from model peptides corresponding to fragment sequences of original rice proteins (Os01g0941500, Os01g0872700, and allergenic protein) after treatment with thermolysin, a microorganism-derived enzyme often used in industrial scale processes. The thermolysin digest also improved cognitive decline after oral administration in mice. Because VYTPG (1.0 mg/kg) potently improved cognitive decline and is enzymatically produced from the rice bran, we named it rice-memolin. Next, we investigated the mechanisms underlying the cognitive decline improvement associated with rice-memolin. Methyllycaconitine, an antagonist for α7 nicotinic acetylcholine receptor, suppressed the rice-memolin-induced effect, suggesting that rice-memolin improved cognitive decline coupled to the acetylcholine system. Rice-memolin increased the number of 5-bromo-2’-deoxyuridine (BrdU)-positive cells and promoted the mRNA expression of EGF and FGF-2 in the hippocampus, implying that these neurotropic factors play a role in hippocampal neurogenesis after rice-memolin administration. Epidemiologic studies demonstrated that diabetes is a risk factor for dementia; therefore, we also examined the effect of rice-memolin on glucose metabolism. Rice-memolin improved glucose intolerance. In conclusion, we identified a novel rice-derived peptide that can improve cognitive decline. The mechanisms are associated with acetylcholine and hippocampal neurogenesis. Rice-memolin is the first rice-brain-derived peptide able to improve cognitive decline.
Similar content being viewed by others
Introduction
Rice, known as one of the three major grains, is a staple food for almost half of the world population1. It is usually served in the form of polished white rice; however, beneficial health effects of non-polished brown rice have also been reported2,3. Rice bran is well-known to be rich in protein and has a high protein efficiency ratio4; however, its availability remains unknown, and it is not utilized industrially. On the other hand, we have found that an enzymatic digest of bran protein exhibits an antihypertensive effect, followed by isolating novel antihypertensive peptides5,6. As such, the rice bran protein is a promising source of functional peptides.
The importance of Quality of Life (QOL) increases with a prolonged life expectancy. In the past 50 years, life expectancy has been prolonged globally by approximately 10 years. Therefore, there is an increasing concern over how long a human can stay healthy. Aging is known to increase inflammation in organs and induce multiple health problems such as high blood pressure, metabolic disorder, and cognitive decline, to which Western style diet might also be related7. In particular, cognitive decline leads to dementia and necessitates care, which has become a serious issue in countries with an aging society. Mild cognitive impairment (MCI) is a preclinical stage of dementia8. Completely recovering from dementia is difficult; however, it is possible to keep or to improve MCI through medical treatment or lifestyle changes such as dietary habits and exercise9. Especially some clinical trials of dietary intervention were previously reported10,11. Thus, we hypothesized that peptide derived from rice bran protein can be used to improve mild cognitive decline.
So far, epidemiological studies have revealed that glucose tolerance impairment is associated with cognitive decline12,13. It was reported that high-fat diet (HFD) intake, known to induce glucose intolerance, accelerates brain aging and induces cognitive decline14,15,16. Suggested causes might be brain damage, such as the reduction of proliferation and decreased numbers of immature neurons in the hippocampus17. We previously reported that short-term HFD intake induced cognitive decline and hippocampal dysfunction in mice16. In this study, we used this experimental system to examine the effect of a rice bran-derived peptide on cognitive function.
We previously isolated Val-Tyr-Thr-Pro-Gly (VYTPG) in the process searching for angiotensin I-converting enzyme (ACE) inhibitory peptides originated from the thermolysin digest of rice bran proteins (unpublished observation). This peptide has homology with YLG, a milk derived peptide that improved cognitive decline in the mice model16 (Fig. 1). We then examined the effect of VYTPG on cognitive function. VYTPG potently improved cognitive decline at a low dose compared with a known food-derived peptide after oral administration, and we termed it as rice-memolin. We also investigated the mechanisms underlying the cognitive decline improvement of rice-memolin.
Materials and methods
Materials
Rice bran obtained from Japonica rice (Oryza japonica) is commercially available in Japan, named High-Bref, was supplied by Sunbran Co., Ltd. (Tendou, Japan). VYTPG was synthesized chemically using the F-moc method and purified by reverse phase high-performance liquid chromatography (RP-HPLC). d-Glucose was purchased from Sigma-Aldrich (St Louis, MO). The plant collection and use were in accordance with all the relevant guidelines.
Preparation of the digest of rice bran protein
Thermolysin digestion of rice bran protein was performed as described previously5. Briefly, 900 ml of ultrapure water and 0.36 g of thermolysin were added to 27 g of rice bran. Enzymatic digestion took place at 37 °C for 20 h. The digest was then heated for 10 min in boiling water to inactivate thermolysin. The supernatant was recovered after centrifugation (8000×g, 15 °C, 15 min) and its pH was adjusted to 7.0 with sodium hydroxide. The supernatant was then filtered using filter paper No.2 (Advantec, Tokyo, Japan) and freeze-dried.
Pepsin and pancreatin digests of VYTPG and LCMS analysis were performed in a similar manner to previous studies (Shobako et al.) with slight modifications to simulate peptide bioavailability. Each enzyme treatment time was 1 h.
Digest-mixed feeding experiment
Male ddY mice (11 weeks old) were obtained from SLC (Shizuoka, Japan). They were kept in a temperature-controlled room (23 °C) on a daily 12-h light: 12-h dark cycle. After one week-acclimatization, these mice were fed MF pellets (Oriental Yeast, Tokyo, Japan) as control diet, HFD60 pellets (Oriental Yeast Co., Ltd, Japan) as high-fat diet, or HFD 60 pellets containing 0.04% rice bran protein digest (specially ordered from Oriental Yeast Co., Ltd, Japan) with free access to water for 7 days. The component of HFD60 was previously reported18. Animal experiments were conducted in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and were approved by the Committee on Animal Experimentation at Kyoto University, Japan. All experiments were approved by the Kyoto University Ethics Committee for Animal Research Use, permission code was R3-13. All experimental procedures were performed in accordance with the ARRIVE guidelines. All efforts were made to minimize the number of animals used and to limit experimentation to what was necessary to produce reliable scientific information.
Administration experiment
Male ddY mice (11 weeks old) were obtained from SLC (Shizuoka, Japan). They were kept in a temperature-controlled room (23 °C) on a daily 12-h light: 12-h dark cycle. After acclimatization, mice were divided into experimental groups: control diet fed group (CD group), high-fat diet fed group (HFD group), and HFD fed plus administration of the rice bran protein digest (digest group) or chemosynthetic rice-memolin group (rice-memolin group). For the CD group, a 10 kcal% fat containing diet (D12450J, Research Diets) was fed. For the HFD group, digest group, and rice-memolin group, 60 kcal% HFD (D12492, Research Diets Inc., New Brunswick, USA) was fed. Each group was fed for 7 days. For the last 3 days, the digest was administered to the digest group and rice-memolin was administered to the rice-memolin group. Mice were euthanized by cervical dislocation after the experiment. All experiments were approved by the Kyoto University Ethics Committee for Animal Research Use, permission code was R3-13. All efforts were made to minimize the number of animals used and to limit experimentation to what was necessary to produce reliable scientific information. The experiment design was referenced to our previous study16.
Object recognition test and object location test
Cognitive function was evaluated on Day 6 and 7 using two behavioral pharmacological tests: novel object recognition test (ORT) and object location test (OLT). The ORT was performed as described previously in Nagai et al.16 with slight modification. Using the ORT, effect of donepezil, a drug currently used for the relief of cognitive deficits associated with mild to moderate Alzheimer’s diseases, was evaluated19. Briefly, the experimental apparatus used in this study was a square open field (50 cm × 50 cm × 50 cm) made of grey polyvinyl chloride. Two typical objects were used: wooden block and falcon tissue culture flask filled with sand. The ORT comprised three sessions: a habituation session, a familiarization session, and a test session. The habituation session was carried out on Day 6. Each mouse was placed into the experimental apparatus without any objects for 5 min. The familiarization session was performed 24 h after the habituation period. In the familiarization session, each mouse was then placed into the open field in the presence of identical two objects and allowed to explore freely until 20 s of total exploration time was reached. Exploration of the objects was considered positive when the animals' nose was facing the objects less than 1 cm away from the object. After 1 h, the test session was performed. At the test session, one object of each pair was replaced with a novel object. Each mouse was again placed into the open field for 20 s of total exploration time, and the exploration time spent on the familiar object and novel object were measured.
BrdU incorporation in the hippocampus
BrdU incorporation was assessed as reported previously in Nagai et al. and Yamamoto et al.16,20 On Day 6, BrdU (100 mg/kg; Sigma Aldrich) was administered intraperitoneally. Twenty-four hours after BrdU administration, mice were euthanized and transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde under anesthesia (n = 4). Brains were postfixed overnight in 4% paraformaldehyde at room temperature and dehydrated in 20% sucrose (4 °C for 1 day). Serial sections of the brains were cut (30-mm sections) on a freezing microtome. After the sections were washed with PBS containing Triton X-100 (PBST), they were incubated in 2 N HCl for 45 min and washed with PBST. After blocking in 3% normal donkey serum–PBST for 1 h, sections were incubated overnight with anti-rat BrdU (1:1,000; Abcam, Cambridge, United Kingdom) and DAPI (1:100,000; Thermo Fisher Scientific, Waltham, MA, USA) at room temperature. They were incubated with the secondary antibody (1:500, donkey anti-rat Alexa 594; Thermo Fisher Scientific) for 2 h. BrdU-positive cells were counted in the hippocampus using an Olympus microscope (Olympus, Tokyo, Japan).
RNA preparation and quantitative RT-PCR
The hippocampus was excised. Total RNA was extracted from the hippocampus using a RNeasy Lipid Tissue Kit (QIAGEN Sciences Inc.) and transcribed into cDNA with random primers using Takara PrimeScript RT Master Mix (Takara, Osaka, Japan). For quantitative PCR, we amplified the cDNA using a LightCycler 96 System (Roche Diagnostics Co., Mannheim, Germany) with THUNDERBIRD qPCR Mix (Toyobo Co., Osaka, Japan). Primer sets specific for mouse brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-3 (NT-3), glial cell line-derived neurotrophic factor (GDNF), epidermal growth factor (EGF), ciliary neurotrophic factor (CNTF), fibroblast growth factor 2 (FGF2), insulin-like growth factor 2 (IGF2), and vascular endothelial growth factor (VEGF) were prepared in accordance with the manufacturer's instructions. The primer sequences are shown in Supplemental Table S1. The reactions were cycled 45 times with denaturation at 95 °C for 10 s, and with annealing and elongation at 65 °C for 60 s each. The relative expression level of each mRNA was normalized using the mRNA level of β-actin.
Oral glucose tolerance test
Glucose lowering effect of the peptide was assessed using the oral glucose tolerance test (OGTT). The OGTT was performed as reported previously in Ogiwara et al.21. Briefly, glucose solution was administered orally at a dose of 2 g/kg to mice fasted for 18 h. Blood was obtained from the tail vein. The blood glucose levels were immediately measured before and 15, 30, 60, and 90 min after glucose administration using a FreeStyle Freedom (Nipro Corp., Osaka Japan). Rice-memolin dissolved in saline was administered p.o. 2 h before glucose administration. The GTT was started from noon during the light phase of the light/dark cycle.
Data analysis
All values are expressed as the means ± SEM. To assess differences among more than three groups, analysis of variance (ANOVA) followed by post-hoc test was performed. Dunnett’ test was used for comparing the HFD group with other groups. P-values less than 0.05 were considered significant.
Results
Rice bran protein-originated pentapeptide VYTPG improved HFD-induced cognitive decline after oral administration
To investigate the effect of rice bran peptide on cognitive function, we performed two behavior tests: the novel ORT and the OLT. Chronic intake of HFD was reported by Lindqvist et al.22 to reduce cognitive function; however, we have recently found that short-term HFD intake has similar effects16. Thus, we used mice treated with HFD for 1 week.
The results related to cognitive function are shown in Fig. 2. In the novel ORT, the approach time to new objects of mice fed HFD decreased compared with that of mice fed the control diet (CD), consistent with previous report (Fig. 2A). In contrast, orally administered VYTPG, a pentapeptide originating from rice bran protein, increased the approach time to the new object. These results suggest that VYTPG improves cognitive decline in mice fed with HFD. In the OLT, VYTPG also increased the approach time to the object placed in a new location (Fig. 2B). Thus, we demonstrated that VYTPG improved cognitive decline in two different paradigms. The effective dose of VYTPG was 1.0 mg/kg/day, and it was administrated for 3 consecutive days, which is 1/10 of the effective dose of previously reported for YLG (10 mg/kg/day for 1 week). To the best of our knowledge, VYTPG is the most potent peptide for improving cognitive decline even after oral administration.
VYTPG improved cognitive decline in mice treated with HFD after oral administration. Mice were fed CD or HFD for a week and orally administered saline or VYTPG at a dose of 1 mg/kg (once a day for 3 days). (A) Thereafter, the novel object recognition test (ORT) was performed. (B) The object location test (OLT) was also performed similarly to the novel ORT. All values are means ± SEM (A, n = 4–6; B, n = 5–7). *P < 0.05, ***P < 0.001 vs. HFD group.
VYTPG, named rice-memolin, is enzymatically released from rice bran proteins
VYTPG was detected and isolated from the thermolysin digest by RP-HPLC. To assess its origin, the amino acid sequence of rice proteins including VYTPG was searched using BLAST online (https://blast.ncbi.nlm.nih.gov/Blast.cgi). We found that VYTPG corresponded to Os01g0941500(9–13), Os01g0872700(9–13), and allergenic protein(35–39) (Fig. 3C). Model peptides, a sub-sequence of Os01g0941500, YFDPPVYTPGIKPCR; Os01g0872700, ILREVVYTPGQQDKC, and allergenic protein, HQDQVVYTPGPLCQP were synthesized using the F-moc method. VYTPG was found in the thermolysin-digested model peptide (Fig. 3D–F). On the other hand, VYTPG itself existed even after digestion by pepsin and pancreatin present in the gastrointestinal tract digestion (Supplemental Fig. S1). These results suggest that VYTPG was resistant against gut proteases.
Effect of rice bran digest on cognitive decline and release of VYTPG, named as rice-memolin, from three model peptides corresponding to partial sequences of the original proteins. (A) Mice were fed CD, HFD, or 0.04% digest-mixed HFD for a week. Thereafter, the novel ORT was performed. (B) Mice were fed HFD for a week and the digest was administered orally at a dose of 50 and 200 mg/kg (once a day for 3 days). Thereafter, the novel ORT was performed. All values are means ± SEM (A, n = 5–8; B, n = 12–13). *P < 0.05, **P < 0.01 vs. HFD group. (C) Each model peptide, corresponding to partial sequence of rice protein candidate containing VYTPG sequence, was synthesized. These HPLC chromatograms showed that VYTPG was released by thermolysin digestion from the model peptides corresponding to the partial sequence of (D) Os01g0941500, (E) Os01g0872700, and (F) allergenic protein.
The effect of the rice bran digest on cognitive function was also tested using the novel ORT. The thermolysin digest increased the approach time to the novel object compared with that of the HFD group (Fig. 3A,B), suggesting that the digest also ameliorated cognitive decline which is similar to VYTPG.
Taken together, VYTPG potently improved cognitive decline, and this peptide was demonstrated to be enzymatically produced from rice bran proteins. We then named it rice-memolin.
Cognitive improving effect of rice-memolin was associated with acetylcholine
Acetylcholine is a transmitter associated with memory and learning. The α7 nicotinic acetylcholine receptor (α7nAChR) is also present in the brain, especially in the cerebrum and hippocampus, and is associated with memory and learning23. To investigate the mechanism of rice-memolin, we performed a novel ORT using methyllycaconitine (MLA; Sigma Aldrich), an antagonist of α7nAChR. Injection of MLA (0.3 mg/kg, i.p.) inhibited the effects of rice-memolin (Fig. 4), suggesting that rice-memolin improves cognitive decline via α7nAChR.
Rice-memolin improves cognitive decline depends on the acetylcholine system. Mice were fed HFD for a week and rice-memolin was administered orally at a dose of 1 mg/kg (once a day for 3 days) with or without administration of methyllycaconitin (MLA), an antagonist for α7 nicotinic acetylcholine receptor, at a dose of 0.3 mg/kg. Thereafter, the novel ORT was performed. All values are means ± SEM (n = 3–5). *P < 0.05, **P < 0.01, ***P < 0.001 vs. HFD group. RM: Rice-memolin.
Rice-memolin increased neurogenesis and expression of neurotrophic factors in the hippocampus
To investigate the effect of rice-memolin on neurogenesis in the hippocampus, hippocampal BrdU-positive cells were examined. We reported previously that 1 week of HFD intake reduced BrdU-positive cells in the hippocampus16. BrdU-positive cells in the hippocampus significantly increased after injection of rice-memolin (Fig. 5A–C), suggesting that rice-memolin increased hippocampal neurogenesis that was depressed by HFD intake.
Rice-memolin increases hippocampal neurogenesis and mRNA expression of neurotrophic factors in mice fed with HFD. Representative histology of the mice administered (A) saline or (B) rice-memolin and (C) the number of BrdU-positive cells. (D) Changes in the hippocampal mRNA expression of neurotrophic factors. All values are means ± SEM (A–C, n = 4; D, n = 6). #P < 0.1, *P < 0.05 vs. saline group. RM: Rice-memolin.
Neurotrophic factors are associated with hippocampal neurogenesis and cognitive function. We examined the hippocampal mRNA expression of neurotrophic factors, including BDNF, NGF, NT-3, GDNF, EGF, CNTF, FGF-2, IGF-2, and VEGF. Administration of rice-memolin significantly increased the mRNA expression of EGF and FGF-2, and slightly increased NGF, NT-3, and GDNF (Fig. 5D). This suggests that changes in neurotrophic factors might contribute to neurogenesis and cognitive function.
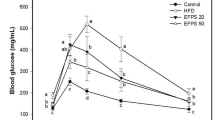
Rice-memolin reduces blood glucose
Impaired glucose tolerance is a factor of cognitive decline12,13. We then performed an OGTT, as shown in Fig. 6A–C. Fasting-glucose levels were not different among all groups (Fig. 6B). HFD intake increased the blood glucose level, and rice-memolin ameliorated such increase (Fig. 6A,C).
Discussion
In this study, we found rice-memolin (VYTPG), a novel rice bran-derived peptide to improve cognitive decline. Rice-memolin potently ameliorated cognitive decline induced by short-term HFD intake after oral administration. Several protease-digested rice bran showed functional properties such as hypotensive24, anti-diabetic25 and anti-inflammatory26 effects. However, a few studies have isolated the functional amino acid sequence27. This is the first rice bran-derived peptide to improve cognitive decline.
Aging reduces hippocampal neurogenesis and synaptic plasticity through multiple factors, including structural alternations such as increasing altered intracellular signaling, gene expression, and neuroinflammation28. Such hippocampal dysfunction leads to cognitive decline. Aging also induces decreases in acetylcholine in brain regions, including the hippocampus, and weakens cholinergic signaling, thereby suppressing cognitive function29,30. For example, the acetylcholine esterase donepezil, which prevents the degradation of acetylcholine and promotes cholinergic neurotransmission, has been widely used to treat dementia. This study suggested that rice-memolin, a peptide contained in the enzymatic digest of rice bran protein, improves cognitive decline by stimulating acetylcholine receptor α7nAChR. Acetylcholine stimulation increased hippocampal neurogenesis31. We also noted an increase in hippocampal neurogenesis by rice-memolin, which may be related to increased acetylcholine signaling. Rice-memolin may be a novel dementia mediation or preventive agent.
It was reported that neurogenesis occurs throughout life even in the adult brain, especially in the hippocampus32,33. We also uncovered that short-term HFD intake suppresses hippocampal neurogenesis and mRNA expression of neurotrophic factors, resulting in cognitive decline, as reported previously16,34. On the other hand, administration of rice-memolin promoted hippocampal neurogenesis, increased mRNA expression of EGF and FGF-2, and slightly increased NGF, NT-3, and GDNF. NGF is a neurotrophic factor produced and secreted by glial cells35,36,37 that plays a role in the maintenance and survival of cholinergic neurons releasing acetylcholine. GDNF is also produced and secreted from glial cells, and it functions in the maintenance and survival of dopaminergic neurons38,39. Both acetylcholine and dopamine are neurotransmitters involved in memory39,40,41. NT-3 is a neurotrophin that promotes the survival and differentiation of neural progenitor cells42,43. Both FGF-2 and EGF are essential for the proliferation and survival of neural precursor cells44,45, and hippocampal neurogenesis depends greatly on FGF-246. An enriched environment, including progenitor cell proliferation and cell survival, improves cognitive performance47,48. Orally administered rice-memolin may alter the hippocampal expression of these genes, thereby affecting neurogenesis and improving cognitive function.
Brain inflammation is thought to play a key role in HFD-induced cognitive loss14, whereas rice-memolin had no effect on the hippocampal mRNA expression of inflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1α, IL-1β, and IL-6 (Supplementary Table S2). Rice-memolin might improve HFD-induced cognitive decline independent of the inflammatory response.
Insulin resistance also induces cognitive dysfunction, and glucose intolerance was suggested to be a risk factor for dementia in a comparative study12. Others reported that activation of AMPK in skeletal muscle improved cognitive disorder49. We found that rice-memolin improved glucose tolerance, suggesting that the cognition-improving effects of rice-memolin are associated with not only an increase in acetylcholine and hippocampal function but also improved glucose tolerance. Further studies will reveal the mechanisms underlying peptide-induced improvement of glucose metabolism, including the involvement of muscle AMPK activation.
Several food-derived peptides have been reported to improve cognitive function16,50,51; however, higher concentrations and more frequent administration than rice-memolin were required. On the other hand, prevention of cognitive decline by processed rice bran was reported, but functional substances were not cleared and some of them were considered as γ-oryzanol52,53,53,55. Rice-memolin is orally active and the most potent cognition-improving peptide derived from food to the best of our knowledge. Cognition-improving molecules may be taken daily as an easy-to-eat food to prevent cognitive impairment at an early stage. To sum up, a schematic diagram of rice-memolin was summarized in Fig. 7.
Other limitations are still existing. Since we tested only a diet related cognition decline model, evaluation of other models such as aged mouse model is needed. The cognitive improvement of rice bran protein digest appears to be saturated at 50 mg/kg (Fig. 3b), but further dose-dependent studies are needed. Evaluation in human trials, especially considering the relation between aging and diets might also be needed. Further studies on metabolism of rice-memolin, especially in the liver, and on its half-life are required, while the safety of the rice bran digest containing this peptide had been checked by human clinical study56.
In conclusion, we found VYTPG named as rice-memolin, a novel pentapeptide derived from rice bran protein, improved cognitive decline in mice. This improvement might be coupled to the acetylcholine system. Rice-memolin tends to induce hippocampal neurogenesis and improve glucose metabolism. We also demonstrated that rice-memolin is enzymatically released from rice bran proteins using model peptides corresponding to partial sequences of known rice bran proteins.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Muthayya, S., Sugimoto, J. D., Montgomery, S. & Maberly, G. F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 1324, 7–14 (2014).
Kondo, K. et al. Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: A randomized controlled trial. PLoS ONE 12, e0179869 (2017).
Takano, Y., Kokubun, K., Saika, K., Nishiyama, N. & Taki, Y. Effect of the intake of brown rice for six months on the cognitive function in healthy elderly persons: A study protocol for a pilot, non-randomized controlled trial. Methods Protoc. 4, 78 (2021).
Han, S. W., Chee, K. M. & Cho, S. J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 172, 766–769 (2015).
Shobako, N. et al. A novel antihypertensive peptide identified in thermolysin-digested rice bran. Mol. Nutr. Food Res. 62, 1–7 (2018).
Shobako, N. et al. Vasorelaxant and Antihypertensive Effects That Are Dependent on the Endothelial NO System Exhibited by Rice Bran-Derived Tripeptide. J. Agric. Food Chem. 67, 1437–1442. https://doi.org/10.1021/acs.jafc.8b06341 (2019).
Więckowska-Gacek, A., Mietelska-Porowska, A., Wydrych, M. & Wojda, U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res. Rev. 70, 101397. https://doi.org/10.1016/j.arr.2021.101397 (2021).
Anderson, N. D. State of the science on mild cognitive impairment (MCI). CNS Spectr. 24, 78–87 (2019).
Tao, J. et al. Mind-body exercise improves cognitive function and modulates the function and structure of the hippocampus and anterior cingulate cortex in patients with mild cognitive impairment. Neuroimage Clin. 23, 101834 (2019).
Igase, M. et al. Auraptene in the peels of citrus kawachiensis (kawachibankan) contributes to the preservation of cognitive function: A randomized, placebo-controlled, double-blind study in healthy volunteers. J. Prev. Alzheimers Dis. https://doi.org/10.14283/jpad.2017.47 (2017).
Sasai, M. et al. Effects of a single dose of tablets containing lactononadecapeptide on cognitive function in healthy adults: A randomized, double-blind, cross-over, placebo-controlled trial. Biosci. Biotechnol. Biochem. 85, 948–956 (2021).
Ohara, T. et al. Glucose tolerance status and risk of dementia in the community: The Hisayama study. Neurology 77, 1126–1134 (2011).
Matsuzaki, T. et al. Insulin resistance is associated with the pathology of Alzheimer disease: The Hisayama study. Neurology 75, 764–770 (2010).
Uranga, R. M. et al. Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J. Neurochem. 114, 344–361. https://doi.org/10.1111/j.1471-4159.2010.06803.x (2010).
Sanguinetti, E. et al. Combined effect of fatty diet and cognitive decline on brain metabolism, food intake, body weight, and counteraction by intranasal insulin therapy in 3×tg mice. Front. Cell Neurosci. 13, 00188 (2019).
Nagai, A., Mizushige, T., Matsumura, S., Inoue, K. & Ohinata, K. Orally administered milk-derived tripeptide improved cognitive decline in mice fed a high-fat diet. FASEB J. 33, 14095–14102 (2019).
Driscoll, I. et al. The aging hippocampus: A multi-level analysis in the rat. Neuroscience 139, 1173–1185 (2006).
Sumi, T. et al. (−)-Epigallocatechin-3-Gallate Suppresses Hepatic Preneoplastic Lesions Developed in A Novel Rat Model of Non-alcoholic Steatohepatitis. http://www.springerplus.com/content/2/1/690 (2013).
Provensi, G. et al. Donepezil, an acetylcholine esterase inhibitor, and ABT-239, a histamine H3 receptor antagonist/inverse agonist, require the integrity of brain histamine system to exert biochemical and procognitive effects in the mouse. Neuropharmacology 109, 139–147. https://doi.org/10.1016/j.neuropharm.2016.06.010 (2016).
Yamamoto, Y. et al. Antidepressant-like effect of food-derived pyroglutamyl peptides in mice. Neuropeptides 51, 25–29 (2015).
Ogiwara, M., Ota, W., Mizushige, T., Kanamoto, R. & Ohinata, K. Enzymatic digest of whey protein and wheylin-1, a dipeptide released in the digest, increase insulin sensitivity in an Akt phosphorylation-dependent manner. Food Funct. 9, 4635–4641 (2018).
Lindqvist, A. et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 13, 1385–1388 (2006).
Valentine, G. & Sofuoglu, M. Cognitive effects of nicotine: Recent progress. Curr. Neuropharmacol. 16, 403–414 (2018).
Ardiansyah, A., Shirakawa, H., Koseki, T., Hashizume, K. & Komai, M. The Driselase-treated fraction of rice bran is a more effective dietary factor to improve hypertension, glucose and lipid metabolism in stroke-prone spontaneously hypertensive rats compared to ferulic acid. Br. J. Nutr. 97, 67–76 (2007).
Boonloh, K. et al. Rice bran protein hydrolysates attenuate diabetic nephropathy in diabetic animal model. Eur. J. Nutr. 57, 761–772 (2018).
Saisavoey, T., Sangtanoo, P., Reamtong, O. & Karnchanatat, A. Antioxidant and anti-inflammatory effects of defatted rice bran (Oryza sativa L.) protein hydrolysates on raw 264.7 macrophage cells. J. Food Biochem. 40, 731–740 (2016).
Shobako, N. Hypotensive peptides derived from plant proteins. Peptides 142, 170573 (2021).
Bettio, L. E. B., Rajendran, L. & Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 79, 66–86. https://doi.org/10.1016/j.neubiorev.2017.04.030 (2017).
Kehr, J. et al. Microdialysis in freely moving mice: Determination of acetylcholine, serotonin and noradrenaline release in galanin transgenic mice. J. Neurosci. Methods 109, 71–80 (2001).
Ikegami, S. Behavioral impairment in radial-arm maze learning and acetylcholine content of the hippocampus and cerebral cortex in aged mice. Behav. Brain Res. 65, 103–111 (1994).
Itou, Y., Nochi, R., Kuribayashi, H., Saito, Y. & Hisatsune, T. Cholinergic activation of hippocampal neural stem cells in aged dentate gyrus. Hippocampus 21, 446–459 (2011).
Kempermann, G., Kuhn, H. G. & Gage, F. H. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997).
Kuhn, H. G., Dickinson-Anson, H. & Gage, F. H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 76, 2027–2033 (1996).
Wang, Q. et al. FGF21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. Mol. Neurobiol. 55, 4702–4717 (2018).
Hirose, M., Kuroda, Y. & Murata, E. NGF/TrkA signaling as a therapeutic target for pain. Pain Pract. 16, 175–182 (2016).
Sofroniew, M. V., Howe, C. L. & Mobley, W. C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 24, 1217–81 (2001).
Schliebs, R. & Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 221, 555–563 (2011).
Lin, L. F., Doherty, D. H., Lile, J. D., Bektesh, S. & Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132 (1993).
Blokland, A. Acetylcholine: a neurotransmitter for learning and memory?. Brain Res. Brain Res. Rev. 21, 285–300 (1995).
Pepeu, G. & Grazia Giovannini, M. The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 1670, 173–184 (2017).
Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 67, 53–83 (2002).
Binder, D. K. Neurotrophins in the dentate gyrus. Prog. Brain Res. 163, 371–397 (2007).
Barnabé-Heider, F. & Miller, F. D. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J. Neurosci. 23, 5149–5160 (2003).
Zechel, S., Werner, S., Unsicker, K. & von Bohlen und Halbach, O. Expression and functions of fibroblast growth factor 2 (FGF-2) in hippocampal formation. The Neuroscientist 16, 357–373 (2010).
Wong, R. W. C. & Guillaud, L. The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 15, 147–156 (2004).
Reuss, B. & von Bohlen und Halbach, O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 313, 139–157 (2003).
Cope, E. C. & Gould, E. Adult neurogenesis, glia, and the extracellular matrix. Cell Stem Cell 24, 690–705 (2019).
Nilsson, M., Perfilieva, E., Johansson, U., Orwar, O. & Eriksson, P. S. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 39, 569–578 (1999).
Kobilo, T. et al. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn. Mem. 21, 119–126 (2014).
Ano, Y. et al. Tryptophan-related dipeptides in fermented dairy products suppress microglial activation and prevent cognitive decline. Aging 11, 2949–2967 (2019).
Ano, Y. et al. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 72, 23–31 (2018).
Hara, T. et al. Black rice bran intake reduces phosphorylated tau levels and enhances insulin signaling in the brain of aged normal mice. Biosci. Biotechnol. Biochem. 86, 1570–1575 (2022).
Mostafa, A. O., Abdel-Kader, R. M. & Heikal, O. A. Enhancement of cognitive functions by rice bran extract in a neuroinflammatory mouse model via regulation of PPARγ. J. Funct. Foods 48, 314–321 (2018).
Hagl, S. et al. Effects of long-term rice bran extract supplementation on survival, cognition and brain mitochondrial function in Aged NMRI mice. Neuromol. Med. 18, 347–363 (2016).
Behl, T. et al. Rice bran, an off-shoot to newer therapeutics in neurological disorders. Biomed. Pharmacother. 140, 11796. https://doi.org/10.1016/j.biopha.2021.111796 (2021).
Ogawa, Y. et al. Rice bran supplement containing a functional substance, the novel peptide Leu-Arg-Ala, has anti-hypertensive effects: A double-blind, randomized, placebo-controlled study. Nutrients 11, 130–139 (2019).
Acknowledgements
This work was financially supported by Sunstar Inc. This study was also supported in part by JSPS KAKENHI Grant (KO/No. 22H02286). We performed a collaborative research to reveal the bioactivity related to the rice bran protein digest.
Funding
The authors received financially support from SUNSTAR Inc.
Author information
Authors and Affiliations
Contributions
K.O. supervised and designed the experiments; M.S., N.S. and B.Z. with help from K.K. performed the experiments and analyzed the data; M.S. and K.O. wrote the paper; and all authors discussed the results and the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shobako, M., Shobako, N., Zhang, B. et al. Rice-memolin, a novel peptide derived from rice bran, improves cognitive function after oral administration in mice. Sci Rep 13, 2887 (2023). https://doi.org/10.1038/s41598-023-30021-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30021-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.